Reticulocyte and Erythrocyte Hemoglobin Parameters for Iron Deficiency and Anemia Diagnostics in Patient Blood Management. A Narrative Review
Abstract
:1. Introduction
2. Patient Blood Management in the ERAS Concept
3. Causes of Anemia
3.1. Iron Deficiency Anemia
3.2. Anemia of Inflammation (ACD, Anemia of Chronic Disease)/Hospital-Acquired Anemia
3.3. Using Diagnostics to Differentiate between Iron Deficiency Anemia and Anemia of Chronic Diseases to Identify Adequate Treatment
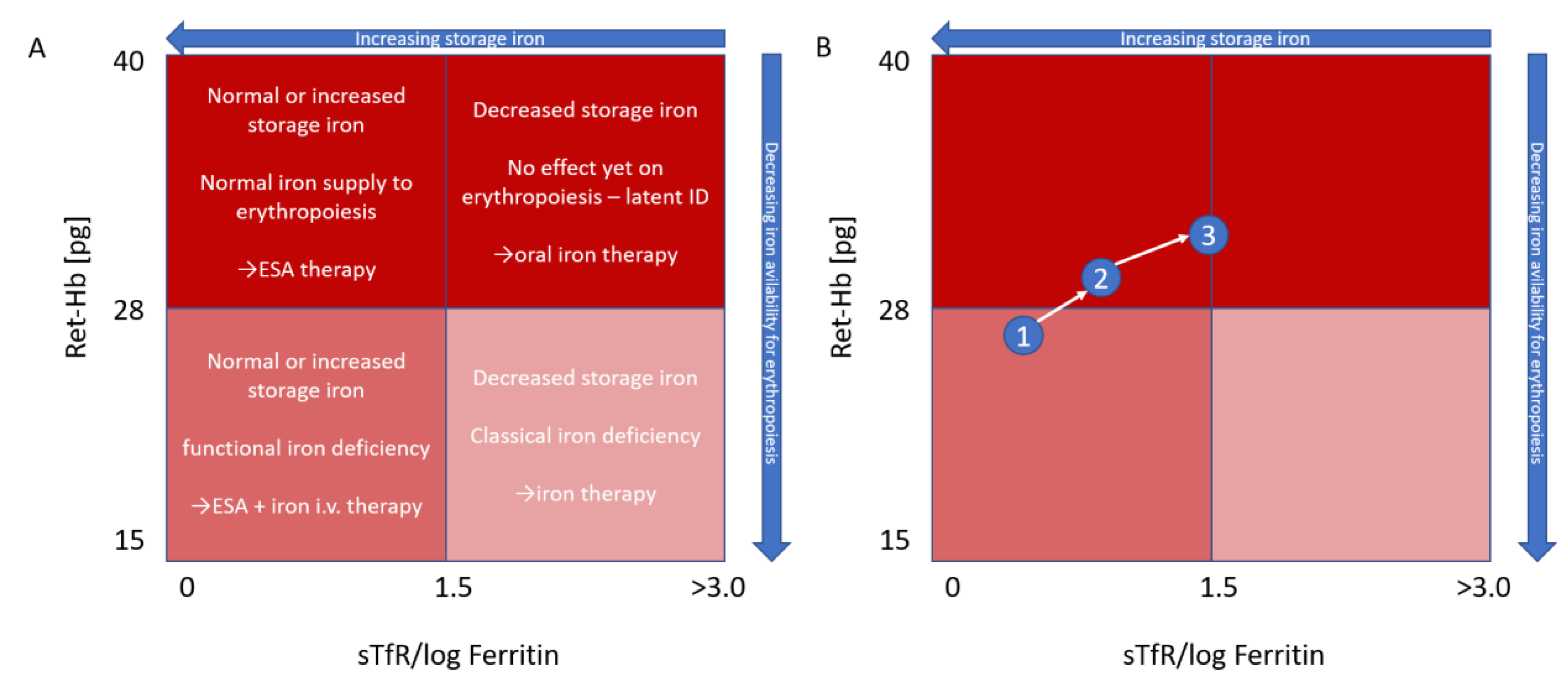
4. Reticulocyte Hemoglobin
5. Treatment
6. Postoperative Iron Deficiency
7. Patient Blood Management (PBM) and Iron Deficiency
8. Potential Role of Ret-He in Treatment of Iron Deficiency in Septic Patients
9. Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kehlet, H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br. J. Anaesth. 1997, 78, 606–617. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, H.; Kettelhack, C. Fast-Track Surgery—Conditions and Challenges in Postsurgical Treatment: A Review of Ele-ments of Translational Research in Enhanced Recovery after Surgery. Eur. Surg. Res. 2012, 49, 24–34. [Google Scholar] [CrossRef]
- Moningi, S.; Patki, A.; Padhy, N.; Ramachandran, G. Enhanced recovery after surgery: An anesthesiologist’s perspective. J. Anaesthesiol. Clin. Pharmacol. 2019, 35, S5–S13. [Google Scholar] [CrossRef] [PubMed]
- Ripollés-Melchor, J.; Rodríguez, J.M.R.; Casans-Francés, R.; Aldecoa, C.; Abad-Motos, A.; Logroño-Egea, M.; García-Erce, J.A.; Camps-Cervantes, Á.; Ferrando, C.; De La Rica, A.S.; et al. Association Between Use of Enhanced Recovery After Surgery Protocol and Postoperative Complications in Colorectal Surgery. JAMA Surg. 2019, 154, 725–736. [Google Scholar] [CrossRef]
- Ripollés-Melchor, J.; Abad-Motos, A.; Díez-Remesal, Y.; Aseguinolaza-Pagola, M.; Padin-Barreiro, L.; Sánchez-Martín, R.; Logro-ño-Egea, M.; Catalá-Bauset, J.C.; García-Orallo, S.; Bisbe, E.; et al. Association Between Use of Enhanced Recovery After Surgery Protocol and Postoperative Complications in Total Hip and Knee Arthroplasty in the Postoperative Outcomes Within En-hanced Recovery After Surgery Protocol in Elective Total Hip and Knee Arthroplasty Study (POWER2). JAMA Surg. 2020, 155, e196024. [Google Scholar]
- Dean, H.F.; Carter, F.; Francis, N.K. Modern perioperative medicine—Past, present, and future. Innov. Surg. Sci. 2019, 4, 123–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musallam, K.M.; Tamim, H.M.; Richards, T.; Spahn, D.R.; Rosendaal, F.R.; Habbal, A.; Khreiss, M.; Dahdaleh, F.S.; Khavandi, K.; Sfeir, P.M.; et al. Preoperative anaemia and postoperative outcomes in non-cardiac surgery: A retrospective cohort study. Lancet Lond. Engl. 2011, 378, 1396–1407. [Google Scholar] [CrossRef]
- Gómez-Ramírez, S.; Bisbe, E.; Shander, A.; Spahn, D.R.; Muñoz, M. Management of Perioperative Iron Deficiency Anemia. Acta Haematol. 2019, 142, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Fowler, A.; Ahmad, T.; Phull, M.K.; Allard, S.; Gillies, M.A.; Pearse, R.M. Meta-analysis of the association between preoperative anaemia and mortality after surgery. Br. J. Surg. 2015, 102, 1314–1324. [Google Scholar] [CrossRef]
- Vincent, J.L.; Baron, J.-F.; Reinhart, K.; Gattinoni, L.; Thijs, L.; Webb, A.; Meier-Hellmann, A.; Nollet, G.; Peres-Bota, D. ABC (Anemia and Blood Transfusion in Critical Care) Investigators. Anemia and blood transfusion in critically ill patients. JAMA 2002, 288, 1499–1507. [Google Scholar] [CrossRef] [Green Version]
- Muñoz, M.; Acheson, A.G.; Bisbe, E.; Butcher, A.; Gómez-Ramírez, S.; Khalafallah, A.A.; Kehlet, H.; Kietaibl, S.; Liumbruno, G.M.; Meybohm, P.; et al. An international consensus statement on the management of postoperative anaemia after major surgical procedures. Anaesthesia 2018, 73, 1418–1431. [Google Scholar] [CrossRef] [Green Version]
- Spahn, D.R. Anemia and patient blood management in hip and knee surgery: A systematic review of the literature. Anesthesiology 2010, 113, 482–495. [Google Scholar] [CrossRef] [Green Version]
- Markowitz, M.A.; Waters, J.H.; Ness, P.M. Patient blood management: A primary theme in transfusion medicine. Transfusion 2014, 54, 2587. [Google Scholar] [CrossRef]
- Ferraris, V.A.; Davenport, D.L.; Saha, S.P.; Austin, P.; Zwischenberger, J.B. Surgical Outcomes and Transfusion of Minimal Amounts of Blood in the Operating Room. Arch. Surg. Chic. III 2012, 147, 49–55. [Google Scholar] [CrossRef] [Green Version]
- Muñoz, M.; Shander, A.; Rijhwani, T.; Dyga, R.; Waters, J. Postoperative blood management strategies. In Patient Blood Management: Multidisciplinary Approaches to Optimizing Care; AABB Press: Bethesda, MD, USA, 2016; pp. 233–258. [Google Scholar]
- Kurniali, P.C.; Curry, S.; Brennan, K.W.; Velletri, K.; Shaik, M.; Schwartz, K.A.; McCormack, E. A Retrospective Study Investigating the Incidence and Predisposing Factors of Hospital-Acquired Anemia. Anemia 2014, 2014, 634582. [Google Scholar] [CrossRef] [Green Version]
- Koch, C.G.; Li, L.; Sun, Z.; Hixson, E.D.; Tang, A.; Phillips, S.C.; Blackstone, E.H.; Henderson, J.M. Hospital-acquired anemia: Prevalence, outcomes, and healthcare implications. J. Hosp. Med. 2013, 8, 506–512. [Google Scholar] [CrossRef]
- Salisbury, A.C.; Alexander, K.P.; Reid, K.J.; Masoudi, F.A.; Rathore, S.S.; Wang, T.Y.; Bach, R.G.; Marso, S.P.; Spertus, J.A.; Kosiborod, M. Incidence, Correlates, and Outcomes of Acute, Hospital-Acquired Anemia in Patients with Acute Myocardial Infarction. Circ. Cardiovasc. Qual. Outcomes 2010, 3, 337–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salisbury, A.C.; Amin, A.P.; Reid, K.J.; Wang, T.Y.; Masoudi, F.A.; Chan, P.S.; Alexander, K.P.; Bach, R.G.; Spertus, J.A.; Kosiborod, M. Hospital-acquired anemia and in-hospital mortality in patients with acute myocardial infarction. Am. Heart J. 2011, 162, 300–309.e3. [Google Scholar] [CrossRef]
- Meroño, O.; Cladellas, M.; Recasens, L.; García-García, C.; Ribas, N.; Bazan, V.; Farré, N.; Sainz, Á.; Comin, J.; Bruguera, J. In-hospital Acquired Anemia in Acute Coronary Syndrome. Predictors, In-hospital Prognosis and One-year Mortality. Rev. Española Cardiol. 2012, 65, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Salisbury, A.C.; Reid, K.J.; Alexander, K.P.; Masoudi, F.A.; Lai, S.-M.; Chan, P.S.; Bach, R.G.; Wang, T.Y.; Spertus, J.A.; Kosiborod, M. Diagnostic blood loss from phlebotomy and hospital-acquired anemia during acute myocardial infarction. Arch. Intern. Med. 2011, 171, 1646–1653. [Google Scholar] [CrossRef] [PubMed]
- Thavendiranathan, P.; Bagai, A.; Ebidia, A.; Detsky, A.S.; Choudhry, N.K. Do blood tests cause anemia in hospitalized patients? J. Gen. Intern. Med. 2005, 20, 520–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rennke, S.; Fang, M.C. Hazards of hospitalization: More than just “never events”. Arch. Intern. Med. 2011, 171, 1653–1654. [Google Scholar] [CrossRef]
- Choi, J.S.; Kim, Y.A.; Kang, Y.U.; Kim, C.S.; Bae, E.H.; Ma, S.K.; Ahn, Y.-K.; Jeong, M.H.; Kim, S.W. Clinical Impact of Hospital-Acquired Anemia in Association with Acute Kidney Injury and Chronic Kidney Disease in Patients with Acute Myocardial Infarction. PLoS ONE 2013, 8, e75583. [Google Scholar] [CrossRef] [Green Version]
- Salisbury, A.C.; Kosiborod, M.; Amin, A.P.; Reid, K.J.; Alexander, K.P.; Spertus, J.A.; Masoudi, F.A. Recovery From Hospital-Acquired Anemia After Acute Myocardial Infarction and Effect on Outcomes. Am. J. Cardiol. 2011, 108, 949–954. [Google Scholar] [CrossRef] [Green Version]
- WHO. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. 2011. Available online: http://www.who.int/vmnis/indicators/haemoglobin/en/ (accessed on 15 November 2020).
- Kassebaum, N.J.; Jasrasaria, R.; Naghavi, M.; Wulf, S.K.; Johns, N.; Lozano, R.; Regan, M.; Weatherall, D.; Chou, D.P.; Eisele, T.P.; et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood 2014, 123, 615–624. [Google Scholar] [CrossRef]
- Soppi, E.T. Iron deficiency without anemia—A clinical challenge. Clin. Case Rep. 2018, 6, 1082–1086. [Google Scholar] [CrossRef]
- Vaucher, P.; Druais, P.-L.; Waldvogel, S.; Favrat, B. Effect of iron supplementation on fatigue in nonanemic menstruating women with low ferritin: A randomized controlled trial. Can. Med. Assoc. J. 2012, 184, 1247–1254. [Google Scholar] [CrossRef] [Green Version]
- Comín-Colet, J.; Enjuanes, C.; González, G.; Torrens, A.; Cladellas, M.; Meroño, O.; Ribas, N.; Ruiz, S.; Gómez, M.; Verdú, J.M.; et al. Iron deficiency is a key determinant of health-related quality of life in patients with chronic heart failure regardless of anae-mia status. Eur. J. Heart Fail. 2013, 15, 1164–1172. [Google Scholar] [CrossRef] [PubMed]
- Carson, J.L.; Adamson, J.W. Iron Deficiency and Heart Disease: Ironclad Evidence? Hematology 2010, 2010, 348–350. [Google Scholar] [CrossRef] [Green Version]
- Nemeth, E.; Tuttle, M.S.; Powelson, J.; Vaughn, M.B.; Donovan, A.; Ward, D.M.; Ganz, T.; Kaplan, J. Hepcidin Regulates Cellular Iron Efflux by Binding to Ferroportin and Inducing Its Internalization. Science 2004, 306, 2090–2093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chapter 1: Diagnosis and evaluation of anemia in CKD. Kidney Int. Suppl. 2012, 2, 288–291. [CrossRef] [PubMed] [Green Version]
- Brugnara, C.; Schiller, B.; Moran, J. Reticulocyte hemoglobin equivalent (Ret He) and assessment of iron-deficient states. Clin. Lab. Haematol. 2006, 28, 303–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wirawan, R.; Tedja, A.T.; Henrika, F.; Lydia, A. Concordance between Reticulocyte Hemoglobin Equivalent and Reticulocyte Hemoglobin Content in CKD Patients Undergoing Hemodialysis. Acta Med. Indones. 2017, 49, 34–40. [Google Scholar] [PubMed]
- Tiwari, A.K.; Bhardwaj, G.; Arora, D.; Aggarwal, G.; Pabbi, S.; Dara, R.C.; Sachdev, R.; Raizada, A.; Sethi, M. Applying newer parameter Ret-He (reticulocyte haemoglobin equivalent) to assess latent iron deficiency (LID) in blood donors-study at a tertiary care hospital in India. Vox Sang. 2018, 113, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Toki, Y.; Ikuta, K.; Kawahara, Y.; Niizeki, N.; Kon, M.; Enomoto, M.; Tada, Y.; Hatayama, M.; Yamamoto, M.; Ito, S.; et al. Reticulocyte hemoglobin equivalent as a potential marker for diagnosis of iron defi-ciency. Int. J. Hematol. 2017, 106, 116–125. [Google Scholar] [CrossRef] [Green Version]
- Thomas, C.; Thomas, L. Biochemical markers and hematologic indices in the diagnosis of functional iron deficiency. Clin. Chem. 2002, 48, 1066–1076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, L.; Franck, S.; Messinger, M.; Linssen, J.; Thomé, M.; Thomas, C. Reticulocyte hemoglobin measurement—Comparison of two methods in the diagnosis of iron-restricted erythropoiesis. Clin. Chem. Lab. Med. 2005, 43, 1193–1202. [Google Scholar] [CrossRef]
- Van Wyck, D.B.; Alcorn, H.; Gupta, R. Analytical and biological variation in measures of anemia and iron status in patients treated with maintenance hemodialysis. Am. J. Kidney Dis. Off. J. Natl. Kidney Found 2010, 56, 540–546. [Google Scholar] [CrossRef]
- Maconi, M.; Cavalca, L.; Danise, P.; Cardarelli, F.; Brini, M. Erythrocyte and reticulocyte indices in iron deficiency in chronic kidney disease: Comparison of two methods. Scand. J. Clin. Lab. Investig. 2009, 69, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, R.; Tubau, I.; Masip, J.; Muñoz, L.; Roig, I.; Artigas, A. Low reticulocyte hemoglobin content is associated with a higher blood transfusion rate in critically ill patients: A cohort study. Anesthesiology 2010, 112, 1211–1215. [Google Scholar] [CrossRef] [Green Version]
- Mikhail, A.; Brown, C.; Williams, J.A.; Mathrani, V.; Shrivastava, R.; Evans, J.; Isaac, H.; Bhandari, S. Renal association clinical practice guideline on Anaemia of Chronic Kidney Disease. BMC Nephrol. 2017, 18, 1–29. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5709852/ (accessed on 4 November 2020). [CrossRef]
- Chapter 2: Use of iron to treat anemia in CKD. Kidney Int. Suppl. 2012, 2, 292–298. [CrossRef] [Green Version]
- Chaparro, C.M.; Suchdev, P. Anemia epidemiology, pathophysiology, and etiology in low- and middle-income countries. Ann. N. Y. Acad. Sci. 2019, 1450, 15–31. [Google Scholar] [CrossRef]
- Chinudomwong, P.; Binyasing, A.; Trongsakul, R.; Paisooksantivatana, K. Diagnostic performance of reticulocyte hemoglobin equivalent in assessing the iron status. J. Clin. Lab. Anal. 2020, 34, e23225. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7307362/ (accessed on 4 November 2020). [CrossRef] [Green Version]
- Cullis, J.O. Diagnosis and management of anaemia of chronic disease: Current status. Br. J. Haematol. 2011, 154, 289–300. [Google Scholar] [CrossRef]
- Enko, D.; Wallner, F.; von Goedecke, A.; Hirschmugl, C.; Auersperg, V.; Halwachs-Baumann, G. The Impact of an Algorithm-Guided Management of Preoperative Anemia in Perioperative Hemoglobin Level and Transfusion of Major Orthopedic Surgery Patients. Anemia 2013, 2013, e641876. Available online: https://www.hindawi.com/journals/anemia/2013/641876/ (accessed on 4 November 2020). [CrossRef] [PubMed] [Green Version]
- Muñoz, M.; García-Erce, J.A.; Remacha, Á.F. Disorders of iron metabolism. Part 1: Molecular basis of iron homoeostasis. J. Clin. Pathol. 2011, 64, 281–286. [Google Scholar] [CrossRef]
- Spahn, D.R.; Schoenrath, F.; Spahn, G.H.; Seifert, B.; Stein, P.; Theusinger, O.M.; Kaserer, A.; Hegemann, I.; Hofmann, A.; Maisano, F.; et al. Effect of ultra-short-term treatment of patients with iron deficiency or anaemia undergoing cardiac surgery: A prospective randomised trial. Lancet 2019, 393, 2201–2212. [Google Scholar] [CrossRef]
- Lee, B.; Kim, E.J.; Song, J.; Jung, Y.-S.; Koo, B.-N. A randomised trial evaluating the effect of intraoperative iron administration. Sci. Rep. 2020, 10, 1–8. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7522208/ (accessed on 25 November 2020).
- Drabinski, T.; Zacharowski, K.; Meybohm, P.; Rüger, A.M.; De Arellano, A.R. Estimating the Epidemiological and Economic Impact of Implementing Preoperative Anaemia Measures in the German Healthcare System: The Health Economic Footprint of Patient Blood Management. Adv. Ther. 2020, 37, 3515–3536. [Google Scholar] [CrossRef]
- Manzini, P.M.; Dall’Omo, A.M.; D’Antico, S.; Valfrè, A.; Pendry, K.; Wikman, A.; Fischer, D.; Borg-Aquilina, D.; LaSpina, S.; Van Pampus, E.C.M.; et al. Patient blood management knowledge and practice among clinicians from seven European university hospitals: A multicentre survey. Vox Sang. 2017, 113, 60–71. [Google Scholar] [CrossRef] [Green Version]
- Wojciechowska, M.; Wisniewski, O.W.; Kolodziejski, P.; Krauss, H. Role of hepcidin in physiology and pathophysiology. Emerging experimental and clinical evidence. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2021, 72. [Google Scholar] [CrossRef]
- Symeonidis, A.; Marangos, M. Iron and Microbial Growth. In Insight and Control of Infectious Disease in Global Scenario; Priti, R., Ed.; InTech: Rijeka, Croatia, 2012; pp. 289–330. Available online: https://cdn.intechopen.com/pdfs/33040/InTech-Iron_and_microbial_growth.pdf (accessed on 18 September 2021).
- Weimann, A.; Cremer, M.; Hernáiz-Driever, P.; Zimmermann, M. Delta-He, Ret-He and a New Diagnostic Plot for Differential Diagnosis and Therapy Monitoring of Patients Suffering from Various Disease-Specific Types of Anemia. Clin. Lab. 2016, 62, 667–677. [Google Scholar] [CrossRef]
- Nierhaus, A.; Linssen, J.; Wichmann, D.; Braune, S.; Kluge, S. Use of a weighted, automated analysis of the differential blood count to differentiate sepsis from non-infectious systemic inflammation: The intensive care infection score (ICIS). Inflamm. Allergy Drug Targets 2012, 11, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Geest, P.J.; van der Mohseni, M.; Linssen, J.; Duran, S.; Jonge, R.; de Groeneveld, A.B.J. The intensive care infection score—A novel marker for the prediction of infection and its severity. Crit. Care 2016, 20, 1–8. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4936267/ (accessed on 15 November 2020).
- Urrechaga, E. Reviewing the value of leukocytes cell population data (CPD) in the management of sepsis. Ann. Transl. Med. 2020, 8, 953. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7475430/ (accessed on 15 November 2020). [CrossRef] [PubMed]
- Linssen, J.; Ermens, A.; Berrevoets, M.; Seghezzi, M.; Previtali, G.; Brugge, S.V.D.S.-V.D.; Russcher, H.; Verbon, A.; Gillis, J.; Riedl, J.; et al. A novel haemocytometric COVID-19 prognostic score developed and validated in an observational multicentre European hospital-based study. eLife 2020, 9, e63195. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7732342/ (accessed on 27 January 2020). [CrossRef]
- Muusze, R.; Corbey, A. Protocol voor transfusievrije grote orthopedische operaties. Ned. Tijdschr. Voor Orthop. 2016, 13, 163–172. [Google Scholar]
- Neef, V.; Meisenzahl, D.; Kessler, P.; Raimann, F.J.; Piekarski, F.; Choorapoikayil, S.; Fleege, C.; Zacharowski, K.D.; Meybohm, P.; Meurer, A. Implementation of an anaemia walk-in clinic: Feasibility and preliminary data from the Orthopedic University Hospital. Transfus. Med. 2020, 30, 467–474. [Google Scholar] [CrossRef] [PubMed]



| Parameter | Elevated | Reduced | Shows | Disadvantages |
|---|---|---|---|---|
| Serum Iron | Iron overload (preanalytical hemolysis) | Iron deficiency and acute/chronic inflammation | Amount of iron (Iron bound to transferrin & non-transferrin bound iron) | High intra- and inter-individual variability Neither sensitive nor specific |
| Ferritin | Acute phase, liver disease, lymphoma | Iron deficiency with depleted iron storage | Iron stores | Acute phase reactant, no direct conclusion for erythropoiesis |
| Transferrin | Iron deficiency, pregnancy (last trimester) | Chronic inflammation, tumors, hemolysis | Transport iron, demand by erythropoiesis | By itself only indicates demand, not supply |
| Transferrin saturation | Iron overload | Iron deficiency, acute phase, pregnancy (last trimester) | Percentage of filled transferrin binding sites | Acute phase reactant, requires two measurements (transferrin, iron) |
| Soluble transferrin receptor | Increased erythropoiesis during iron deficiency | Chronic kidney disease with reduced EPO | Secreted fragment of transferrin receptor | Not elevated during acute phase, reference interval highly dependent on specific test, expensive |
| Transferrin receptor | Iron overload | Iron deficiency, acute phase, pregnancy (last trimester) | Target of transferrin iron transport, mediates iron uptake by endocytosis | Acute phase reactant |
| sTfR-Ferritin index | Iron deficiency | Anemia of chronic disease | sTfR/log Ferritin | Requires two measurements, thus complex and costly |
| Hepcidin | Iron deficiency anemia, CKD, inflammation | Iron overload | Iron absorption and release from storage | Complex measuring methods, reference interval highly dependent on specific technology |
| RET-He | Iron deficient erythropoiesis | Functional availability of iron | Not available from all manufacturers |
| Cellular assessment | Hb < 11 g/dL RBC indices (MCH, MCHC, MCV) White blood cell and differential count Platelet and reticulocyte count |
| Iron assessment | Hypochromic cells % (if sample < 6 h old) Reticulocyte Hb (RET-He) Serum ferritin C-reactive protein |
| Iron therapy targets | Hypochromic cells < 6% Reticulocyte Hb (RET-He) > 29 pg Ferritin > 100 µg/L TSAT > 20% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoenemann, C.; Ostendorf, N.; Zarbock, A.; Doll, D.; Hagemann, O.; Zimmermann, M.; Luedi, M. Reticulocyte and Erythrocyte Hemoglobin Parameters for Iron Deficiency and Anemia Diagnostics in Patient Blood Management. A Narrative Review. J. Clin. Med. 2021, 10, 4250. https://doi.org/10.3390/jcm10184250
Hoenemann C, Ostendorf N, Zarbock A, Doll D, Hagemann O, Zimmermann M, Luedi M. Reticulocyte and Erythrocyte Hemoglobin Parameters for Iron Deficiency and Anemia Diagnostics in Patient Blood Management. A Narrative Review. Journal of Clinical Medicine. 2021; 10(18):4250. https://doi.org/10.3390/jcm10184250
Chicago/Turabian StyleHoenemann, Christian, Norbert Ostendorf, Alexander Zarbock, Dietrich Doll, Olaf Hagemann, Mathias Zimmermann, and Markus Luedi. 2021. "Reticulocyte and Erythrocyte Hemoglobin Parameters for Iron Deficiency and Anemia Diagnostics in Patient Blood Management. A Narrative Review" Journal of Clinical Medicine 10, no. 18: 4250. https://doi.org/10.3390/jcm10184250
APA StyleHoenemann, C., Ostendorf, N., Zarbock, A., Doll, D., Hagemann, O., Zimmermann, M., & Luedi, M. (2021). Reticulocyte and Erythrocyte Hemoglobin Parameters for Iron Deficiency and Anemia Diagnostics in Patient Blood Management. A Narrative Review. Journal of Clinical Medicine, 10(18), 4250. https://doi.org/10.3390/jcm10184250







