Using MRI Measurement to Improve Accuracy of Femoral Component Sizing in Oxford Unicompartmental Knee Arthroplasty
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Price, A.J.; Webb, J.; Topf, H.; Dodd, C.A.; Goodfellow, J.W.; Murray, D.W.; Oxford, H.; Knee, G. Rapid recovery after Oxford unicompartmental arthroplasty through a short incision. J. Arthroplasty 2001, 16, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Rees, J.L.; Price, A.J.; Beard, D.J.; Dodd, C.A.; Murray, D.W. Minimally invasive Oxford unicompartmental knee arthroplasty: Functional results at 1 year and the effect of surgical inexperience. Knee 2004, 11, 363–367. [Google Scholar] [CrossRef]
- Sun, X.W.; Lu, F.F.; Zou, K.; Hong, M.; Zhang, Q.D.; Guo, W.S. Does new instrument for Oxford unicompartmental knee arthroplasty improve short-term clinical outcome and component alignment? A meta-analysis. J. Orthop. Surg. Res. 2020, 15, 386. [Google Scholar] [CrossRef]
- Cheng, J.; Feng, M.; Cao, G.; Li, Z.; An, S.; Lu, S. Patient outcomes in Anteromedial osteoarthritis patients over 80 years old undergoing Oxford Unicompartmental knee Arthroplasty in China. BMC Musculoskelet. Disord. 2020, 21, 446. [Google Scholar] [CrossRef] [PubMed]
- Fisher, D.A.; Dalury, D.F.; Adams, M.J.; Shipps, M.R.; Davis, K. Unicompartmental and total knee arthroplasty in the over 70 population. Orthopedics 2010, 33, 668. [Google Scholar] [CrossRef]
- Fawzy, E.; Pandit, H.; Jenkins, C.; Dodd, C.A.; Murray, D.W. Determination of femoral component size in unicompartmental knee replacement. Knee 2008, 15, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Bothra, V.; Lemon, G.; Lang, D.; Smith, D.M.; Ali, A.M. Reliability of templating in estimating the size of uni-condylar knee arthroplasty. J. Arthroplasty 2003, 18, 780–783. [Google Scholar] [CrossRef]
- Edmondson, M.C.; Isaac, D.; Wijeratna, M.; Brink, S.; Gibb, P.; Skinner, P. Oxford unicompartmental knee arthroplasty: Medial pain and functional outcome in the medium term. J. Orthop. Surg. Res. 2011, 6, 52. [Google Scholar] [CrossRef] [Green Version]
- Vajapey, S.P.; Alvarez, P.M.; Chonko, D. Bearing failure in a mobile bearing unicompartmental knee arthroplasty: An uncommon presentation of an implant-specific complication. J. Arthroplasty 2021, 3, 16. [Google Scholar] [CrossRef]
- Sun, X.; Liu, P.; Lu, F.; Wang, W.; Guo, W.; Zhang, Q. Bearing dislocation of mobile bearing unicompartmental knee arthroplasty in East Asian countries: A systematic review with meta-analysis. J. Orthop. Surg. Res. 2021, 16, 28. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Xue, H.; Cai, M.; Ma, T.; Liu, X.; Xia, Z. Improvement of femoral component size prediction using a C-arm intensifier guide and our established algorithm in unicompartmental knee arthroplasty: A report from a Chinese population. Knee 2014, 21, 435–438. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, R.; Gaba, S.; Wahal, N.; Kumar, V.; Srivastava, D.N.; Pandit, H. Femoral Component Sizing in Oxford Unicompartmental Knee Replacement: Existing Guidelines Do Not Work for Indian Patients. J. Knee Surg. 2019, 32, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Kasis, A.G.; Pacheco, R.J.; Hekal, W.; Farhan, M.J.; Smith, D.M.; Ali, A.M. The precision and accuracy of templating the size of unicondylar knee arthroplasty. Knee 2004, 11, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, T.W.; Pandit, H.G.; Inabathula, A.; Ostlere, S.J.; Jenkins, C.; Mellon, S.J.; Dodd, C.A.; Murray, D.W. Unsatisfactory outcomes following unicompartmental knee arthroplasty in patients with partial thickness cartilage loss: A medium-term follow-up. Bone Jt. J. 2017, 99-B, 475–482. [Google Scholar] [CrossRef]
- Pandit, H.; Gulati, A.; Jenkins, C.; Barker, K.; Price, A.J.; Dodd, C.A.; Murray, D.W. Unicompartmental knee replacement for patients with partial thickness cartilage loss in the affected compartment. Knee 2011, 18, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Hurst, J.M.; Berend, K.R.; Morris, M.J.; Lombardi, A.V., Jr. Abnormal preoperative MRI does not correlate with failure of UKA. J. Arthroplasty 2013, 28, 184–186. [Google Scholar] [CrossRef]
- Tuecking, L.R.; Savov, P.; Richter, T.; Windhagen, H.; Ettinger, M. Clinical validation and accuracy testing of a radiographic decision aid for unicondylar knee arthroplasty patient selection in midterm follow-up. Knee Surg. Sports Traumatol. Arthrosc. 2020, 28, 2082–2090. [Google Scholar] [CrossRef]
- Goodfellow, J.W.; Kershaw, C.J.; Benson, M.K.; O’Connor, J.J. The Oxford Knee for unicompartmental osteoarthritis. The first 103 cases. J. Bone Jt. Surg. Br. 1988, 70, 692–701. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.G.; Kotti, M.; Wiik, A.V.; Collins, R.; Brevadt, M.J.; Strachan, R.K.; Cobb, J.P. Gait comparison of unicompartmental and total knee arthroplasties with healthy controls. Bone Jt. J. 2016, 98-B, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Tille, E.; Beyer, F.; Auerbach, K.; Tinius, M.; Lützner, J. Better short-term function after unicompartmental compared to total knee arthroplasty. BMC Musculoskelet. Disord. 2021, 22, 326. [Google Scholar] [CrossRef]
- Li, K.; Saffarini, M.; Valluy, J.; Desseroit, M.C.; Morvan, Y.; Telmon, N.; Cavaignac, E. Sexual and ethnic polymorphism render prosthetic overhang and under-coverage inevitable using off-the shelf TKA implants. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 2130–2139. [Google Scholar] [CrossRef] [PubMed]
- Mathon, P.; Micicoi, G.; Seil, R.; Kacaoglu, B.; Cerciello, S.; Ahmad, F.; LiArno, S.; Teitge, R.; Ollivier, M. Healthy middle-aged Asian and Caucasian populations present with large intra- and inter-individual variations of lower limb torsion. Knee Surg. Sports Traumatol. Arthrosc. 2020. [Google Scholar] [CrossRef] [PubMed]
- Murgier, J.; Chantalat, E.; Li, K.; Chiron, P.; Telmon, N.; Huang, W.; Berard, E.; Cavaignac, E. Distal femoral torsion: Differences between caucasians and asians. A multicentre computed tomography study of 515 distal femurs. Orthop. Traumatol. Surg. Res. 2018, 104, 997–1001. [Google Scholar] [CrossRef]
- Charng, J.R.; Chen, A.C.; Chan, Y.S.; Hsu, K.Y.; Wu, C.T. Proximal tibial morphology and risk of posterior tibial cortex impingement in patients with AA-sized Oxford unicompartmental knee arthroplasty tibial implants. J. Orthop. Surg. Res. 2020, 15, 380. [Google Scholar] [CrossRef]
- Hamilton, T.W.; Pandit, H.G.; Lombardi, A.V.; Adams, J.B.; Oosthuizen, C.R.; Clave, A.; Dodd, C.A.; Berend, K.R.; Murray, D.W. Radiological Decision Aid to determine suitability for medial unicompartmental knee arthroplasty: Development and preliminary validation. Bone Jt. J. 2016, 98-B, 3–10. [Google Scholar] [CrossRef]
- Jacobs, C.A.; Berend, K.R.; Lombardi, A.V., Jr.; Christensen, C.P. The location and severity of preoperative subchondral bone marrow lesions were not associated with inferior postoperative outcomes after medial unicompartmental knee arthroplasty or total knee arthroplasty. J. Arthroplasty 2016, 31, 2476–2480. [Google Scholar] [CrossRef] [PubMed]
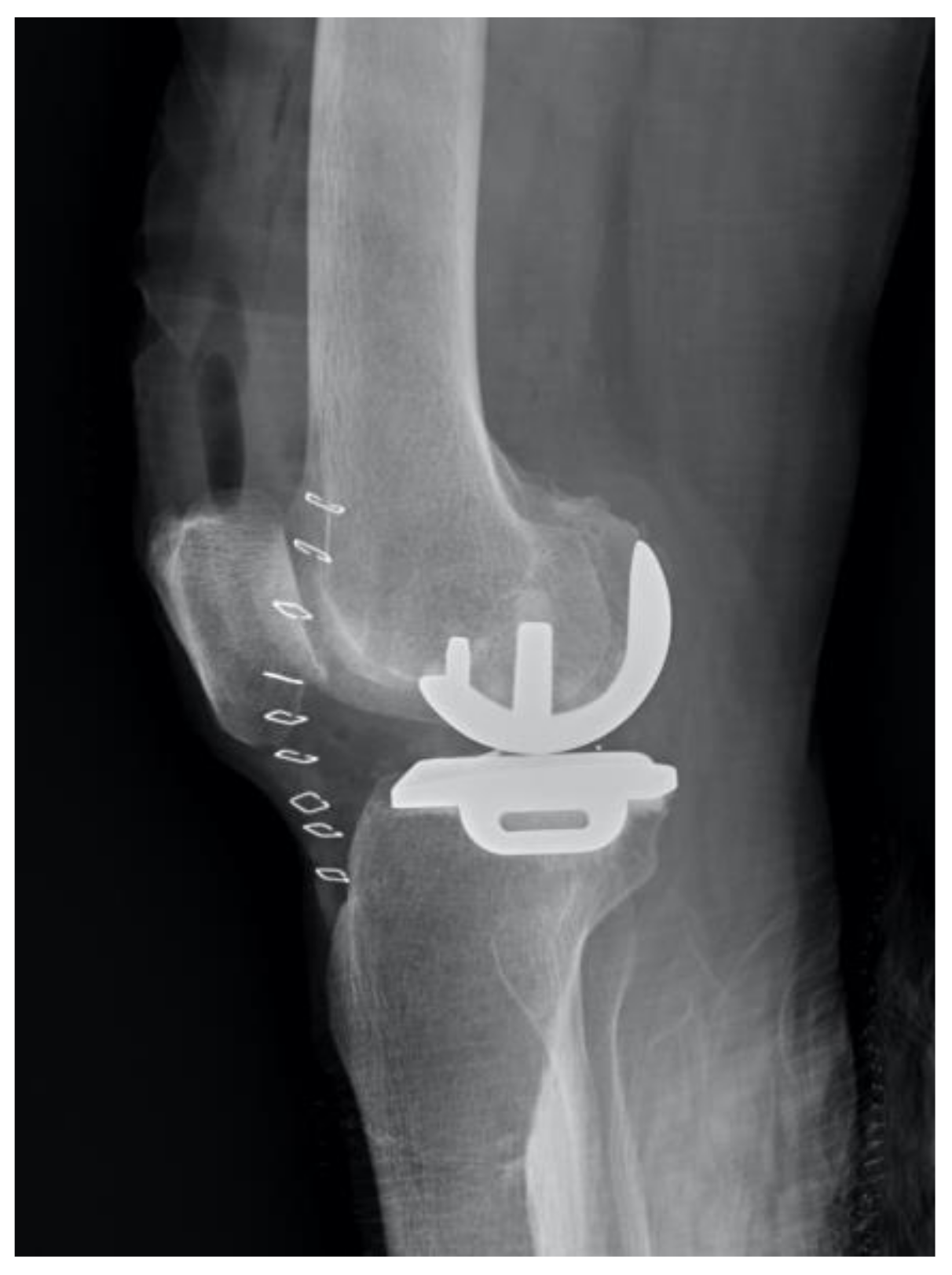

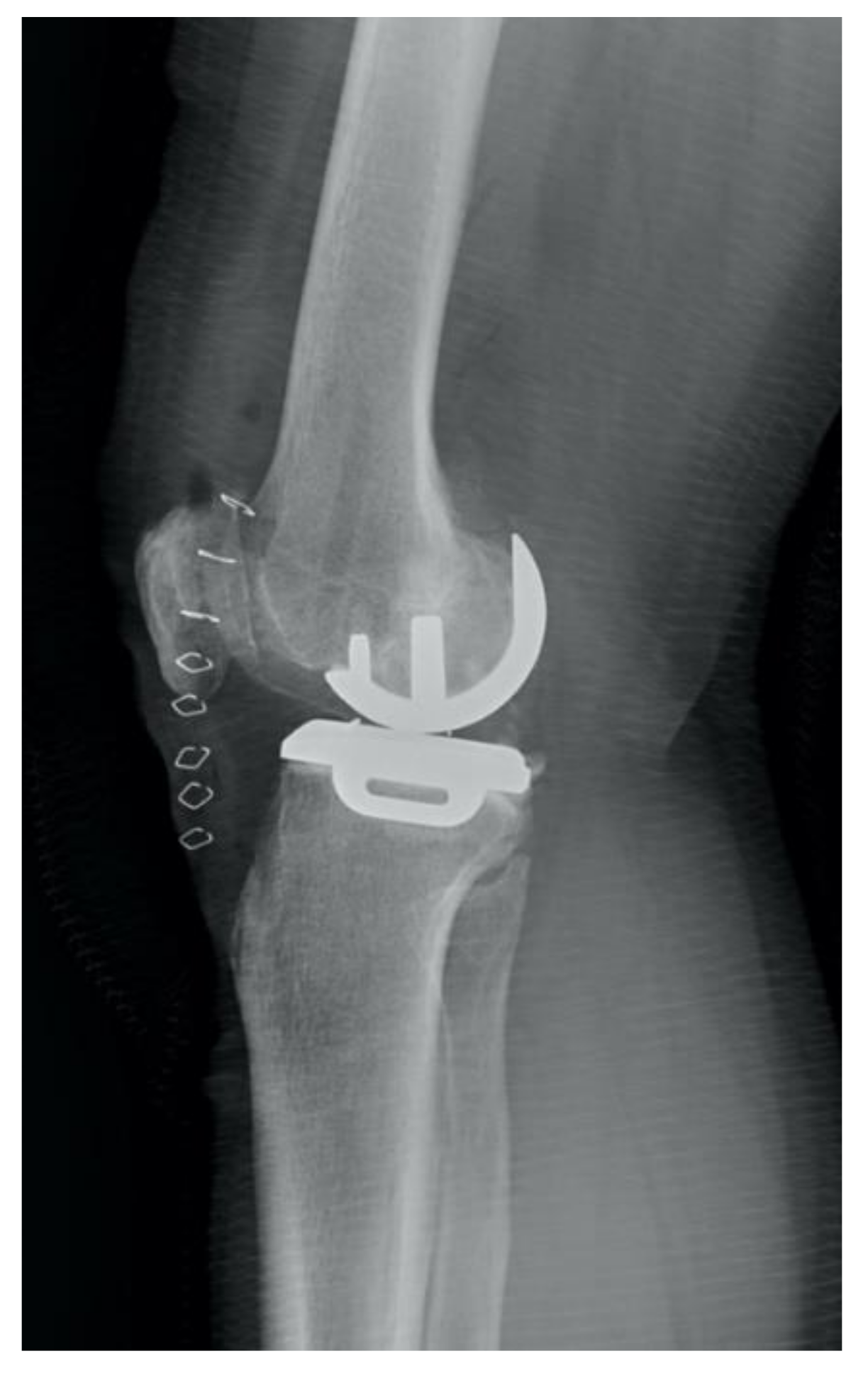


| Correct | Incorrect | Accuracy of Prediction | |
|---|---|---|---|
| Templating | 22 | 30 (3 two size out) | 42.3% |
| Spoon | 35 | 17 (2 two size out) | 67.3% |
| Fawzy et al. [6] | 22 | 30 (2 two size out) | 42.3% |
| Tu et al. [11] | 12 | 40 (10 two size out) | 23% |
| Malhotra et al. [12] | 13 | 39 (11 two size out) | 25% |
| MRI measurement | 47 | 5 (0 two size out) | 90.3% |
| p-Value (Fisher’s Exact Test) | |
|---|---|
| MRI versus templating | <0.001 |
| MRI versus spoon | 0.007 |
| MRI versus Fawzy et al. | <0.001 |
| MRI versus Tu et al. | <0.001 |
| MRI versus Malhotra et al. | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.-P.; Lai, Y.-C.; Wu, C.-T.; Hung, K.-T.; Chan, Y.-S.; Chen, A.C.-Y.; Hsu, K.-Y. Using MRI Measurement to Improve Accuracy of Femoral Component Sizing in Oxford Unicompartmental Knee Arthroplasty. J. Clin. Med. 2021, 10, 4284. https://doi.org/10.3390/jcm10184284
Yang C-P, Lai Y-C, Wu C-T, Hung K-T, Chan Y-S, Chen AC-Y, Hsu K-Y. Using MRI Measurement to Improve Accuracy of Femoral Component Sizing in Oxford Unicompartmental Knee Arthroplasty. Journal of Clinical Medicine. 2021; 10(18):4284. https://doi.org/10.3390/jcm10184284
Chicago/Turabian StyleYang, Cheng-Pang, Ying-Chieh Lai, Chen-Te Wu, Kung-Tseng Hung, Yi-Sheng Chan, Alvin Chao-Yu Chen, and Kuo-Yao Hsu. 2021. "Using MRI Measurement to Improve Accuracy of Femoral Component Sizing in Oxford Unicompartmental Knee Arthroplasty" Journal of Clinical Medicine 10, no. 18: 4284. https://doi.org/10.3390/jcm10184284
APA StyleYang, C.-P., Lai, Y.-C., Wu, C.-T., Hung, K.-T., Chan, Y.-S., Chen, A. C.-Y., & Hsu, K.-Y. (2021). Using MRI Measurement to Improve Accuracy of Femoral Component Sizing in Oxford Unicompartmental Knee Arthroplasty. Journal of Clinical Medicine, 10(18), 4284. https://doi.org/10.3390/jcm10184284






