Less Known Gastrointestinal Manifestations of Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) Syndrome: A Systematic Review of the Literature
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Demographic Characteristics (Age, Sex, Race) and Co-Morbidities
3.2. Medications, Eosinophilia and Latency
3.2.1. Medications
3.2.2. Eosinophilia
3.2.3. Latency
3.3. GIT Involvement
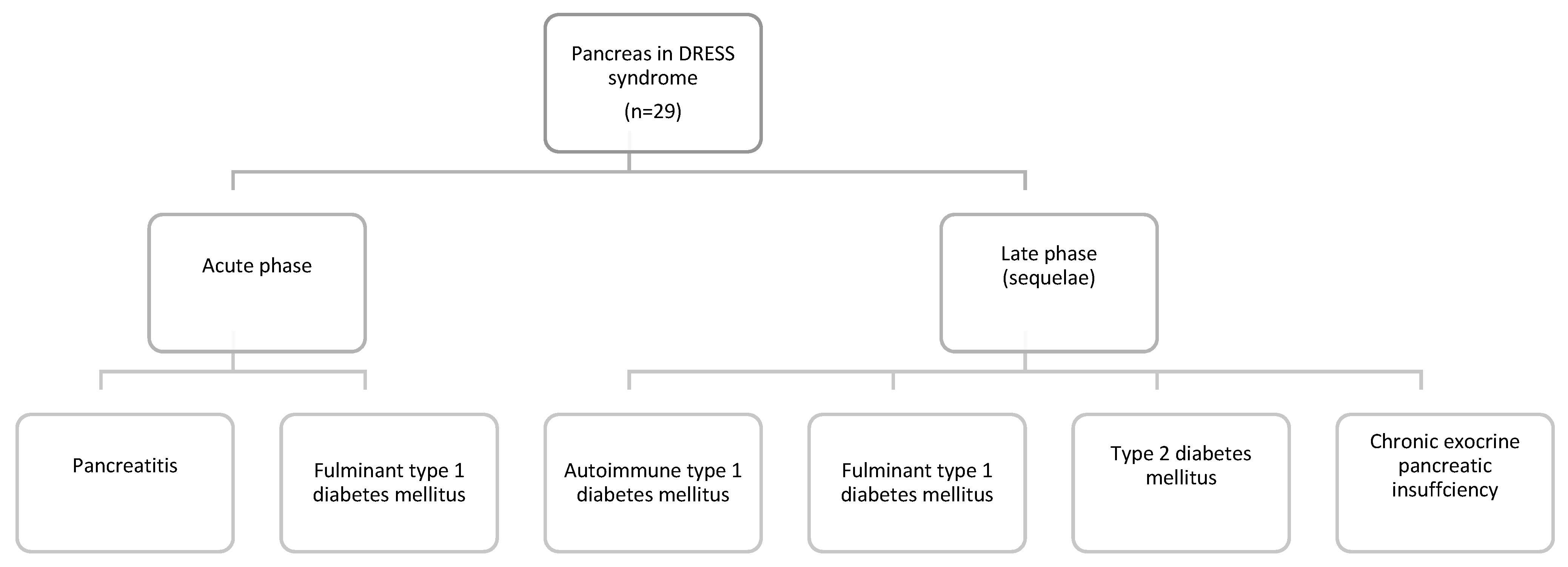
3.3.1. Pancreas in DRESS Syndrome
3.3.2. DRESS Colitis
3.3.3. DRESS Enteritis
3.3.4. DRESS Esophagitis
3.3.5. Gastric Involvement in DRESS Syndrome
3.4. Other Internal Organ Involvement in DRESS Syndrome
3.5. Viral Involvement
3.6. Therapy and Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cacoub, P.; Musette, P.; Descamps, V.; Meyer, O.; Speirs, C.; Finzi, L.; Roujeau, J.C. The DRESS syndrome: A literature review. Am. J. Med. 2011, 124, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Kardaun, S.H.; Sidoroff, A.; Valeyrie-Allanore, L.; Halevy, S.; Davidovici, B.B.; Mockenhaupt, M.; Roujeau, J.C. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: Does a DRESS syndrome really exist? Br. J. Dermatol. 2007, 156, 609–611. [Google Scholar] [CrossRef] [PubMed]
- Soria, A.; Bernier, C.; Veyrac, G.; Barbaud, A.; Puymirat, E.; Milpied, B. Drug reaction with eosinophilia and systemic symptoms may occur within 2 weeks of drug exposure: A retrospective study. J. Am. Acad. Dermatol. 2020, 82, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Wu, J.; Chen, G.; Yang, Y.; Yi, C. Fulminant type 1 diabetes mellitus caused by drug reaction with eosinophilia and systemic symptoms (DRESS): A case report and review of the literature. Front. Endocrinol. 2019, 10, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Erdem, S.B.; Nacaroglu, H.T.; Bag, O.; Karkiner, C.S.U.; Korkmaz, H.A.; Can, D. DRESS syndrome associated with type 2 diabetes in a child. Cent. Eur. J. Immunol. 2015, 40, 493–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kano, Y.; Ishida, T.; Hirahara, K.; Shiohara, T. Visceral involvements and long-term sequelae in drug-induced hypersensitivity syndrome. Med. Clin. North Am. 2010, 94, 743–759. [Google Scholar] [CrossRef] [PubMed]
- Roquin, G.; Peres, M.; Lerolle, N.; Dib, N.; Mercat, A.; Croue, A.; Augusto, J.F. First report of lamotrigine-induced drug rash with eosinophilia and systemic symptoms syndrome with pancreatitis. Ann. Pharmacother. 2010, 44, 1998–2000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adike, A.; Boppana, V.; Lam-Himlin, D.; Stanton, M.; Nelson, S.; Ruff, K.C. A mysterious DRESS case: Autoimmune enteropathy associated with DRESS syndrome. Case Rep. Gastrointest. Med. 2017, 2017, 7861857. [Google Scholar] [CrossRef] [Green Version]
- Bocquet, H.; Bagot, M.; Roujeau, J.C. Drug-induced pseudolymphoma and drug hypersensitivity syndrome (Drug Rash with Eosinophilia and Systemic Symptoms: DRESS). Semin. Cutan. Med. Surg. 1996, 15, 250–257. [Google Scholar] [CrossRef]
- Taweesedt, P.T.; Nordstrom, C.W.; Stoeckel, J.; Dumic, I. Pulmonary manifestations of drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome: A systematic review. Biomed. Res. Int. 2019, 2019, 7863815. [Google Scholar] [CrossRef] [Green Version]
- Husain, Z.; Reddy, B.Y.; Schwartz, R.A. DRESS syndrome: Part, I. Clinical perspectives. J. Am. Acad. Dermatol. 2013, 68, 693.e1–14. [Google Scholar] [CrossRef] [PubMed]
- Kardaun, S.H.; Sekula, P.; Valeyrie-Allanore, L.; Liss, Y.; Chu, C.Y.; Creamer, D.; Sidoroff, A.; Naldi, L.; Mockehaupt, M.; Roujeau, J.C. Drug reaction with eosinophilia and systemic symptoms (DRESS): An original multisystem adverse drug reaction. Results from the prospective RegiSCAR study Funding source. Br. J. Dermatol. 2013, 169, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Cabriales, S.A.; Shear, N.H.; Gonzalez-Moreno, E.I. Liver involvement in the drug reaction, eosinophilia, and systemic symptoms syndrome. World J. Clin. Cases 2019, 7, 705–716. [Google Scholar] [CrossRef]
- Chiou, C.C.; Chung, W.H.; Hung, S.I.; Yang, L.C.; Hong, H.S. Fulminant type 1 diabetes mellitus caused by drug hypersensitivity syndrome with human herpesvirus 6 infection. J. Am. Acad. Dermatol. 2006, 54 (Suppl. S2), S14–S17. [Google Scholar] [CrossRef] [PubMed]
- Darban, M.; Bagheri, B. Drug reaction with eosinophilia and systemic symptoms induced by valproic acid: A case report. Iran Red Crescent Med. J. 2016, 18, 1–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, A.; Jawed, Q. Drug-induced acute pancreatitis: A rare manifestation of an incomplete “dapsone syndrome”. Indian J. Pharmacol. 2014, 46, 455–457. [Google Scholar] [CrossRef] [PubMed]
- Descamps, V.; Mahe, E.; Houhou, N.; Abramowitz, L.; Rozenberg, F.; Ranger-Rogez, S.; Crickx, B. Drug-induced hypersensitivity syndrome associated with Epstein-Barr virus infection. Br. J. Dermatol. 2003, 148, 1032–1034. [Google Scholar] [CrossRef]
- Do-Pham, G.; Charachon, A.; Duong, T.A.; Thille, A.W.; Benhaiem, N.; Bagot, M.; Chosidow, O.; Roujeau, J.C.; Wolenstein, P.; Valeyrie-Allanore, L. Drug reaction with eosinophilia and systemic symptoms and severe involvement of digestive tract: Description of two cases. Br. J. Dermatol. 2011, 165, 207–209. [Google Scholar] [CrossRef]
- Dubois-Laforgue, D.; Moachon, L.; Laude, H.; Timsit, J. Fulminant type 1 diabetes in the course of drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome. Diabetes Care 2013, 36, 2291. [Google Scholar] [CrossRef] [Green Version]
- Eland, I.A.; Dofferhoff, A.S.M.; Vink, R.; Zondervan, P.E.; Stricker, B.H.C. Colitis may be part of the antiepileptic drug hypersensitivity syndrome. Epilepsia 1999, 40, 1780–1783. [Google Scholar] [CrossRef]
- Eshki, M.; Allanore, L.; Musette, P.; Milpied, B.; Grange, A.; Guillaume, J.C.; Chosidow, O.; Guillot, I.; Paradis, V.; Joly, P.; et al. Twelve-year analysis of severe cases of drug reaction with eosinophilia and systemic symptoms: A cause of unpredictable multiorgan failure. Arch. Dermatol. 2009, 145, 67–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fathallah, N.; Ben Salem, C.; Slim, R.; Kaabia, N.; Letaief, A.; Bouraoui, K. Fatal allopurinol-induced hypersensitivity syndrome associated with pancreatic abnormalities. J. Clin. Rheumatol. 2010, 16, 170–171. [Google Scholar] [CrossRef] [PubMed]
- Fervenza, F.C.; Sharan, K.; Kunau, R.T.; Richard, G.; Lager, D.J. Acute granulomatous interstitial nephritis and colitis in anticonvulsant hypersensitivity syndrome associated with lamotrigine treatment. Am. J. Kidney Dis. 2000, 36, 1034–1040. [Google Scholar] [CrossRef]
- Fujiya, A.; Ochiai, H.; Mizukoshi, T.; Kiyota, A.; Shibata, T.; Suzuki, A.; Ohashi, N.; Sobajima, H. Fulminant type 1 diabetes mellitus associated with a reactivation of Epstein-Barr virus that developed in the course of chemotherapy of multiple myeloma. J. Diabetes Investig. 2010, 1, 286–289. [Google Scholar] [CrossRef]
- Ghislain, P.D.; Bodarwe, A.D.; Vanderdonckt, O.; Tennstedt, D.; Marot, L.; Lachapelle, J.M. Drug-induced Eosinophilia and multisystemic failure with positive patch-test reaction to spironolactone: DRESS syndrome. Acta Derm. Venereol. 2004, 84, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Macías, A.; Lizarralde-Palacios, E.; Martínez-Odriozola, P.; Miguel-De La Villa, F. Lesson of the week: Fatal allopurinol hypersensitivity syndrome after treatment of asymptomatic hyperuricaemia. Br. Med. J. 2005, 331, 623–624. [Google Scholar] [CrossRef] [PubMed]
- Haddad, R.; O’Brien, B.; Evans, J.; Orr, C. Novel finding of carbamazepine induced gall bladder granulomatous vasculitis. Intern. Med. J. 2014, 44, 700–703. [Google Scholar] [CrossRef]
- Sekine, N.; Motokura, T.; Oki, T.; Umeda, Y.; Sasaki, N.; Hayashi, M.; Sato, H.; Fujita, T.; Kaneko, T.; Asano, Y.; et al. Rapid loss of insulin secretion in a patient with fulminant type 1 diabetes mellitus and carbamazepine hypersensitivity syndrome. JAMA 2001, 285, 1153–1154. [Google Scholar] [CrossRef]
- Jackson, C.W.; Haboubi, N.Y.; Whorwell, P.J.; Schofield, P.F. Gold induced enterocolitis. Gut 1986, 27, 452–456. [Google Scholar] [CrossRef]
- Ozaki, N.; Miura, Y.; Oiso, Y. A case of type 1 diabetes followed by methimazole-induced hypersensitivity syndrome. Diabetes Care May 2006, 29, 1179–1180. [Google Scholar] [CrossRef]
- Klassen, B.D.; Sadler, R.M. Induction of hypersensitivity to a previously tolerated antiepileptic drug by a second antiepileptic drug. Epilepsia 2001, 42, 433–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krummenacher, M.; Banovic, T.; Kette, F.; Smith, W.; Hissaria, P. Drug reaction with eosinophilia and systemic symptoms and cytomegalovirus colitis. Ann. Allergy Asthma Immunol. 2019, 123, 401–403. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.; Lahoti, A.; Lew, D.B. A severe case of minocycline-induced DRESS resulting in liver transplantation and autoimmune sequelae. Ann. Allergy Asthma Immunol. 2016, 116, 367–368. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.A.; Knowles, S.R.; Cohen, L.B.; Werb, M.R.; Shear, N.H. Pancreatic insufficiency due to antituberculous therapy. Ann. Pharmacother. 1997, 31, 724–726. [Google Scholar] [CrossRef]
- Marchese, M.; Leinung, M.; Shawa, H. Drug-induced hypersensitivity reaction: A case of simultaneous thyroiditis and fulminant type 1 diabetes. Avicenna J. Med. 2017, 7, 67–70. [Google Scholar]
- Minegaki, Y.; Higashida, Y.; Ogawa, M.; Miyachi, Y.; Fujii, H.; Kabashima, K. Drug-induced hypersensitivity syndrome complicated with concurrent fulminant type 1 diabetes mellitus and Hashimoto’s thyroiditis. Int. J. Dermatol. 2013, 52, 355–357. [Google Scholar] [CrossRef]
- Nawaz, F.; Wall, B.M. Drug rash with eosinophilia and systemic symptoms (DRESS) syndrome: Suspected association with titanium bioprosthesis. Am. J. Med. Sci. 2007, 334, 215–218. [Google Scholar] [CrossRef]
- Oskay, T.; Karademir, A.; Ertürk, Ö.I. Association of anticonvulsant hypersensitivity syndrome with Herpesvirus 6, 7. Epilepsy Res. 2006, 70, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Parsi, M.; Daniel, C. Lamotrigine-induced DRESS syndrome manifesting as ‘eosinophilic colitis’: An uncommon presentation of a very uncommon condition. Cureus 2020, 12, e7570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quidley, A.M.; Pharm, D.; Bookstaver, P.B.; Gainey, A.B.; Gainey, M.D. Fatal clindamycin-induced drug rash with eosinophilia. Pharmacopherapy 2012, 32, e387–e392. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, J.; Alex, G.; Roberts, H.; Orchard, D. Drug rash with eosinophilia and systemic symptoms secondary to sulfasalazine. J. Paediatr. Child Health 2010, 46, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Sommers, L.M.; Schoene, R.B. Allopurinol hypersensitivity syndrome associated with pancreatic exocrine abnormalities and new-onset diabetes mellitus. Arch. Intern. Med. 2002, 162, 1190–1192. [Google Scholar] [CrossRef]
- Seino, Y.; Yamauchi, M.; Hirai, C.; Okumura, A.; Kondo, K.; Yamamoto, M.; Okazaki, Y. A case of fulminant Type 1 diabetes associated with mexiletine hypersensitivity syndrome. Diabetes Med. 2004, 21, 1156–1157. [Google Scholar] [CrossRef] [PubMed]
- Alkhateeb, H.; Said, S.; Cooper, C.J.; Gaur, S.; Porres-Aguilar, M. DRESS syndrome following ciprofloxacin exposure: An unusual association. Am. J. Case Rep. 2013, 14, 526–528. [Google Scholar] [CrossRef] [PubMed]
- Swanson, E.A.; Low, L.; Naini, B.V. Severe enterocolitis associated with antiepileptic-induced drug reaction with eosinophilia and systemic symptoms. Hum. Pathol. 2014, 45, 1973–1977. [Google Scholar] [CrossRef] [PubMed]
- Takeno, A.; Kanazawa, I.; Morita, M.; Takedani, K.; Miyake, H.; Yamamoto, M.; Nogami, K.; Kaneko, S.; Sugimoto, T. A case report of fulminant type 1 diabetes mellitus associated with drug-induced hypersensitivity syndrome in an elderly patient with coxsackie B4 virus infection and human leukocyte antigen-A24 haplotype. Endocr. J. 2018, 65, 129–132. [Google Scholar] [CrossRef] [Green Version]
- Tohyama, M.; Yahata, Y.; Yasukawa, M.; Inagi, R.; Urano, Y.; Yamanishi, K.; Hashimoto, K. Severe hypersensitivity syndrome due to sulfasalazine associated with reactivation of human herpesvirus 6. Arch. Dermatol. 1998, 134, 1113–1117. [Google Scholar] [CrossRef] [Green Version]
- Walsh, S.; Diaz-Cano, S.; Higgins, E.; Morris-Jones, R.; Bashir, S.; Bernal, W.; Creamer, D. Drug reaction with eosinophilia and systemic symptoms: Is cutaneous phenotype a prognostic marker for outcome? A review of clinicopathological features of 27 cases. Br. J. Dermatol. 2013, 168, 391–401. [Google Scholar] [CrossRef]
- Singer, E.M.; Wanat, K.A.; Rosenbach, M.A. A case of recalcitrant DRESS syndrome with multiple autoimmune sequelae treated with intravenous immunoglobulins. JAMA Dermatol. 2013, 149, 494–495. [Google Scholar] [CrossRef]
- Yoneda, S.; Imagawa, A.; Fukui, K.; Uno, S.; Kozawa, J.; Sakai, M.; Yumioka, T.; Iwahashi, H.; Shimomura, I. A histological study of fulminant type 1 diabetes mellitus related to human cytomegalovirus reactivation. J. Clin. Endocrinol. Metab. 2017, 102, 2394–2400. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.C.; Liang, L.; Fu, J.F. Type 1 diabetes mellitus in a child with phenobarbital hypersensitivity syndrome. J. Endocrinol. Invest. 2008, 31, 360–363. [Google Scholar] [CrossRef] [PubMed]
- Alexander, T.; Iglesia, E.; Park, Y.; Duncan, D.; Peden, D.; Sheikh, S.; Ferris, M. Severe DRESS syndrome managed with therapeutic plasma exchange. Pediatrics 2013, 131, e945–e949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atkinson, R.J.; Dennis, G.; Cross, S.S.; McAlindon, M.E.; Sharrack, B.; Sanders, D.S. Eosinophilic colitis complicating anti-epileptic hypersensitivity syndrome: An indication for colonoscopy? Gastrointest. Endosc. 2004, 60, 1034–1036. [Google Scholar] [CrossRef]
- Balatsinou, C.; Milano, A.; Caldarella, M.P.; Laterza, F.; Pierdomenico, S.D.; Cuccurullo, F.; Neri, M. Eosinophilic esophagitis is a component of the anticonvulsant hypersensitivity syndrome: Description of two cases. Dig. Liver Dis. 2008, 40, 145–148. [Google Scholar] [CrossRef]
- Bridges, A.J.; Marshall, J.B.; Diaz-Arias, A.A. Acute eosinophilic colitis and hypersensitivity reaction associated with naproxen therapy. Am. J. Med. 1990, 89, 526–527. [Google Scholar] [CrossRef]
- Brown, R.J.; Rother, K.I.; Artman, H.; Mercurio, M.G.; Wang, R.; Looney, R.J.; Cowen, E.W. Minocycline-induced drug hypersensitivity syndrome followed by multiple autoimmune sequelae. Arch. Dermatol. 2009, 145, 63–66. [Google Scholar] [CrossRef] [Green Version]
- Chiang, A.; Shiu, J.; Elsensohn, A.N.; Chapman, L.W.; de Feraudy, S.; Smith, J. Classic autoimmune type 1 diabetes mellitus after a case of drug reaction with eosinophilia and systemic symptoms (DRESS). JAAD Case Rep. 2018, 4, 295–297. [Google Scholar] [CrossRef] [Green Version]
- Illing, P.T.; Purcell, A.W.; McCluskey, J. The role of HLA genes in pharmacogenomics: Unravelling HLA associated adverse drug reactions. Immunogenetics 2017, 69, 617–630. [Google Scholar] [CrossRef]
- Hung, S.I.; Chung, W.H.; Liou, L.B.; Chu, C.C.; Lin, M.; Huang, H.P.; Lin, Y.L.; Lan, J.L.; Yang, L.C.; Hong, H.S.; et al. HLA-B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc. Natl. Acad. Sci. USA 2005, 102, 4134–4139. [Google Scholar] [CrossRef] [Green Version]
- Somogyi, A.A.; Barratt, D.T.; Phillips, E.J.; Moore, K.; Ilyas, F.; Gabb, G.M. High and variable population prevalence of HLA-B*56:02 in indigenous Australians and relation to phenytoin-associated drug reaction with eosinophilia and systemic symptoms. Br. J. Clin. Pharmacol. 2019, 85, 2163–2169. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, P.; Barrett, S.; Mcevoy, L.; Daly, A.K.; Aithal, G.; Lucena, M.I.; Andrade, R.J.; Wadelius, M.; Hallberg, P.; Stephens, C.; et al. Shared genetic risk factors across carbamazepine-induced hypersensitivity reactions. Clin. Pharmacol. Ther. 2019, 106, 1028–1036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Séguéla, P.E.; Iriart, X.; Acar, P.; Montaudon, M.; Roudaut, R.; Thambo, J.B. Eosinophilic cardiac disease: Molecular, clinical and imaging aspects. Arch. Cardiovasc. Dis. 2015, 108, 258–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- James, J.; Sammou, Y.M.; Virata, A.R.; Nordin, T.A.; Dumic, I. Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome secondary to furosemide: Case report and review of literature. Am. J. Case Rep. 2018, 19, 163–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.C.; Chang, C.Y.; Cho, Y.T.; Chiu, H.C.; Chu, C.Y. Long-term sequelae of drug reaction with eosinophilia and systemic symptoms: A retrospective cohort study from Taiwan. J. Am. Acad. Dermatol. 2013, 68, 459–465. [Google Scholar] [CrossRef]
- Takahashi, R.; Kano, Y.; Yamazaki, Y.; Kimishima, M.; Mizukawa, Y.; Shiohara, T. Defective regulatory T cells in patients with severe drug eruptions: Timing of the dysfunction is associated with the pathological phenotype and outcome. J. Immunol. 2009, 182, 8071–8079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fida, M.; Hamdi, A.M.; Bryson, A.; Razonable, R.R.; Abu Saleh, O. Long-term outcomes of patients with human herpesvirus 6 encephalitis. Open Forum Infect. Dis. 2019, 6, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.R.; Hall, O.M.; Kaye, A.M.; Kaye, A.D. Drug-induced acute pancreatitis: A review. Ochsner J. 2015, 15, 45–51. [Google Scholar]
- Hung, W.Y. Contemporary review of drug-induced pancreatitis: A different perspective. World J. Gastrointest. Pathophysiol. 2014, 5, 405–415. [Google Scholar] [CrossRef]
- Sadr-Azodi, O.; Mattsson, F.; Bexlius, T.S.; Lindblad, M.; Lagergren, J.; Ljung, R. Association of oral glucocorticoid use with an increased risk of acute pancreatitis: A population-based nested case-control study. JAMA Intern. Med. 2013, 173, 444–449. [Google Scholar] [CrossRef] [Green Version]
- Furuta, G.T.; Katzka, D.A. Eosinophilic esophagitis definition and differential diagnosis. N. Engl. J. Med. 2016, 373, 1640–1648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dellon, E.S. Eosinophilic esophagitis: Diagnostic tests and criteria. Curr. Opin. Gastroenterol. 2012, 28, 382–388. [Google Scholar] [CrossRef] [Green Version]
- Shiohara, T.; Iijima, M.; Ikezawa, Z.; Hashimoto, K. The diagnosis of a DRESS syndrome has been sufficiently established on the basis of typical clinical features and viral reactivations. Br. J. Dermatol. 2007, 156, 1083–1084. [Google Scholar] [CrossRef]
- Tohyama, M.; Hashimoto, K.; Yasukawa, M.; Kimura, H.; Horikawa, T.; Nakajima, K.; Urano, Y.; Matsumoto, K.; Ilijima, M.; Shear, N.H. Association of human herpesvirus 6 reactivation with the flaring and severity of drug-induced hypersensitivity syndrome. Br. J. Dermatol. 2007, 157, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Husain, Z.; Reddy, B.Y.; Schwartz, R.A. DRESS syndrome: Part II. Management and therapeutics. J. Am. Acad. Dermatol. 2013, 68, 709.e1–e9. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, E.; Yanes, D.; Imadojemu, S.; Kroshinsky, D. Evaluation of cyclosporine for the treatment of DRESS syndrome. JAMA Dermatol. 2020, 156, 704–706. [Google Scholar] [CrossRef]
- Kim, D.; Kobayashi, T.; Voisin, B.; Jo, J.H.; Sakamoto, K.; Jin, S.P.; Kelly, M.; Pasieka, H.B.; Naff, J.L.; Meyerle, J.H.; et al. Targeted therapy guided by single-cell transcriptomic analysis in drug-induced hypersensitivity syndrome: A case report. Nat. Med. 2020, 26, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Schmid-Grendelmeier, P.; Steiger, P.; Naegeli, M.C.; Kolm, I.; Lang, C.C.V.; Maverakis, E.; Bruggen, M.C. Benralizumab for severe DRESS in two COVID-19 patients. J. Allergy Clin. Immunol. Pract. 2021, 9, 481–483.e2. [Google Scholar] [CrossRef]

| Chronic Co-Morbid Conditions | Number of Patients |
|---|---|
| None | 11/51 (21.6%) |
| Epilepsy/seizure disorder | 11/51 (21.6%) |
| Hypertension | 7/51 (13.7%) |
| Hyperuricemia/Gout | 5/51 (9.8%) |
| Bipolar disorder | 4/51 (7.8%) |
| Thyroid disease | 4/51 (7.8%) |
| Chronic kidney disease | 4/51 (7.8%) |
| Diabetes/pre-diabetes | 3/51 (5.9%) |
| Arrhythmia | 2/51 (3.9%) |
| Psoriatic arthritis | 2/51 (3.9%) |
| Other less common comorbidities: asthma, alcohol abuse, arrhythmia, chronic heart failure, chronic cough, coronary artery disease, duodenal ulcer, dyslipidemia, idiopathic peripheral neuropathy, mitral valve prolapse, multiple myeloma, multiple sclerosis, opiate use disorder, pulmonary hypertension, pemphigus, primary sclerosing cholangitis, psoriatic arthritis, rheumatoid arthritis, schizophrenia, severe pes excavatum, trigeminal neuralgia, ulcerative colitis and tuberculosis. | All other comorbidities listed were present in only one patient. |
| Medication | Number of Cases |
|---|---|
| Carbamazepine | 11/51 (21.6%) |
| Allopurinol | 5/51 (9.8%) |
| Lamotrigine | 5/51 (9.8%) |
| Dapsone | 2/51 (3.9%) |
| Mexiletine | 2/51 (3.9%) |
| Minocycline | 2/51 (3.9%) |
| Sulfasalazine | 2/51 (3.9%) |
| Vancomycin | 2/51 (3.9%) |
| Amoxicillin–Clavulanate | 1/51 (1.9%) |
| Ciprofloxacin | 1/51 (1.9%) |
| Clindamycin | 1/51 (1.9%) |
| Leflunomide | 1/51 (1.9%) |
| Methicillin sodium | 1/51 (1.9%) |
| Methimazole | 1/51 (1.9%) |
| Naproxen | 1/51 (1.9%) |
| Piperacillin–Tazobactam | 1/51 (1.9%) |
| Phenytoin | 1/51 (1.9%) |
| Phenobarbital | 1/51 (1.9%) |
| Sodium Aurothiomalate (Gold) | 1/51 (1.9%) |
| Spironolactone | 1/51 (1.9%) |
| Valproic acid | 1/51 (1.9%) |
| Antituberculotics | 1/51 (1.9%) |
| Titanium bioprothesis/Minocycline/Rifampicin | 1/51 (1.9%) |
| Diclofenac/Ibuprofen | 1/51 (1.9%) |
| Lamotrigine/Bupropion | 1/51 (1.9%) |
| Phenytoin/Lamotrigine | 1/51 (1.9%) |
| Chemotherapeutics (vincristine, doxorubicin, melphalan) | 1/51 (1.9%) |
| Unspecified antibiotic | 1/51 (1.9%) |
| Signs/Symptoms | Number of Cases | |
|---|---|---|
| Diarrhea (n = 22) | Unspecified type | 10 (19.6%) |
| Non-bloody | 4 (7.8%) | |
| Bloody | 4 (7.8%) | |
| Watery with positive occult blood | 2 (3.9%) | |
| Non-bloody becoming bloody | 2 (3.9%) | |
| Vomiting | 10/51 (19.6%) | |
| Abdominal pain | 8/51 (15.7%) | |
| Nausea | 7/51 (13.7%) | |
| Epigastric pain/chest pain ** | 3/51 (5.9%) | |
| Dysphagia | 2/51 (3.9%) | |
| Organ Affected | Number of Cases |
|---|---|
| Pancreas | 29/51 (56.9%) |
| Colon | 21/51 (41.2%) |
| Esophagus | 4/51 (7.8%) |
| Small bowel | 3/51 (5.9%) |
| Stomach | 1/51 (1.9%) |
| Pancreas Involvement | Number of Cases | |
|---|---|---|
| Acute pancreatitis | 11/29 (37.9%) | |
| T1DM 18/29 (62.1%) | Sequela: autoimmune T1DM | 7/29 (24.1%) |
| Sequela: fulminant T1DM | 7/29 (24.1%) | |
| Admission: fulminant T1DM | 4/29 (13.8%) | |
| Sequela: type 2 diabetes mellitus | 1/29 (3.4%) | |
| Sequela: chronic pancreatic insufficiency | 1/29 (3.4%) | |
| Therapy Option | Number of Cases |
|---|---|
| Steroids (IV or PO) | 39/51 (76.5%) |
| Antihistamines | 5/51 (9.8%) |
| IVIG | 5/51 (9.8%) |
| Ganciclovir | 3/51 (5.9%) |
| Plasma exchange | 2/51 (3.9%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jevtic, D.; Dumic, I.; Nordin, T.; Singh, A.; Sulovic, N.; Radovanovic, M.; Jecmenica, M.; Milovanovic, T. Less Known Gastrointestinal Manifestations of Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) Syndrome: A Systematic Review of the Literature. J. Clin. Med. 2021, 10, 4287. https://doi.org/10.3390/jcm10184287
Jevtic D, Dumic I, Nordin T, Singh A, Sulovic N, Radovanovic M, Jecmenica M, Milovanovic T. Less Known Gastrointestinal Manifestations of Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) Syndrome: A Systematic Review of the Literature. Journal of Clinical Medicine. 2021; 10(18):4287. https://doi.org/10.3390/jcm10184287
Chicago/Turabian StyleJevtic, Djordje, Igor Dumic, Terri Nordin, Amteshwar Singh, Nadezda Sulovic, Milan Radovanovic, Mladen Jecmenica, and Tamara Milovanovic. 2021. "Less Known Gastrointestinal Manifestations of Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) Syndrome: A Systematic Review of the Literature" Journal of Clinical Medicine 10, no. 18: 4287. https://doi.org/10.3390/jcm10184287
APA StyleJevtic, D., Dumic, I., Nordin, T., Singh, A., Sulovic, N., Radovanovic, M., Jecmenica, M., & Milovanovic, T. (2021). Less Known Gastrointestinal Manifestations of Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) Syndrome: A Systematic Review of the Literature. Journal of Clinical Medicine, 10(18), 4287. https://doi.org/10.3390/jcm10184287










