Meteorin-Like Protein (Metrnl) in Obesity, during Weight Loss and in Adipocyte Differentiation
Abstract
:1. Introduction
- -
- Circulating Metrnl concentrations (before intervention) with respect to correlate them with anthropometric and biochemical parameters;
- -
- Metrnl gene expression in subcutaneous and visceral adipose tissue compartments of morbidly obese patients undergoing bariatric surgery;
- -
- Circulating Metrnl concentrations longitudinally over 12 months following bariatric surgery or start of LCD;
- -
- Metrnl gene expression in murine adipose tissues and in the murine 3T3-L1 cell line upon treatment with metabolites such as glucose, insulin, fatty acids, bile acids, and incretins.
2. Materials and Methods
2.1. Adipocyte Cell Culture and Stimulation Experiments
2.2. Preparation of mRNA and Real-Time PCR Analysis of Metrnl Gene Expression in Murine Cells and in Murine and Human Adipose Tissue
2.3. ROBS (Research in Obesity and Bariatric Surgery) Study Cohort
2.4. Measurement of Serum Metrnl Levels
2.5. Statistical Analysis
3. Results
3.1. Quantification of Baseline Metrnl Serum Levels in Patients Undergoing LCD or Bariatric Surgery
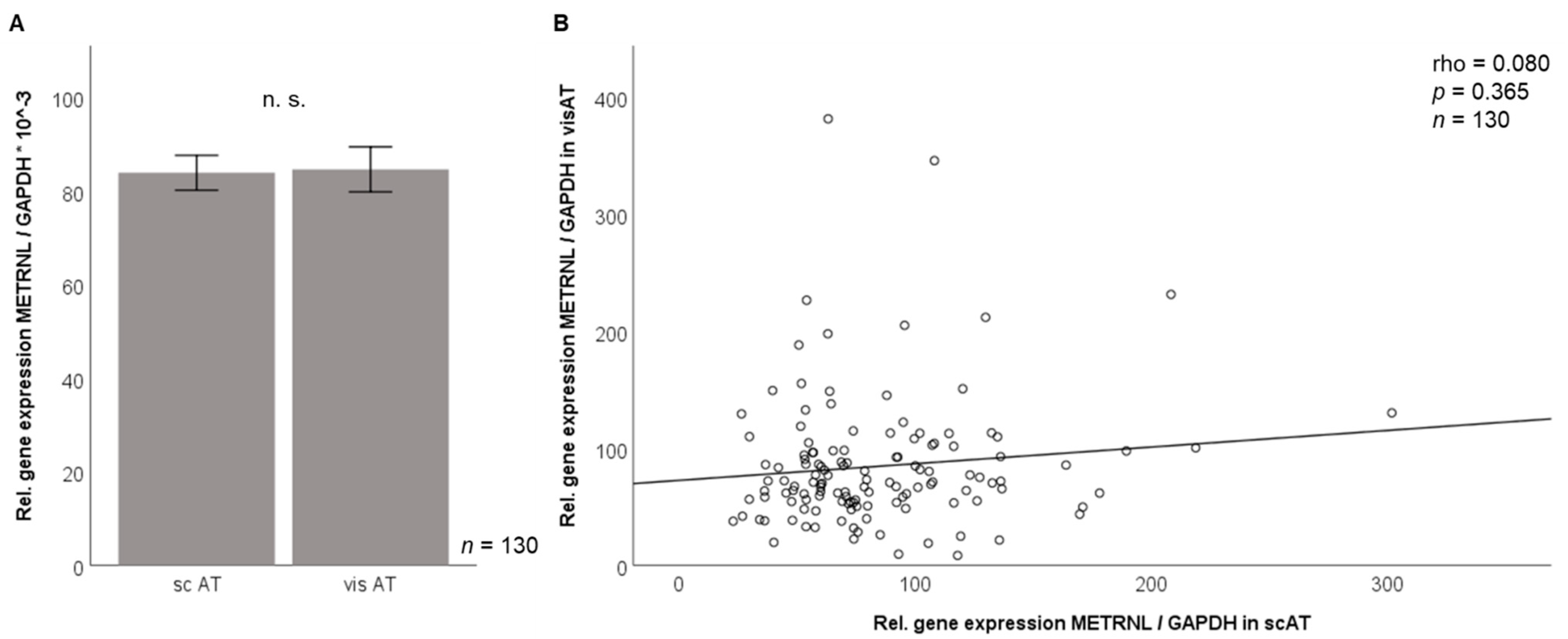
3.2. Metrnl Gene Expression Is Not Different between Visceral and Subcutaneous Adipose Tissue in Morbidly Obese Patients
3.3. Correlation of Adipose Tissue Metrnl Gene Expression with Anthropometric and Biochemical Parameters
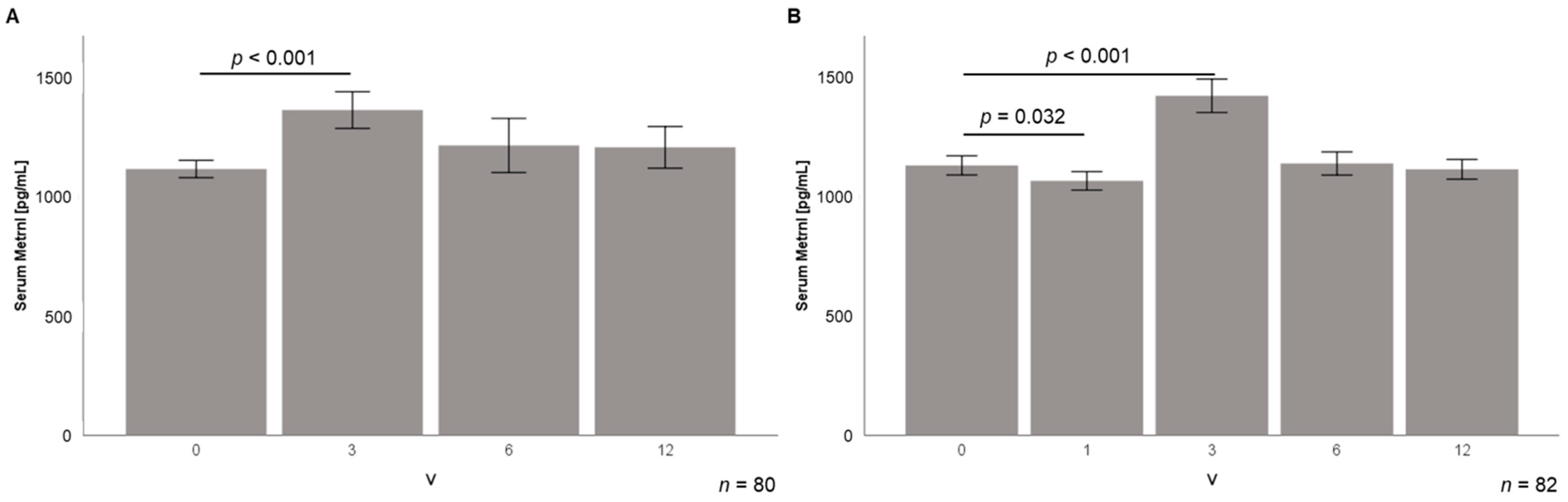
3.4. Serum Metrnl Levels Are Transiently Elevated during Early Stages of Weight Loss
3.5. Correlation Analysis of Serum Metrnl during Weight Loss
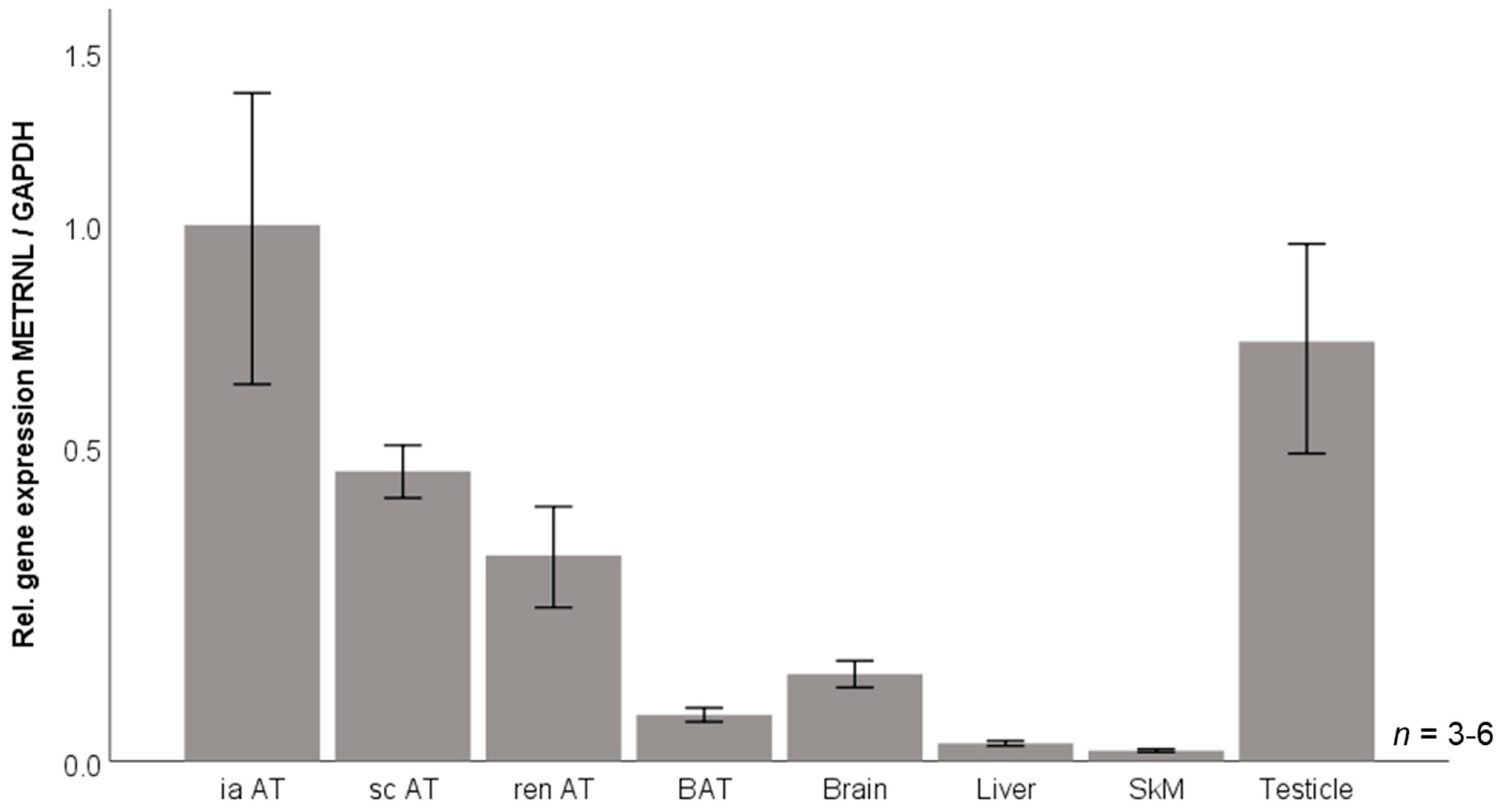
3.6. Murine Tissue Expression of Metrnl mRNA
3.7. Metrnl Gene Expression Is Transiently Downregulated during 3T3-L1 Adipocyte Differentiation
3.8. Effects of Metabolic Stimuli on Metrnl Expression in Adipocytes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kershaw, E.E.; Flier, J.S. Adipose Tissue as an Endocrine Organ. J. Clin. Endocrinol. Metab. 2004, 89, 2548–2556. [Google Scholar] [CrossRef] [PubMed]
- Ahima, R.S. Adipose tissue as an endocrine organ. Obesity 2006, 14 (Suppl. 5), 242S–249S. [Google Scholar] [CrossRef]
- Trayhurn, P.; Wood, I.S. Adipokines: Inflammation and the pleiotropic role of white adipose tissue. Br. J. Nutr. 2004, 92, 347–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schäffler, A.; Schölmerich, J.; Büchler, C. Mechanisms of Disease: Adipocytokines and visceral adipose tissue—emerging role in intestinal and mesenteric diseases. Nat. Clin. Pr. Gastroenterol. Hepatol. 2005, 2, 103–111. [Google Scholar] [CrossRef]
- Fasshauer, M.; Blüher, M. Adipokines in health and disease. Trends Pharmacol. Sci. 2015, 36, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Fuster, J.J.; Walsh, K. Adipokines: A link between obesity and cardiovascular disease. J. Cardiol. 2014, 63, 250–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maury, E.; Brichard, S. Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol. Cell. Endocrinol. 2010, 314, 1–16. [Google Scholar] [CrossRef]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Unamuno, X.; Gómez-Ambrosi, J.; Rodríguez, A.; Becerril, S.; Frühbeck, G.; Catalán, V. Adipokine dysregulation and adipose tissue inflammation in human obesity. Eur. J. Clin. Investig. 2018, 48, e12997. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.-Y.; Zheng, S.-L.; Wang, P.; Xu, T.-Y.; Guan, Y.-F.; Zhang, Y.-J.; Miao, C.-Y. Subfatin is a Novel Adipokine and Unlike Meteorin in Adipose and Brain Expression. CNS Neurosci. Ther. 2014, 20, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, J.R.; Fransson, A.; Fjord-Larsen, L.; Thompson, L.; Houchins, J.P.; Andrade, N.; Torp, M.; Kalkkinen, N.; Andersson, E.; Lindvall, O.; et al. Cometin is a novel neurotrophic factor that promotes neurite outgrowth and neuroblast migration in vitro and supports survival of spiral ganglion neurons in vivo. Exp. Neurol. 2012, 233, 172–181. [Google Scholar] [CrossRef] [Green Version]
- Berghoff, M.; Höpfinger, A.; Rajendran, R.; Karrasch, T.; Schmid, A.; Schäffler, A. Evidence of a Muscle–Brain Axis by Quantification of the Neurotrophic Myokine METRNL (Meteorin-Like Protein) in Human Cerebrospinal Fluid and Serum. J. Clin. Med. 2021, 10, 3271. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.R.; Long, J.Z.; White, J.P.; Svensson, K.J.; Lou, J.; Lokurkar, I.; Jedrychowski, M.P.; Ruas, J.; Wrann, C.D.; Lo, J.C.; et al. Meteorin-like Is a Hormone that Regulates Immune-Adipose Interactions to Increase Beige Fat Thermogenesis. Cell 2014, 157, 1279–1291. [Google Scholar] [CrossRef] [Green Version]
- Zheng, S.-L.; Li, Z.-Y.; Song, J.; Liu, J.-M.; Miao, C.-Y. Metrnl: A secreted protein with new emerging functions. Acta Pharmacol. Sin. 2016, 37, 571–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baht, G.S.; Bareja, A.; Lee, D.E.; Rao, R.R.; Huang, R.; Huebner, J.L.; Bartlett, D.B.; Hart, C.R.; Gibson, J.R.; Lanza, I.R.; et al. Meteorin-like facilitates skeletal muscle repair through a Stat3/IGF-1 mechanism. Nat. Metab. 2020, 2, 278–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ushach, I.; Burkhardt, A.M.; Martinez, C.; Hevezi, P.A.; Gerber, P.A.; Buhren, B.A.; Schrumpf, H.; Valle-Rios, R.; Vazquez, M.I.; Homey, B.; et al. METEORIN-LIKE is a cytokine associated with barrier tissues and alternatively activated macrophages. Clin. Immunol. 2015, 156, 119–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuo, L.; Ge, S.; Ge, Y.; Li, J.; Zhu, B.; Zhang, Z.; Jiang, C.; Li, J.; Wang, S.; Liu, M.; et al. The Adipokine Metrnl Ameliorates Chronic Colitis in Il-10–/– Mice by Attenuating Mesenteric Adipose Tissue Lesions During Spontaneous Colitis. J. Crohn’s Colitis 2019, 13, 931–941. [Google Scholar] [CrossRef]
- Qi, Q.; Hu, W.-J.; Zheng, S.-L.; Zhang, S.-L.; Le, Y.-Y.; Li, Z.-Y.; Miao, C.-Y. Metrnl deficiency decreases blood HDL cholesterol and increases blood triglyceride. Acta Pharmacol. Sin. 2020, 41, 1568–1575. [Google Scholar] [CrossRef] [PubMed]
- Kerget, B.; Afşin, D.E.; Kerget, F.; Aşkın, S.; Akgün, M. Is Metrnl an Adipokine İnvolved in the Anti-inflammatory Response to Acute Exacerbations of COPD? Lung 2020, 198, 307–314. [Google Scholar] [CrossRef]
- Liu, Z.-X.; Ji, H.-H.; Yao, M.-P.; Wang, L.; Wang, Y.; Zhou, P.; Liu, Y.; Zheng, X.-F.; He, H.-W.; Wang, L.-S.; et al. Serum Metrnl is associated with the presence and severity of coronary artery disease. J. Cell. Mol. Med. 2018, 23, 271–280. [Google Scholar] [CrossRef]
- Li, Z.-Y.; Song, J.; Zheng, S.-L.; Fan, M.-B.; Guan, Y.-F.; Qu, Y.; Xu, J.; Wang, P.; Miao, C.-Y. Adipocyte Metrnl Antagonizes Insulin Resistance Through PPARγ Signaling. Diabetes 2015, 64, 4011–4022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Q.; Dan, Y.-L.; He, Y.-S.; Xiang, K.; Hu, Y.-Q.; Zhao, C.-N.; Zhong, X.; Wang, D.-G.; Pan, H.-F. Circulating Meteorin-like Levels in Patients with Type 2 Diabetes Mellitus: A Meta-Analysis. Curr. Pharm. Des. 2020, 26, 5732–5738. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Lin, P.; Wang, C.; Wang, K.; Sun, Y. Administration of metrnl delays the onset of diabetes in non-obese diabetic mice. Endocr. J. 2021, 68, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Alkhairi, I.; Cherian, P.; Abu-Farha, M.; Al Madhoun, A.; Nizam, R.; Melhem, M.; Jamal, M.; Al-Sabah, S.; Ali, H.; Tuomilehto, J.; et al. Increased Expression of Meteorin-Like Hormone in Type 2 Diabetes and Obesity and Its Association with Irisin. Cells 2019, 8, 1283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pellitero, S.; Piquer-Garcia, I.; Ferrer-Curriu, G.; Puig, R.; Martínez, E.; Moreno, P.; Tarascó, J.; Balibrea, J.M.; Lerin, C.; Domingo, M.P.; et al. Opposite changes in meteorin-like and oncostatin m levels are associated with metabolic improvements after bariatric surgery. Int. J. Obes. 2018, 42, 919–922. [Google Scholar] [CrossRef]
- Jamal, M.H.; Abu-Farha, M.; Al-Khaledi, G.; Al-Sabah, S.; Ali, H.; Cherian, P.; Al-Khairi, I.; Alotaibi, F.; Al-Ali, W.; Bosso, M.; et al. Effect of sleeve gastrectomy on the expression of meteorin-like (METRNL) and Irisin (FNDC5) in muscle and brown adipose tissue and its impact on uncoupling proteins in diet-induced obesity rats. Surg. Obes. Relat. Dis. 2020, 16, 1910–1918. [Google Scholar] [CrossRef]
- Green, H.; Kehinde, O. An established preadipose cell line and its differentiation in culture II. Factors affecting the adipose conversion. Cell 1975, 5, 19–27. [Google Scholar] [CrossRef]
- Schmid, A.; Hochberg, A.; Kreiß, A.F.; Gehl, J.; Patz, M.; Thomalla, M.; Hanses, F.; Karrasch, T.; Schäffler, A. Role of progranulin in adipose tissue innate immunity. Cytokine 2020, 125, 154796. [Google Scholar] [CrossRef]
- Zaitsu, H.; Serrero, G. Pedersen fetuin contains three adipogenic factors with distinct biochemical characteristics. J. Cell. Physiol. 1990, 144, 485–491. [Google Scholar] [CrossRef]
- Bachmeier, M.; Löffler, G. Adipogenic Activities in Commercial Preparations of Fetuin. Horm. Metab. Res. 1994, 26, 92–96. [Google Scholar] [CrossRef]
- Green, H.; Kehinde, O. Formation of normally differentiated subcutaneous fat pads by an established preadipose cell line. J. Cell. Physiol. 1979, 101, 169–171. [Google Scholar] [CrossRef]
- Green, H.; Meuth, M. An established pre-adipose cell line and its differentiation in culture. Cell 1974, 3, 127–133. [Google Scholar] [CrossRef]
- MacDougald, O.A.; Lane, M.D. Transcriptional Regulation of Gene Expression During Adipocyte Differentiation. Annu. Rev. Biochem. 1995, 64, 345–373. [Google Scholar] [CrossRef] [PubMed]
- Cornelius, P.; MacDougald, O.; Lane, M.D. Regulation of Adipocyte Development. Annu. Rev. Nutr. 1994, 14, 99–129. [Google Scholar] [CrossRef] [PubMed]
- Schmid, A.; Leszczak, S.; Ober, I.; Karrasch, T.; Schäffler, A. Short-term Regulation of Resistin in vivo by Oral Lipid Ingestion and in vitro by Fatty Acid Stimulation. Exp. Clin. Endocrinol. Diabetes 2015, 123, 553–560. [Google Scholar] [CrossRef]
- Singh, P.; Zhang, Y.; Sharma, P.; Covassin, N.; Soucek, F.; Friedman, P.A.; Somers, V.K. Statins decrease leptin expression in human white adipocytes. Physiol. Rep. 2018, 6, e13566. [Google Scholar] [CrossRef] [Green Version]
- Brock, J.; Schmid, A.; Karrasch, T.; Pfefferle, P.; Schlegel, J.; Busse, I.; Hauenschild, A.; Schmidt, B.; Koukou, M.; Arapogianni, E.; et al. Progranulin serum levels and gene expression in subcutaneous vs visceral adipose tissue of severely obese patients undergoing bariatric surgery. Clin. Endocrinol. 2019, 91, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Docherty, N.G.; Le Roux, C.W. Physiological adaptations following Roux-en-Y gastric bypass and the identification of targets for bariatric mimetic pharmacotherapy. Curr. Opin. Pharmacol. 2015, 25, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, H.; Kolodziejczyk, A.A.; Halstuch, D.; Elinav, E. Bile acids in glucose metabolism in health and disease. J. Exp. Med. 2018, 215, 383–396. [Google Scholar] [CrossRef]
- Schmid, A.; Neumann, H.; Karrasch, T.; Liebisch, G.; Schäffler, A. Bile Acid Metabolome after an Oral Lipid Tolerance Test by Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS). PLoS ONE 2016, 11, e0148869. [Google Scholar] [CrossRef]
- Schmid, A.; Schlegel, J.; Thomalla, M.; Karrasch, T.; Schäffler, A. Evidence of functional bile acid signaling pathways in adipocytes. Mol. Cell. Endocrinol. 2019, 483, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bala, M.; Martin, J.; Kopp, A.; Hanses, F.; Buechler, C.; Schäffler, A. In vivo Suppression of Visfatin by Oral Glucose Uptake: Evidence for a Novel Incretin-Like Effect by Glucagon-Like Peptide-1 (GLP-1). J. Clin. Endocrinol. Metab. 2011, 96, 2493–2501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmid, A.; Gehl, J.; Thomalla, M.; Hochberg, A.; Kreiß, A.; Patz, M.; Karrasch, T.; Schäffler, A. Downregulation of CTRP-3 by Weight Loss In Vivo and by Bile Acids and Incretins in Adipocytes In Vitro. Int. J. Mol. Sci. 2020, 21, 8168. [Google Scholar] [CrossRef] [PubMed]



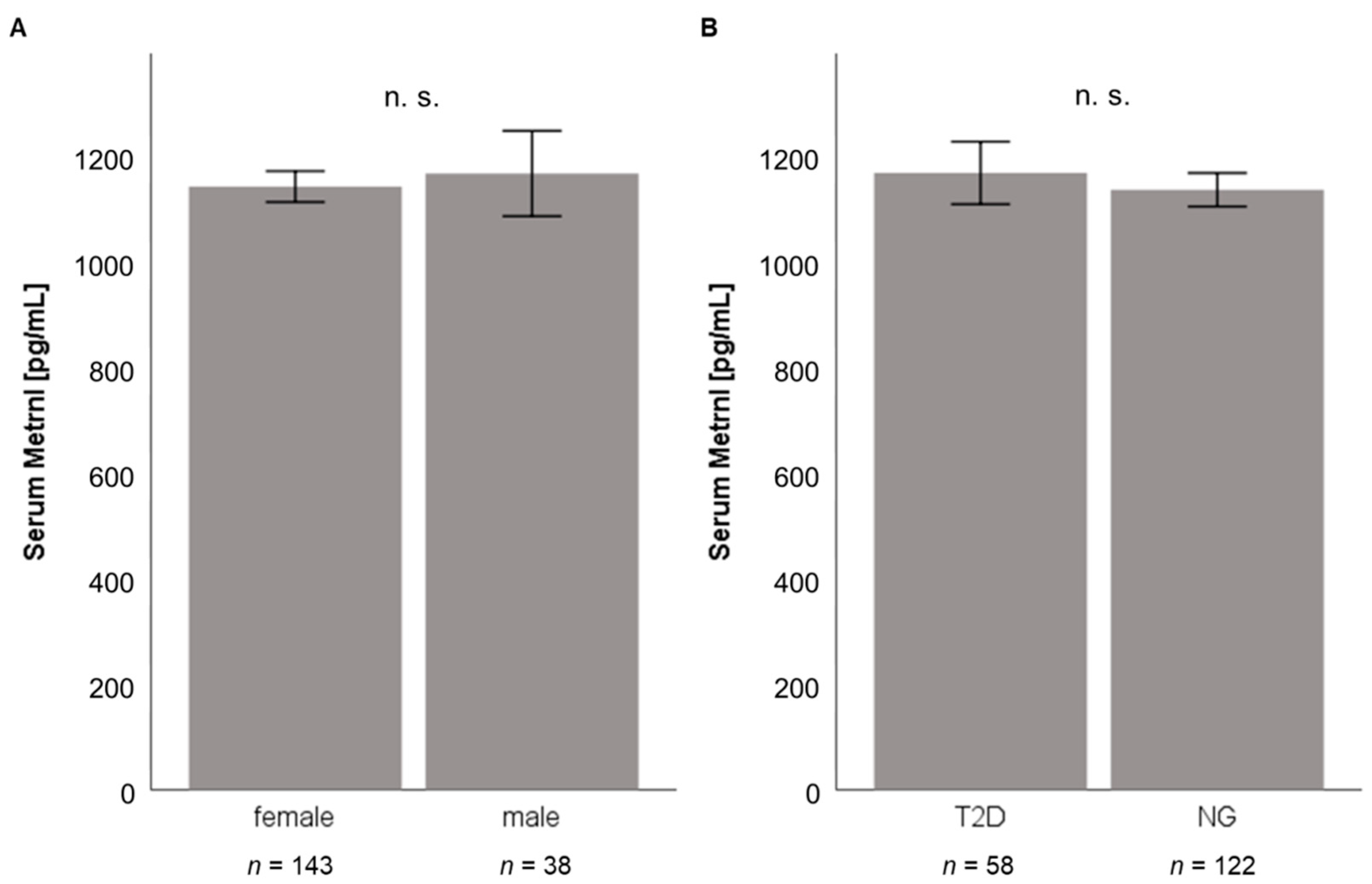

| (A) Low Calorie Diet n = 131 | |
| Females | 88 (67.2%) |
| Males | 43 (32.8%) |
| Age [years] (±SD) | 42.1 ± 12.0 |
| BMI [kg/m2] (±SD) | 43.5 ± 6.7 |
| Serum Metrnl [pg/mL] (±SD) | 1117 ± 378 |
| (B) Bariatric Surgery n = 181 | |
| Females | 143 (79.0%) |
| Males | 38 (21.0%) |
| Age [years] (±SD) | 39.8 ± 11.1 |
| BMI [kg/m2] (±SD) | 53.4 ± 6.8 |
| Serum Metrnl [pg/mL] (±SD) | 1143 ± 383 |
| (A) Low Calorie Diet n = 131 | ||
| Correlation of serum Metrnl with: | rho | p |
| Metabolism/Inflammation | ||
|---|---|---|
| BMI | +0.049 | 0.579 |
| Body fat (%) | +0.130 | 0.145 |
| Glucose | −0.078 | 0.379 |
| Insulin | −0.113 | 0.130 |
| HbA1c | −0.269 | 0.002 |
| Total cholesterol | −0.038 | 0.664 |
| LDL cholesterol | −0.077 | 0.380 |
| HDL cholesterol | +0.191 | 0.029 |
| Triglycerides | −0.131 | 0.137 |
| CRP | −0.051 | 0.563 |
| Classical adipokines | ||
| Adiponectin | +0.135 | 0.124 |
| Leptin | +0.269 | 0.002 |
| Resistin | +0.187 | 0.032 |
| Novel immune-regulatory adipokines | ||
| Progranulin | +0.180 | 0.048 |
| CTRP-3 | −0.017 | 0.845 |
| CAMP | −0.115 | 0.315 |
| Fibroblast growth factors | ||
| FGF19 | +0.101 | 0.259 |
| FGF21 | +0.066 | 0.462 |
| Natriuretic peptides | ||
| NT-proANP | −0.048 | 0.585 |
| (B) Bariatric Surgery n = 181 | ||
| Correlation of Serum Metrnl with: | rho | p |
| Metabolism/Inflammation | ||
| BMI | −0.005 | 0.950 |
| Body fat (%) | +0.045 | 0.579 |
| Glucose | −0.077 | 0.308 |
| Insulin | +0.023 | 0.785 |
| HbA1c | −0.100 | 0.208 |
| Total cholesterol | −0.111 | 0.158 |
| LDL cholesterol | −0.110 | 0.162 |
| HDL cholesterol (n = 163) | +0.180 | 0.022 |
| Triglycerides (n = 163) | −0.164 | 0.037 |
| CRP | −0.035 | 0.643 |
| Classical adipokines | ||
| Adiponectin | −0.019 | 0.804 |
| Leptin | +0.222 | 0.003 |
| Resistin | +0.316 | <0.001 |
| Novel immune-regulatory adipokines | ||
| Progranulin | +0.083 | 0.308 |
| CTRP-3 | −0.122 | 0.105 |
| CAMP | +0.003 | 0.966 |
| Fibroblast growth factors | ||
| FGF19 | +0.101 | 0.180 |
| FGF21 | +0.137 | 0.070 |
| Natriuretic peptides | ||
| NT-proANP | +0.095 | 0.202 |
| (A) Low Calorie Diet (n = 80) | V3 | V6 | V12 | |||
| Correlated Parameters | rho | p | rho | p | rho | p |
|---|---|---|---|---|---|---|
| Body fat (%) | −0.191 | 0.096 | −0.005 | 0.968 | −0.170 | 0.141 |
| BMI | −0.109 | 0.340 | +0.090 | 0.427 | −0.227 | 0.043 |
| Glucose | −0.145 | 0.207 | ||||
| HbA1c | +0.006 | 0.957 | −0.218 | 0.055 | −0.149 | 0.191 |
| Total cholesterol | −0.106 | 0.351 | ||||
| LDL cholesterol | −0.056 | 0.623 | ||||
| HDL cholesterol | +0.002 | 0.987 | ||||
| Triglycerides | −0.142 | 0.214 | ||||
| CRP | −0.056 | 0.624 | +0.002 | 0.983 | −0.062 | 0.585 |
| (B) Bariatric Surgery (n = 82) | V3 | V6 | V12 | |||
| Correlated Parameters | rho | p | rho | p | rho | p |
| Body fat (%) | −0.096 | 0.416 | −0.070 | 0.557 | −0.161 | 0.171 |
| BMI | −0.009 | 0.936 | −0.045 | 0.691 | −0.074 | 0.509 |
| Glucose | +0.232 | 0.040 | ||||
| HbA1c | −0.004 | 0.972 | −0.010 | 0.929 | +0.107 | 0.343 |
| Total cholesterol | −0.008 | 0.945 | ||||
| LDL cholesterol | +0.031 | 0.782 | ||||
| HDL cholesterol | +0.046 | 0.687 | ||||
| Triglycerides | +0.096 | 0.395 | ||||
| CRP | −0.006 | 0.960 | +0.190 | 0.089 | +0.254 | 0.023 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmid, A.; Karrasch, T.; Schäffler, A. Meteorin-Like Protein (Metrnl) in Obesity, during Weight Loss and in Adipocyte Differentiation. J. Clin. Med. 2021, 10, 4338. https://doi.org/10.3390/jcm10194338
Schmid A, Karrasch T, Schäffler A. Meteorin-Like Protein (Metrnl) in Obesity, during Weight Loss and in Adipocyte Differentiation. Journal of Clinical Medicine. 2021; 10(19):4338. https://doi.org/10.3390/jcm10194338
Chicago/Turabian StyleSchmid, Andreas, Thomas Karrasch, and Andreas Schäffler. 2021. "Meteorin-Like Protein (Metrnl) in Obesity, during Weight Loss and in Adipocyte Differentiation" Journal of Clinical Medicine 10, no. 19: 4338. https://doi.org/10.3390/jcm10194338
APA StyleSchmid, A., Karrasch, T., & Schäffler, A. (2021). Meteorin-Like Protein (Metrnl) in Obesity, during Weight Loss and in Adipocyte Differentiation. Journal of Clinical Medicine, 10(19), 4338. https://doi.org/10.3390/jcm10194338





