NT-proBNP as a Potential Marker of Cardiovascular Damage in Children with Chronic Kidney Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Group
2.2. Ethical Issues
2.3. Clinical Parameters
2.4. NT-proBNP and Biochemical Parameters
2.5. Blood Pressure and Parameters of Cardiovascular Damage
2.6. Statistical Analysis
3. Results
3.1. Clinical Characteristics
3.2. NT-proBNP and Biochemical Parameters
3.3. Blood Pressure and Markers of Arterial and Heart Damage
3.4. Correlations of NT-proBNP and Markers of Arterial and Heart Damage
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weaver, D.J.; Mitsnefes, M. Cardiovascular Disease in Children and Adolescents with Chronic Kidney Disease. Semin. Nephrol. 2018, 38, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Shroff, R.; Dégi, A.; Kerti, A.; Kis, E.; Cseprekál, O.; Tory, K.; Szabó, A.J.; Reusz, G.S. Cardiovascular risk assessment in children with chronic kidney disease. Pediatr. Nephrol. 2013, 28, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Maisel, A.S.; Duran, J.M.; Wettersten, N. Natriuretic Peptides in Heart Failure: Atrial and B-type Natriuretic Peptides. Heart Fail. Clin. 2018, 14, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Goetze, J.P.; Bruneau, B.G.; Ramos, H.R.; Ogawa, T.; de Bold, M.K.; de Bold, A.J. Cardiac natriuretic peptides. Nat. Rev. Cardiol. 2020, 17, 698–717. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Zhang, S.; Chen, Z.; Adhikari, B.K.; Zhang, Y.; Zhang, J.; Sun, J.; Wang, Y. Cardiac biomarkers of heart failure in chronic kidney disease. Clin. Chim. Acta 2020, 510, 298–310. [Google Scholar] [CrossRef]
- Forte, M.; Madonna, M.; Schiavon, S.; Valenti, V.; Versaci, F.; Zoccai, G.B.; Frati, G.; Sciarretta, S. Cardiovascular Pleiotropic Effects of Natriuretic Peptides. Int. J. Mol. Sci. 2019, 20, 3874. [Google Scholar] [CrossRef] [Green Version]
- Felker, G.M.; Petersen, J.W.; Mark, D.B. Natriuretic peptides in the diagnosis and management of heart failure. CMAJ 2006, 175, 611–617. [Google Scholar] [CrossRef] [Green Version]
- Colbert, G.; Jain, N.; de Lemos, J.A.; Hedayati, S.S. Utility of traditional circulating and imaging-based cardiac biomarkers in patients with predialysis CKD. Clin. J. Am. Soc. Nephrol. 2015, 10, 515–529. [Google Scholar] [CrossRef] [Green Version]
- Harrison, T.G.; Shukalek, C.B.; Hemmelgarn, B.R.; Zarnke, K.B.; Ronksley, P.E.; Iragorri, N.; Graham, M.M.; James, M.T. Association of NT-proBNP and BNP with Future Clinical Outcomes in Patients with ESKD: A Systematic Review and Meta-analysis. Am. J. Kidney Dis. 2020, 76, 233–247. [Google Scholar] [CrossRef]
- Li, X.; Yang, X.C.; Sun, Q.M.; Chen, X.D.; Li, Y.C. Brain natriuretic peptide and copeptin levels are associated with cardiovascular disease in patients with chronic kidney disease. Chin. Med. J. 2013, 126, 823–827. [Google Scholar]
- Rinat, C.; Becker-Cohen, R.; Nir, A.; Feinstein, S.; Algur, N.; Ben-Shalom, E.; Farber, B.; Frishberg, Y. B-type natriuretic peptides are reliable markers of cardiac strain in CKD pediatric patients. Pediatr. Nephrol. 2012, 27, 617–625. [Google Scholar] [CrossRef]
- Nalcacioglu, H.; Ozkaya, O.; Kafali, H.C.; Tekcan, D.; Avci, B.; Baysal, K. Is N-terminal pro-brain natriuretic peptide a reliable marker for body fluid status in children with chronic kidney disease? Arch. Med. Sci. 2020, 16, 802. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. Suppl. 2013, 3, 1–150. [Google Scholar]
- Harambat, J.; van Stralen, K.J.; Kim, J.J.; Tizard, E.J. Epidemiology of chronic kidney disease in children. Pediatr. Nephrol. 2012, 27, 363–373. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, G.J.; Muñoz, A.; Schneider, M.F.; Mak, R.H.; Kaskel, F.; Warady, B.A.; Furth, S.L. New equations to estimate GFR in children with CKD. J. Am. Soc. Nephrol. 2009, 20, 629–637. [Google Scholar] [CrossRef] [Green Version]
- Kułaga, Z.; Litwin, M.; Tkaczyk, M.; Palczewska, I.; Zajączkowska, M.; Zwolińska, D.; Krynicki, T.; Wasilewska, A.; Moczulska, A.; Morawiec-Knysak, A.; et al. Polish 2010 growth references for school-aged children and adolescents. Eur. J. Pediatr. 2011, 170, 599–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drüeke, T.B.; Parfrey, P.S. Summary of the KDIGO guideline on anemia and comment: Reading between the (guide)line(s). Kidney Int. 2012, 82, 952–960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int. Suppl. 2017, 7, 1–59. [CrossRef] [Green Version]
- Stewart, J.; McCallin, T.; Martinez, J.; Chacko, S.; Yusuf, S. Hyperlipidemia. Pediatr. Rev. 2020, 41, 393–402. [Google Scholar] [CrossRef]
- Feig, D.I.; Kang, D.H.; Johnson, R.J. Uric acid and cardiovascular risk. N. Engl. J. Med. 2008, 359, 1811–1821. [Google Scholar] [CrossRef]
- Kułaga, Z.; Litwin, M.; Grajda, A.; Kułaga, K.; Gurzkowska, B.; Góźdź, M.; Pan, H. Oscillometric blood pressure percentiles for Polish normal-weight school-aged children and adolescents. J. Hypertens. 2012, 30, 1942–1954. [Google Scholar] [CrossRef]
- Skrzypczyk, P.; Przychodzień, J.; Mizerska-Wasiak, M.; Kuźma-Mroczkowska, E.; Okarska-Napierała, M.; Górska, E.; Stelmaszczyk-Emmel, A.; Demkow, U.; Pańczyk-Tomaszewska, M. Renalase in Children with Glomerular Kidney Diseases. Adv. Exp. Med. Biol. 2017, 1021, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Doyon, A.; Kracht, D.; Bayazit, A.K.; Deveci, M.; Duzova, A.; Krmar, R.T.; Litwin, M.; Niemirska, A.; Oguz, B.; Schmidt, B.M.; et al. Carotid artery intima-media thickness and distensibility in children and adolescents: Reference values and role of body dimensions. Hypertension 2013, 62, 550–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reusz, G.S.; Cseprekal, O.; Temmar, M.; Kis, E.; Cherif, A.B.; Thaleb, A.; Fekete, A.; Szabó, A.J.; Benetos, A.; Salvi, P. Reference values of pulse wave velocity in healthy children and teenagers. Hypertension 2010, 56, 217–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Simone, G.; Daniels, S.R.; Devereux, R.B.; Meyer, R.A.; Roman, M.J.; de Divitiis, O.; Alderman, M.H. Left ventricular mass and body size in normotensive children and adults: Assessment of allometric relations and impact of overweight. J. Am. Coll. Cardiol. 1992, 20, 1251–1260. [Google Scholar] [CrossRef] [Green Version]
- Khoury, P.R.; Mitsnefes, M.; Daniels, S.R.; Kimball, T.R. Age-specific reference intervals for indexed left ventricular mass in children. J. Am. Soc. Echocardiogr. 2009, 22, 709–714. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Querfeld, U.; Schaefer, F. Cardiovascular risk factors in children on dialysis: An update. Pediatr. Nephrol. 2020, 35, 41–57. [Google Scholar] [CrossRef]
- Nir, A.; Lindinger, A.; Rauh, M.; Bar-Oz, B.; Laer, S.; Schwachtgen, L.; Koch, A.; Falkenberg, J.; Mir, T.S. NT-pro-B-type natriuretic peptide in infants and children: Reference values based on combined data from four studies. Pediatr. Cardiol. 2009, 30, 3–8. [Google Scholar] [CrossRef]
- Lam, E.; Higgins, V.; Zhang, L.; Chan, M.K.; Bohn, M.K.; Trajcevski, K.; Liu, P.; Adeli, K.; Nathan, P.C. Normative Values of High-Sensitivity Cardiac Troponin T and N-Terminal pro-B-Type Natriuretic Peptide in Children and Adolescents: A Study from the CALIPER Cohort. J. Appl. Lab. Med. 2020, 6, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Niizuma, S.; Iwanaga, Y.; Yahata, T.; Tamaki, Y.; Goto, Y.; Nakahama, H.; Miyazaki, S. Impact of left ventricular end-diastolic wall stress on plasma B-type natriuretic peptide in heart failure with chronic kidney disease and end-stage renal disease. Clin. Chem. 2009, 55, 1347–1353. [Google Scholar] [CrossRef] [Green Version]
- Apple, F.S.; Murakami, M.M.; Pearce, L.A.; Herzog, C.A. Multi-Biomarker Risk Stratification of N-Terminal Pro-B-Type Natriuretic Peptide, High-Sensitivity C-Reactive Protein, and Cardiac Troponin T and I in End-Stage Renal Disease for All-Cause Death. Clin. Chem. 2004, 50, 2279–2285. [Google Scholar] [CrossRef] [PubMed]
- Shafi, T.; Zager, P.G.; Sozio, S.M.; Grams, M.E.; Jaar, B.G.; Christenson, R.H.; Boulware, L.E.; Parekh, R.S.; Powe, N.R.; Coresh, J. Troponin I and NT-proBNP and the association of systolic blood pressure with outcomes in incident hemodialysis patients: The Choices for Healthy Outcomes in Caring for ESRD (CHOICE) Study. Am. J. Kidney Dis. 2014, 64, 443–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.S.; Yang, J.W.; Yoo, J.S.; Choi, S.O.; Han, B.G. Association between E/e ratio and fluid overload in patients with predialysis chronic kidney disease. PLoS ONE 2017, 12, e0184764. [Google Scholar] [CrossRef] [PubMed]
- Litwin, M.; Niemirska, A. Intima-media thickness measurements in children with cardiovascular risk factors. Pediatr. Nephrol. 2009, 24, 707–719. [Google Scholar] [CrossRef] [PubMed]
- Scandale, G.; Dimitrov, G.; Recchia, M.; Carzaniga, G.; Perilli, E.; Carotta, M.; Catalano, M. Arterial stiffness and 5-year mortality in patients with peripheral arterial disease. J. Hum. Hypertens. 2020, 34, 505–511. [Google Scholar] [CrossRef]
- Willeit, P.; Tschiderer, L.; Allara, E.; Reuber, K.; Seekircher, L.; Gao, L.; Liao, X.; Lonn, E.; Gerstein, H.C.; Yusuf, S.; et al. Carotid Intima-Media Thickness Progression as Surrogate Marker for Cardiovascular Risk: Meta-Analysis of 119 Clinical Trials Involving 100 667 Patients. Circulation 2020, 142, 621–642. [Google Scholar] [CrossRef]
- Sasaki, N.; Yamamoto, H.; Ozono, R.; Maeda, R.; Kihara, Y. Association of Common Carotid Artery Measurements with N-terminal Pro B-type Natriuretic Peptide in Elderly Participants. Intern. Med. 2020, 59, 917–925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, W.; Ni, Z.; Yu, Z.; Shi, B.; Wang, Q. Brain natriuretic peptide is related to carotid plaques and predicts atherosclerosis in pre-dialysis patients with chronic kidney disease. Eur. J. Intern. Med. 2012, 23, 539–544. [Google Scholar] [CrossRef]
- Hayashi, M.; Yasuda, Y.; Suzuki, S.; Tagaya, M.; Ito, T.; Kamada, T.; Yoshinaga, M.; Sugishita, Y.; Fujiwara, W.; Yokoi, H.; et al. Brain natriuretic peptide as a potential novel marker of salt-sensitivity in chronic kidney disease patients without cardiac dysfunction. Heart Vessels 2017, 32, 279–286. [Google Scholar] [CrossRef]
- Sanchez, O.A.; Duprez, D.A.; Bahrami, H.; Daniels, L.B.; Folsom, A.R.; Lima, J.A.; Maisel, A.; Peralta, C.A.; Jacobs, D.R., Jr. The associations between metabolic variables and NT-proBNP are blunted at pathological ranges: The Multi-Ethnic Study of Atherosclerosis. Metabolism 2014, 63, 475–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmid, A.; Albrecht, J.; Brock, J.; Koukou, M.; Arapogianni, E.; Schäffler, A.; Karrasch, T. Regulation of natriuretic peptides postprandially in vivo and of their receptors in adipocytes by fatty acids in vitro. Mol. Cell. Endocrinol. 2018, 473, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Hirata, Y.; Matsumoto, A.; Aoyagi, T.; Yamaoki, K.; Komuro, I.; Suzuki, T.; Ashida, T.; Sugiyama, T.; Hada, Y.; Kuwajima, I.; et al. Measurement of plasma brain natriuretic peptide level as a guide for cardiac overload. Cardiovasc. Res. 2001, 51, 585–591. [Google Scholar] [CrossRef] [Green Version]
- Redfield, M.M.; Rodeheffer, R.J.; Jacobsen, S.J.; Mahoney, D.W.; Bailey, K.R.; Burnett, J.C., Jr. Plasma brain natriuretic peptide concentration: Impact of age and gender. J. Am. Coll. Cardiol. 2002, 40, 976–982. [Google Scholar] [CrossRef] [Green Version]
- Tsuji, H.; Nishino, N.; Kimura, Y.; Yamada, K.; Nukui, M.; Yamamoto, S.; Iwasaka, T.; Takahashi, H. Haemoglobin level influences plasma brain natriuretic peptide concentration. Acta Cardiol. 2004, 59, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Larson, M.G.; Levy, D.; Benjamin, E.J.; Leip, E.P.; Wilson, P.W.; Vasan, R.S. Impact of obesity on plasma natriuretic peptide levels. Circulation 2004, 109, 594–600. [Google Scholar] [CrossRef] [Green Version]
- Marwaha, R.K.; Khadgawat, R.; Tandon, N.; Kanwar, R.; Narang, A.; Sastry, A.; Bhadra, K.; Kalaivani, M. Reference intervals of serum calcium, ionized calcium, phosphate and alkaline phosphatase in healthy Indian school children and adolescents. Clin. Biochem. 2010, 43, 1216–1219. [Google Scholar] [CrossRef]
- Bakkaloglu, S.A.; Bacchetta, J.; Lalayiannis, A.D.; Leifheit-Nestler, M.; Stabouli, S.; Haarhaus, M.; Reusz, G.; Groothoff, J.; Schmitt, C.P.; Evenepoel, P.; et al. Bone evaluation in paediatric chronic kidney disease: Clinical practice points from the European Society for Paediatric Nephrology CKD-MBD and Dialysis working groups and CKD-MBD working group of the ERA-EDTA. Nephrol. Dial. Transplant. 2021, 36, 413–425. [Google Scholar] [CrossRef]
- Gąsowski, J.; Piotrowicz, K.; Messerli, F.H. Arterial hypertension after age 65: From epidemiology and pathophysiology to therapy Do we know where we stand? Kardiol. Pol. 2018, 76, 723–730. [Google Scholar] [CrossRef] [Green Version]
- Thurn, D.; Doyon, A.; Sözeri, B.; Bayazit, A.K.; Canpolat, N.; Duzova, A.; Querfeld, U.; Schmidt, B.M.; Schaefer, F.; Wühl, E.; et al. Aortic Pulse Wave Velocity in Healthy Children and Adolescents: Reference Values for the Vicorder Device and Modifying Factors. Am. J. Hypertens. 2015, 28, 1480–1488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hidvégi, E.V.; Illyés, M.; Molnár, F.T.; Cziráki, A. Influence of body height on aortic systolic pressure augmentation and wave reflection in childhood. J. Hum. Hypertens. 2015, 29, 495–501. [Google Scholar] [CrossRef] [PubMed]

| Analyzed Parameter | Value (Mean ± SD or Median and Q1–Q3) |
|---|---|
| Age (years) | 12.3 (8.6–16.3) |
| Gender (males/females) | 21/17 (55%/45%) |
| CKD GRADE (n (%)) | |
| G2 | 14 (37%) |
| G3 | 11 (29%) |
| G4 | 6 (16%) |
| G5 | 7 (18%) |
| Primary kidney disease (n (%)) | |
| CAKUT | 18 (47%) |
| Glomerulonephritis | 7 (18%) |
| Hereditary nephropathy | 3 (8%) |
| Toxic/ischemic kidney injury | 3 (8%) |
| Cystic kidney disease | 2 (5%) |
| Hemolytic uremic syndrome | 1 (3%) |
| Other | 1 (3%) |
| Unknown | 3 (8%) |
| BMI Z-score | −0.1 ± 1.3 |
| Overweight (BMI Z-score 1–2) | 6 (16%) |
| Obesity (BMI Z-score >2) | 2 (5%) |
| Underweight (BMI Z-score <2) | 3 (8%) |
| Arterial hypertension | 26 (68%) |
| Number of antihypertensive medications | 1 (1–2) |
| Medications 1 | |
| Angiotensin-converting enzyme inhibitor | 16 (42%) |
| Angiotensin receptor antagonist | 2 (5%) |
| Calcium channel antagonist | 19 (50%) |
| Beta-adrenolytic | 7 (18%) |
| Erythropoiesis-stimulating agents | 11 (29%) |
| Vitamin D3 | 29 (76%) |
| Alfacalcidol | 12 (32%) |
| Calcium carbonate | 18 (47%) |
| Erythropoiesis-stimulating agents | 11 (29%) |
| Analyzed Parameter | Value (Mean ± SD or Median and Q1–Q3) |
|---|---|
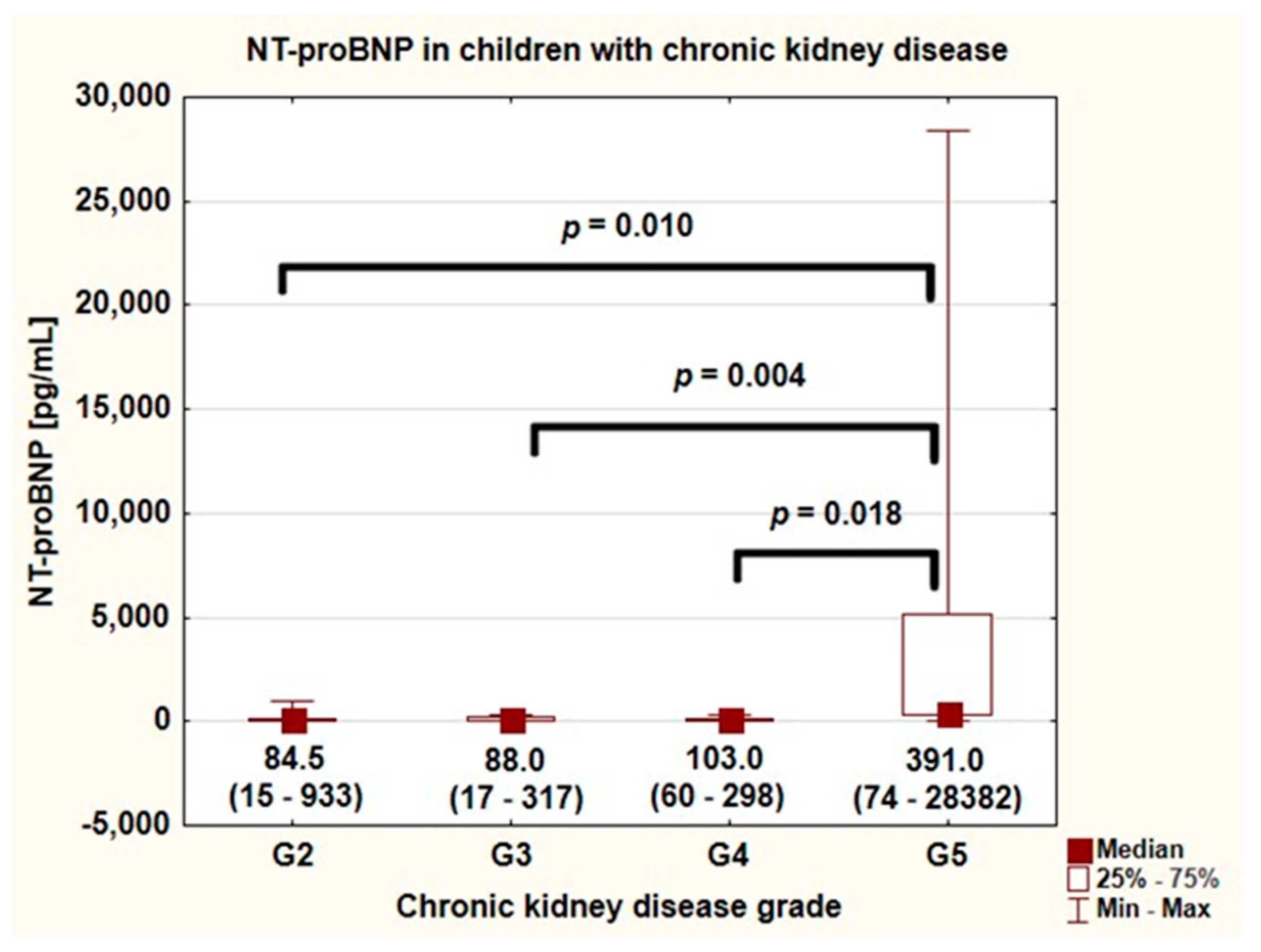
| NT-proBNP (pg/mL) | 95.0 (52–298) |
| NT-proBNP G2 (pg/mL) | 84.5 (32–140) 1 |
| NT-proBNP G3 (pg/mL) | 88.0 (44–174) 2 |
| NT-proBNP G4 (pg/mL) | 103.0 (72–171) 3 |
| NT-proBNP G5 (pg/mL) | 391.0 (317–5146) 1,2,3 |
| Creatinine (mg/dL) | 1.4 (0.9–2.4) |
| GFR (mL/min/1.73 m2) | 43.7 ± 27.3 |
| Urea (mg/dL) | 45.0 (37.0–89.0) |
| Hemoglobin (g/dL) | 12.4 ± 1.4 |
| Albumin (g/dL) | 4.4 (4.2–4.7) |
| cholesterol (mg/dL) | 170.0 (157.0–208.0) |
| LDL-cholesterol (mg/dL) | 96.0 (74.2–115.0) |
| HDL-cholesterol (mg/dL) | 58.2 ± 16.9 |
| Triglyceride (mg/dL) | 101.0 (77.0–152.0) |
| Calcium (mg/dL) | 10.0 ± 0.4 |
| Inorganic phosphate (mg/dL) | 4.7 ± 0.8 |
| Intact parathormone (pg/mL) | 53.5 (29.6–111.0) |
| Alkaline phosphatase (IU/L) | 180.1 ± 77.6 |
| 25(OH)D (ng/mL) | 21.2 (16.3–29.6) |
| Uric acid (mg/dL) | 6.3 ± 1.3 |
| pH | 7.41 ± 0.04 |
| HCO3− (mmol/L) | 24.6 (22.8–25.6) |
| BE (mmol/L) | −0.35 ± 3.16 |
| Parameter | Children with Primary Hypertension |
|---|---|
| Blood pressure and heart rate | |
| Peripheral office SBP (mmHg) | 116.4 ± 12.9 |
| Peripheral office SBP Z-score | 0.99 ± 1.28 |
| Peripheral office DBP (mmHg) | 71.7 ± 12.7 |
| Peripheral office DBP Z-score | 0.82 ± 1.1 |
| Peripheral office MAP (mmHg) | 87.5 ± 12.3 |
| Peripheral office PP (mmHg) | 44.7 ± 7.7 |
| Aortic office SBP (mmHg) | 101.5 ± 13.9 |
| Aortic office DBP (mmHg) | 73.4 ± 12.8 |
| Aortic office MAP (mmHg) | 87.5 ± 12.3 |
| Aortic office PP (mmHg) | 27.4 ± 5.3 |
| Heart rate [bpm] | 82 ± 14.2 |
| Arterial structure and function | |
| cIMT (mm) | 0.47 ± 0.06 |
| cIMT Z-score | 1.77 ± 1.21 |
| AP (mmHg) | 1.5 (−1.3–5.3) |
| AP/PP (AIx) (%) | 6.5 ± 16.2 |
| P2/P1 (AIx) (%) | 108.3 ± 25.4 |
| AIx75HR (%) | 12.4 ± 18.9 |
| SEVR (%) | 151.3 (139.3–173) |
| PWV (m/s) | 4.56 ± 0.86 |
| PWV Z-score | −0.37 ± 1.27 |
| Heart structure and function | |
| IVSDd (mm) | 6.0 (5–7) |
| LVDd (mm) | 44.3 ± 7.0 (39–50) |
| LVPWd (mm) | 6.0 (4.6–6.5) |
| LAD (mm) | 28.2 ± 4.3 |
| RWT | 0.24 (0.22–0.28) |
| LVM (g) | 79.8 (53.4–114.5) |
| LVMI (g/m2.7) | 28.7 (26.4–33.3) |
| SF (%) | 40.1 ± 5.8 |
| EF (%) | 70.5 ± 6.69 |
| E (cm/s) | 89.38 ± 13.43 |
| A (cm/s) | 59.97 ± 11.4 |
| E/A | 1.55 ± 0.37 |
| Edt (ms) | 165 (148–192) |
| E’ (cm/s) | 13.11 ± 2.67 |
| A’ (cm/s) | 6.20 (5.5–6.5) |
| E/E’ | 6.94 (5.83–7.49) |
| IVRT (ms) | 68.4 ± 22.2 |
| IVCT (ms) | 77.2 ± 19.58 |
| C’ (m/s) | 6.0 ± 1.2 |
| Analyzed Parameter | R | p |
|---|---|---|
| Alfacalcidol dose (µg/24 h) | −0.365 | 0.043 |
| Creatinine (mg/dL) | 0.367 | 0.023 |
| GFR (mL/min/1.73 m2) | −0.423 | 0.008 |
| Urea (mg/dL) | 0.407 | 0.008 |
| Inorganic phosphate (mg/dL) | 0.443 | 0.005 |
| Intact parathormone (pg/mL) | 0.435 | 0.006 |
| Triglyceride (mg/dL) | 0.492 | 0.002 |
| AP/PP (AIx) (%) | 0.451 | 0.018 |
| P2/P1 (AIx) (%) | 0.460 | 0.016 |
| cIMT Z-score | 0.504 | 0.020 |
| E/E’ | 0.400 | 0.032 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skrzypczyk, P.; Okarska-Napierała, M.; Pietrzak, R.; Pawlik, K.; Waścińska, K.; Werner, B.; Pańczyk-Tomaszewska, M. NT-proBNP as a Potential Marker of Cardiovascular Damage in Children with Chronic Kidney Disease. J. Clin. Med. 2021, 10, 4344. https://doi.org/10.3390/jcm10194344
Skrzypczyk P, Okarska-Napierała M, Pietrzak R, Pawlik K, Waścińska K, Werner B, Pańczyk-Tomaszewska M. NT-proBNP as a Potential Marker of Cardiovascular Damage in Children with Chronic Kidney Disease. Journal of Clinical Medicine. 2021; 10(19):4344. https://doi.org/10.3390/jcm10194344
Chicago/Turabian StyleSkrzypczyk, Piotr, Magdalena Okarska-Napierała, Radosław Pietrzak, Katarzyna Pawlik, Katarzyna Waścińska, Bożena Werner, and Małgorzata Pańczyk-Tomaszewska. 2021. "NT-proBNP as a Potential Marker of Cardiovascular Damage in Children with Chronic Kidney Disease" Journal of Clinical Medicine 10, no. 19: 4344. https://doi.org/10.3390/jcm10194344






