The Two-Step Treatment for Giant Hepatic Hemangiomas
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Design
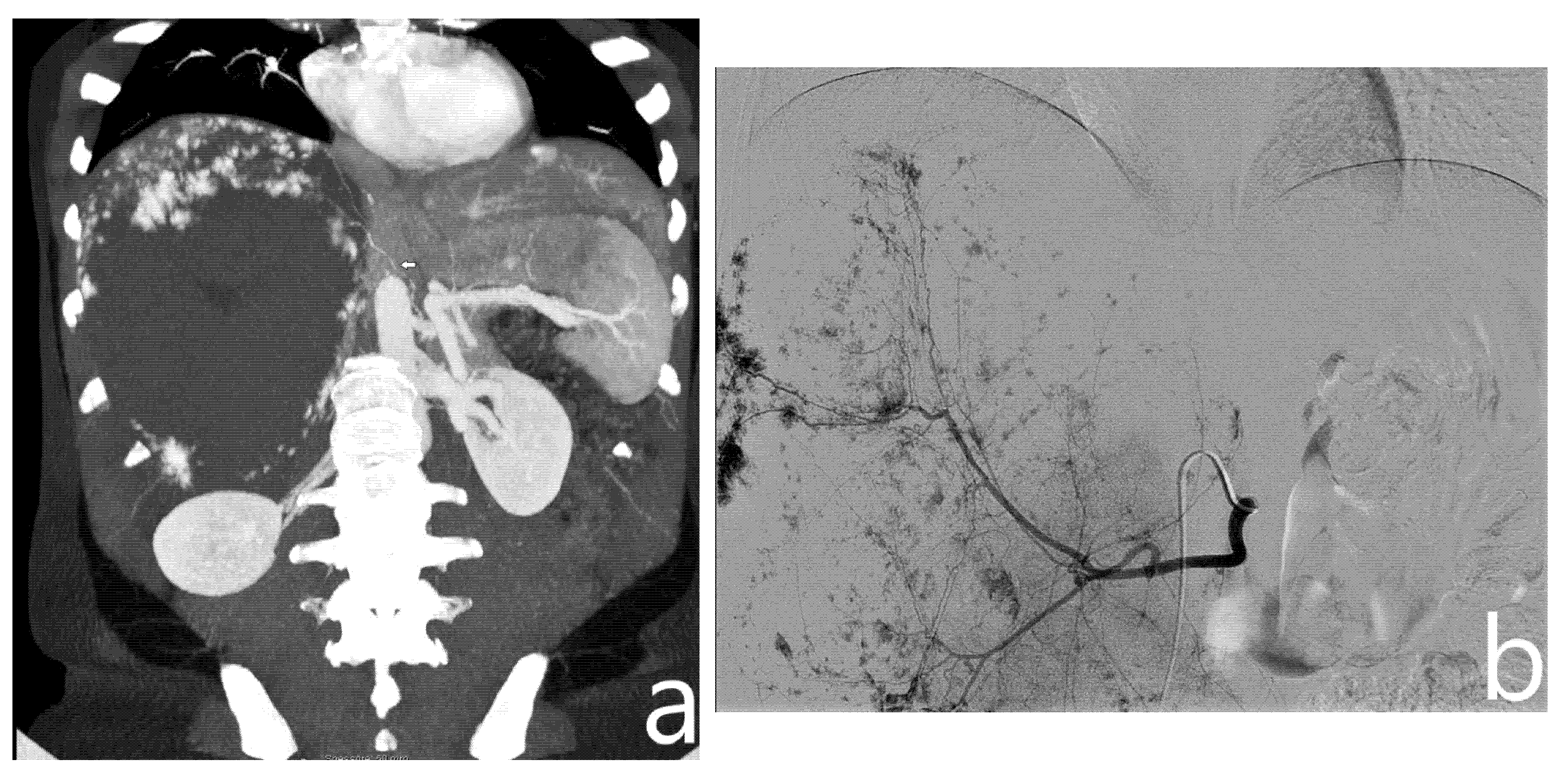
2.2. Transcatheter Arterial Embolization
2.3. Surgical Technique
2.4. Perioperative Management
2.5. Outcome Evaluation
2.6. Statistical Analysis
3. Results
3.1. Baseline
3.2. Embolization Outcome
3.3. Surgical Outcomes
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bo, Y.C.; Nguyen, M.H. The diagnosis and management of benign hepatic tumors. J. Clin. Gastroenterol. 2005, 39, 401–412. [Google Scholar] [CrossRef]
- Toro, A.; Mahfouz, A.E.; Ardiri, A.; Malaguarnera, M.; Malaguarnera, G.; Loria, F.; Bertino, G.; Carlo, I. Di What is changing in indications and treatment of hepatic hemangiomas. A review. Ann. Hepatol. 2014, 13, 327–339. [Google Scholar] [CrossRef]
- Xie, Q.S.; Chen, Z.X.; Zhao, Y.J.; Gu, H.; Geng, X.P.; Liu, F.B. Outcomes of surgery for giant hepatic hemangioma. BMC Surg. 2021, 21, 186. [Google Scholar] [CrossRef] [PubMed]
- Donati, M.; Stavrou, G.A.; Donati, A.; Oldhafer, K.J. The risk of spontaneous rupture of liver hemangiomas: A critical review of the literature. J. Hepatobiliary Pancreat. Sci. 2011, 18, 797–805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wakabayashi, G.; Cherqui, D.; Geller, D.A.; Buell, J.F.; Kaneko, H.; Han, H.S.; Asbun, H.; O’Rourke, N.; Tanabe, M.; Koffron, A.J.; et al. Recommendations for laparoscopic liver resection: A report from the second international consensus conference held in morioka. Ann. Surg. 2015, 261, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.S.; Kwon, C.H.D. Feasibility of laparoscopic liver resection for giant hemangioma of greater than 6 cm in diameter. Korean J. Hepato-Biliary-Pancreat. Surg. 2014, 18, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanthaler, M.; Freund, M.; Nehoda, H. Laparoscopic resection of a giant liver hemangioma. J. Laparoendosc. Adv. Surg. Tech. Part A 2005, 15, 624–626. [Google Scholar] [CrossRef]
- Okumura, Y.; Noda, T.; Eguchi, H.; Hanaki, T.; Iwagami, Y.; Akita, H.; Asaoka, T.; Gotoh, K.; Kobayashi, S.; Umeshita, K.; et al. Pure laparoscopic liver resection for giant liver hemangioma with extrahepatic growth based on preoperative 3-dimensional simulation: A case report. Surg. Case Rep. 2019, 5, 51. [Google Scholar] [CrossRef] [Green Version]
- Veerankutty, F.H.; Rather, S.A.; Yeldho, V.; Zacharia, B.M.; TU, S.A.; Venugopal, B. Totally Laparoscopic Resection of an Extremely Giant Hepatic Hemangioma. Surg. J. 2019, 5, e110–e112. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Gao, J.; Yang, M.; Ke, S.; Ding, X.; Kong, J.; Xu, L.; Sun, W. Intratumoral coagulation by radiofrequency ablation facilitated the laparoscopic resection of giant hepatic hemangioma: A surgical technique report of two cases. Oncotarget 2017, 8, 52006–52011. [Google Scholar] [CrossRef] [Green Version]
- Jinhuan, Y.; Gang, D.; Binyao, S.; Huan, M.; Bin, J. Is laparoscopic hepatectomy suitable for giant hepatic hemangioma larger than 10 cm in diameter? Surg. Endosc. 2020, 34, 1224–1230. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Chen, K.; Zhang, X.; Li, C.; Song, D.; Liu, R. Robotic, laparoscopic or open hemihepatectomy for giant liver haemangiomas over 10 cm in diameter. BMC Surg. 2020, 20, 93. [Google Scholar] [CrossRef] [PubMed]
- Hoekstra, L.T.; Bieze, M.; Erdogan, D.; Roelofs, J.J.T.H.; Beuers, U.H.W.; Gulik, T.M.V. Management of giant liver hemangiomas: An update. Expert Rev. Gastroenterol. Hepatol. 2013, 7, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Bajenaru, N.; Balaban, V.; Săvulescu, F.; Campeanu, I.; Patrascu, T. Hepatic hemangioma -review. J. Med. Life 2015, 8, 4–11. [Google Scholar] [PubMed]
- Yamamoto, T.; Kawarada, Y.; Yano, T.; Noguchi, T.; Mizumoto, R. Spontaneous Rupture of Hemangioma of the Liver: Treatment with Transcatheter Hepatic Arterial Embolization. Am. J. Gastroenterol. 1991, 86, 1645–1649. [Google Scholar] [CrossRef]
- Topaloğlu, S.; Oğuz, Ş.; Kalaycı, O.; Öztürk, M.H.; Çalık, A.; Dinç, H.; Çobanoğlu, Ü. Preoperative arterial embolization of large liver hemangiomas. Diagn. Interv. Radiol. 2015, 21, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Filippiadis, D.K.; Binkert, C.; Pellerin, O.; Hoffmann, R.T.; Krajina, A.; Pereira, P.L. Cirse Quality Assurance Document and Standards for Classification of Complications: The Cirse Classification System. Cardiovasc. Intervent. Radiol. 2017, 40, 1141–1146. [Google Scholar] [CrossRef]
- Ratti, F.; Cipriani, F.; Reineke, R.; Catena, M.; Comotti, L.; Beretta, L.; Aldrighetti, L. Impact of ERAS approach and minimally-invasive techniques on outcome of patients undergoing liver surgery for hepatocellular carcinoma. Dig. Liver Dis. 2016, 48, 1243–1248. [Google Scholar] [CrossRef]
- Pang, Y.Y. The Brisbane 2000 terminology of liver anatomy and resections. Hpb 2000, 2, 333–339. [Google Scholar] [CrossRef] [Green Version]
- Clavien, P.A.; Barkun, J.; De Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; De Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The clavien-dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Huang, Z.Y.; Ke, C.S.; Wu, C.; Zhang, Z.W.; Zhang, B.X.; Chen, Y.F.; Zhang, W.G.; Zhu, P.; Chen, X.P. Surgical Treatment of Giant Liver Hemangioma Larger Than 10cm: A Single Center’s Experience with 86 Patients. Medicine 2015, 94, e1420. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, Z.; Tan, H.; Liu, L.; Xu, L.; Sun, Y.; Si, S.; Huang, J.; Zhou, W. Characteristics and operative treatment of extremely giant liver hemangioma >20 cm. Surgery 2017, 161, 1514–1524. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Chen, Z.; Prasoon, P.; Wu, H.; Zeng, Y. Surgical management for giant liver hemangiomas greater than 20 cm in size. Gut Liver 2011, 5, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.T.; Gamblin, T.C.; Geller, D.A. World review of laparoscopic liver resection-2,804 patients. Ann. Surg. 2009, 250, 831–841. [Google Scholar] [CrossRef]
- Cipriani, F.; Ratti, F.; Fiorentini, G.; Catena, M.; Paganelli, M.; Aldrighetti, L. Pure laparoscopic right hepatectomy: A risk score for conversion for the paradigm of difficult laparoscopic liver resections. A single centre case series. Int. J. Surg. 2020, 82, 108–115. [Google Scholar] [CrossRef]
- Ardito, F.; Aldrighetti, L.; Guglielmi, A.; Jovine, E.; Cillo, U.; Ferrero, A.; De Carlis, L.; Belli, G.; Dalla Valle, R.; Slim, A.; et al. Surgical Management of Hepatic Benign Disease: Have the Number of Liver Resections Increased in the Era of Minimally Invasive Approach? Analysis from the I Go MILS (Italian Group of Minimally Invasive Liver Surgery) Registry. J. Gastrointest. Surg. 2020, 24, 2233–2243. [Google Scholar] [CrossRef]
- Furumaya, A.; van Rosmalen, B.V.; Takkenberg, R.B.; van Delden, O.M.; Dejong, C.H.C.; Verheij, J.; van Gulik, T.M. Transarterial (Chemo-)Embolization and Lipiodolization for Hepatic Haemangioma. Cardiovasc. Intervent. Radiol. 2019, 42, 800–811. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.Q.; Huang, Z.Q.; Duan, W.D.; Zhou, N.X.; Feng, Y.Q. Severe biliary complications after hepatic artery embolization. World J. Gastroenterol. 2002, 8, 119–123. [Google Scholar] [CrossRef]
- Seo, H., II; Jo, H.J.; Sim, M.S.; Kim, S. Right trisegmentectomy with thoracoabdominal approach after transarterial embolization for giant hepatic hemangioma. World J. Gastroenterol. 2009, 15, 3437–3439. [Google Scholar] [CrossRef]
- Venturini, M.; Lanza, C.; Marra, P.; Colarieti, A.; Panzeri, M.; Augello, L.; Gusmini, S.; Salvioni, M.; De Cobelli, F.; Del Maschio, A. Transcatheter embolization with Squid, combined with other embolic agents or alone, in different abdominal diseases: A single-center experience in 30 patients. CVIR Endovasc. 2019, 2, 8. [Google Scholar] [CrossRef] [Green Version]
- Venturini, M.; Marra, P.; Augello, L.; Colarieti, A.; Guazzarotti, G.; Palumbo, D.; Lanza, C.; Melissano, G.; Chiesa, R.; De Cobelli, F. Elective Embolization of Splenic Artery Aneurysms with an Ethylene Vinyl Alcohol Copolymer Agent (Squid) and Detachable Coils. J. Vasc. Interv. Radiol. 2020, 31, 1110–1117. [Google Scholar] [CrossRef]
- Venturini, M.; Della Corte, A.; Lanza, C.; Fontana, F.; Chiesa, R.; De Cobelli, F. Embolization of 2 Coexisting Intraparenchymal Renal Artery Aneurysms with an Ethylene Vinyl Alcohol Copolymer Agent (Squid) and Coils. Cardiovasc. Intervent. Radiol. 2020, 43, 942–944. [Google Scholar] [CrossRef]
- Buell, J.F.; Cherqui, D.; Geller, D.A.; O’Rourke, N.; Iannitti, D.; Dagher, I.; Koffron, A.J.; Thomas, M.; Gayet, B.; Han, H.S.; et al. Position on laparoscopic liver surgery. Ann. Surg. 2009, 250, 825–830. [Google Scholar] [CrossRef]



| Population Baseline Characteristics (n = 10) | Group A (n = 10) | Group B (n = 10) | p |
|---|---|---|---|
| Age (years) * | 45 ± 10 | 43.7 ± 8.5 | 0.67 |
| BMI * | 23.2 ± 4.5 | 24.1 ± 5.7 | 0.82 |
| Female/Male | 9 (90%)/1 (10%) | 10 (100%)/0 (0%) | 0.56 |
| Maximum GH diameter (cm) * | 13.8 ± 3 | 12.2 ± 1.5 | 0.77 |
| GH located in the Right Hepatic Lobe | 6 (60%) | 4 (40%) | 0.48 |
| GH located in the Left Hepatic Lobe | 3 (30%) | 4 (40%) | 0.54 |
| Bilobar | 1 (10%) | 2 (20%) | 0.58 |
| Proximity to large vessels (<1 cm) | 11 (100%) | 10 (100%) | ns |
| ≥3 Segments involved | 4 (36%) | 4 (40%) | 0.62 |
| Liver Steatosis | 2 (20%) | 1 10%) | 0.58 |
| Previous Abdominal Surgery | 2 (20%) | 1 (10%) | 0.58 |
| Embolization Outcome | |
|---|---|
| Vascularization from RHA only | 5 (45.4%) |
| Vascularization from LHA only | 3 (27.3%) |
| Vascularization from RHA + RPA | 2 (18.2%) |
| Vascularization from LHA + RPA | 1 (9.1%) |
| Embolization performed with PVA (355–500 μm) | 7 (70%) |
| Embolization performed with PVA (355–500 μm) + Squid-18 | 3 (30%) |
| Complications | 0 (0%) |
| Time from embolization to surgery (days) * | 2.2 ± 0.7 |
| Surgical Outcome | |||
|---|---|---|---|
| Group A (n = 10) | Group B (n = 10) | p | |
| Pringle Maneuver, n (%) | 10 (100%) | 10 (100%) | ns |
| Right hepatectomy § | 2 (20%) | 3 (30%) | 0.61 |
| Left hepatectomy § | 1 (10%) | 3 (30%) | 0.29 |
| Left lateral sectionectomy § | 1 (10%) | 1 (10%) | ns |
| Right posterior sectionectomy § | 3 (30%) | 1 (10%) | 0.29 |
| Single segment anatomic resection § | 2 (20%) | 2 (20%) | ns |
| Associated procedures, n (%) | 0 (0%) | 2 (20%) | 0.24 |
| Intraoperative adverse events, n (%) | 2 (20%) | 2 (20%) | ns |
| Intraoperative blood transfusions, n (%) | 2 (20%) | 3 (30%) | 0.61 |
| Postoperative blood transfusions, n (%) | 2 (20%) | 4 (40%) | 0.31 |
| Conversion, n (%) | 0 (0%) | 2 (20%) | 0.24 |
| Morbidity, n (%) | 2 (20%) | 5 (50%) | 0.18 |
| Complications n (%) | Grade I 0 (0%) | Grade I 0 (0%) | ns |
| Grade II (20%) | Grade II 4 (40%) | 0.31 | |
| Grade ≥ III (0%) | Grade ≥ III 1 (10%) | 0.32 | |
| Operative time (min) * | 145 ± 60 | 420 ± 60 | 0.027 |
| Intraoperative Blood Loss (mL) * | 250 ± 200 | 400 ± 300 | 0.039 |
| Length of Stay (days) * | 5 ± 1 | 8 ± 2 | 0.046 |
| Mortality, n (%) | 0 (0%) | 0 (0%) | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Della Corte, A.; Marino, R.; Ratti, F.; Palumbo, D.; Guazzarotti, G.; Gusmini, S.; Augello, L.; Cipriani, F.; Fiorentini, G.; Venturini, M.; et al. The Two-Step Treatment for Giant Hepatic Hemangiomas. J. Clin. Med. 2021, 10, 4381. https://doi.org/10.3390/jcm10194381
Della Corte A, Marino R, Ratti F, Palumbo D, Guazzarotti G, Gusmini S, Augello L, Cipriani F, Fiorentini G, Venturini M, et al. The Two-Step Treatment for Giant Hepatic Hemangiomas. Journal of Clinical Medicine. 2021; 10(19):4381. https://doi.org/10.3390/jcm10194381
Chicago/Turabian StyleDella Corte, Angelo, Rebecca Marino, Francesca Ratti, Diego Palumbo, Giorgia Guazzarotti, Simone Gusmini, Luigi Augello, Federica Cipriani, Guido Fiorentini, Massimo Venturini, and et al. 2021. "The Two-Step Treatment for Giant Hepatic Hemangiomas" Journal of Clinical Medicine 10, no. 19: 4381. https://doi.org/10.3390/jcm10194381







