Identifying the Location of an Accessory Pathway in Pre-Excitation Syndromes Using an Artificial Intelligence-Based Algorithm
Abstract
:1. Introduction
2. Materials and Methods
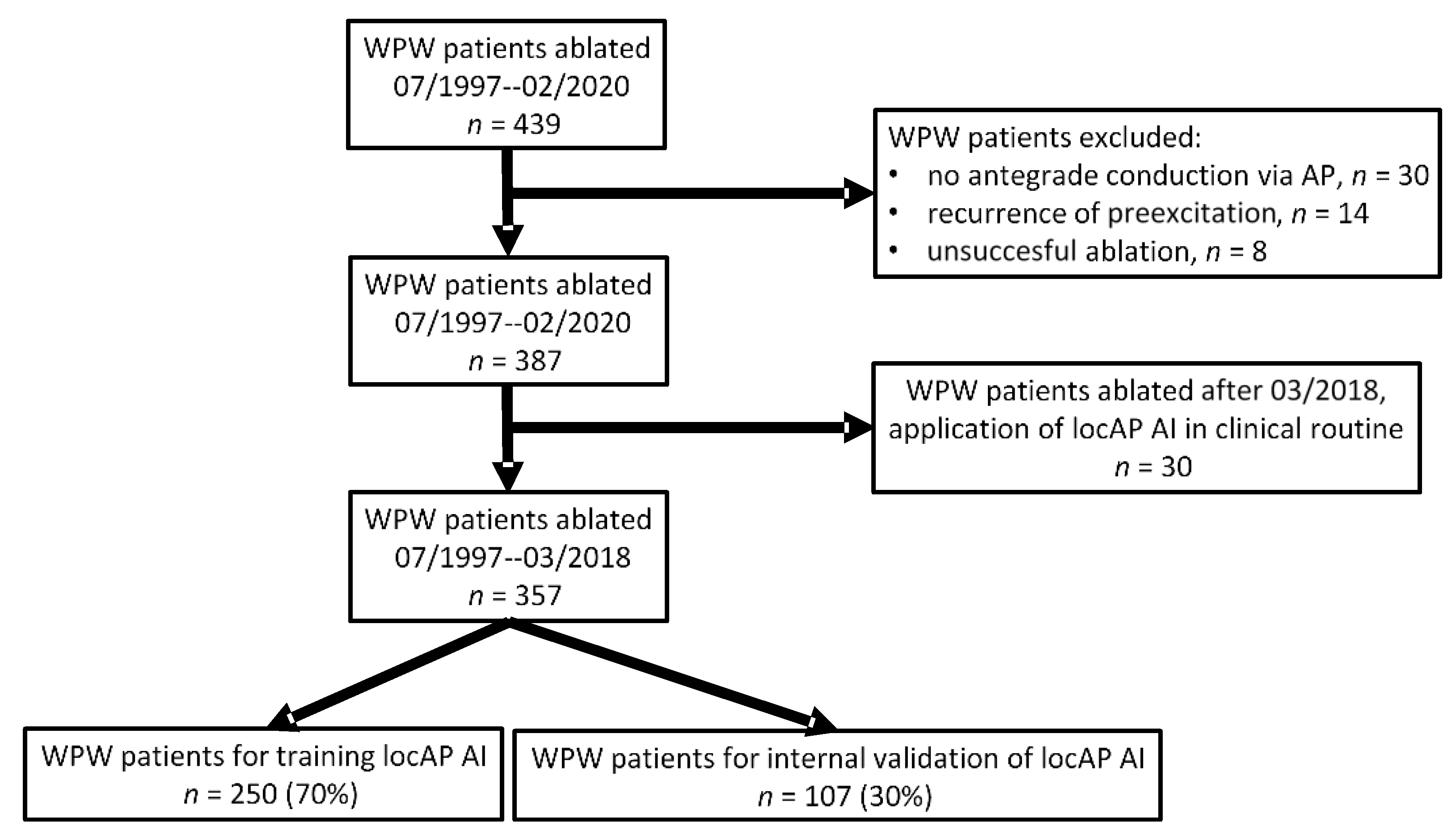
2.1. Study Design and Participants
2.2. Statistical Analysis
2.3. LocAP AI
2.4. Validation
3. Results
4. Discussion
4.1. Artificial Intelligence—Neural Network
4.2. LocAP AI
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brugada, J.; Katritsis, D.G.; Arbelo, E.; Arribas, F.; Bax, J.J.; Blomstrom-Lundqvist, C.; Calkins, H.; Corrado, D.; Deftereos, S.G.; Diller, G.P.; et al. 2019 ESC Guidelines for themanagement of patients with supraventricular tachycardia. Eur. Heart J. 2020, 41, 655–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackman, W.M.; Wang, X.; Friday, K.J.; Roman, C.A.; Moulton, K.P.; Beckman, K.J.; McClelland, J.H.; Twidale, N.; Hazlitt, H.A.; Prior, M.I.; et al. Catheter Ablation of Accessory Atrioventricular Pathways (Wolff–Parkinson–White Syndrome) by Radiofrequency Current. N. Engl. J. Med. 1991, 324, 1605–1611. [Google Scholar] [CrossRef] [PubMed]
- Pappone, C.; Vicedomini, G.; Manguso, F.; Saviano, M.; Baldi, M.; Pappone, A.; Ciaccio, C.; Giannell, L.; Ionescu, B.; Petretta, A.; et al. Wolff-parkinson-white syndrome in the era of catheter ablation insights from a registry study of 2169 patients. Circulation 2014, 130, 811–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arruda, M.S.; McClelland, J.H.; Wang, X.; Beckman, K.J.; Widman, L.E.; Gonzalez, M.D.; Nakagawa, H.; Lazzara, R.; Jackman, W.M. Development and validation of an ECG algorithm for identifying accessory pathway ablation site in Wolff-Parkinson-White syndrome. J. Cardiovasc. Electrophysiol. 1998, 9, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Milstein, S.; Sharma, A.D.; Guiraudon, G.M.; Klein, G.J. An algorithm for the electrocardiographic localization of accessory pathways in the Wolff-Parkinson-White syndrome. Pacing Clin. Electrophysiol. 1987, 10, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, A.P.; Gonzales, R.P.; Lesh, M.D.; Modin, G.W.; Lee, R.J.; Scheinman, M.M. New algorithm for the localization of accessory atrioventricular connections using a baseline electrocardiogram. J. Am. Coll. Cardiol. 1994, 23, 107–116. [Google Scholar] [CrossRef] [Green Version]
- Cosío, F.G.; Anderson, R.H.; Kuck, K.H.; Becker, A.; Borggrefe, M.; Campbell, R.W.; Gaita, F.; Guiraudon, G.M.; Haïssaguerre, M.; Rufilanchas, J.J.; et al. Living anatomy of the atrioventricular junctions. A guide to electrophysiologic mapping. A Consensus Statement from the Cardiac Nomenclature Study Group, Working Group of Arrhythmias, European Society of Cardiology, and the Task Force on Cardiac Nomenclature from NASPE. Circulation 1999, 100, e31–e37. [Google Scholar] [PubMed]
- Rantner, L.J.; Stühlinger, M.C.; Nowak, C.N.; Spuller, K.; Etsadashvili, K.; Stühlinger, X.; Berger, T.; Dichtl, W.; Gothe, R.M.; Fischer, G.; et al. Localizing the Accessory Pathway in Ventricular Preexcitation Patients Using a Score Based Algorithm. Methods Inf. Med. 2012, 51, 3–12. [Google Scholar] [PubMed]
- Sacher, F.; Wright, M.; Tedrow, U.B.; O’neill, M.D.; Jais, P.; Hocini, M.; Macdonald, R.; Davies, D.W.; Kanagaratnam, P.; Derval, N.; et al. Wolff-Parkinson-White ablation after a prior failure: A 7-year multicentre experience. Europace 2010, 12, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Runger, G.; Tuv, E. Bias of importance measures for multi-valued attributes and solutions. In Proceedings of the Lecture Notes in Computer Science; Springer: Berlin, Heidelberg, 2011; Volume 6792 LNCS, pp. 293–300. [Google Scholar]
- Maden, O.; Balci, K.G.; Selcuk, M.T.; Balci, M.M.; Açar, B.; Unal, S.; Kara, M.; Selcuk, H. Comparison of the accuracy of three algorithms in predicting accessory pathways among adult Wolff-Parkinson-White syndrome patients. J. Interv. Card. Electrophysiol. 2015, 44, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Jordan, M.I.; Mitchell, T.M. Machine learning: Trends, perspectives, and prospects. Science 2015, 349, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Fox, D.J.; Klein, G.J.; Skanes, A.C.; Gula, L.J.; Yee, R.; Krahn, A.D. How to identify the location of an accessory pathway by the 12-lead ECG. Heart Rhythm. 2008, 5, 1763–1766. [Google Scholar] [CrossRef] [PubMed]

| Variables | Study Population (n = 357) |
|---|---|
| Male, n (%) | 228 (63.9) |
| Age, y, mean ± SD | 34.3 ± 15 |
| QRS duration, ms, mean ± SD | 121 ± 21 |
| PR interval, ms, mean ± SD | 114 ± 21 |
| Patients < 18 y of age, n (%) | 55 (15.4) |
| QRS duration ≥ 120 ms and PR interval ≤ 120 ms, n (%) | 140 (39.2) |
| Abbreviation | Attitudinally Correct | Attitudinally Incorrect |
|---|---|---|
| RS | Right superior | Right anterior |
| RSA | Right superoanterior | Right anterolateral |
| RA | Right anterior | Right lateral |
| RIA | Right inferoanterior | Right posterolateral |
| RI | Right inferior | Right posterior |
| LS | Left superior | Left anterior |
| LSP | Left superoposterior | Left anterolateral |
| LP | Left posterior | Left lateral |
| LIP | Left inferoposterior | Left posterolateral |
| LI | Left inferior | Left posterior |
| SPS | Superoparaseptal | Anteroseptal |
| IPS | Inferoparaseptal | Posteroseptal |
| S | Septal | Midseptal |
| PH | Parahisian |
| Location | Frequency | Percentage (%) |
|---|---|---|
| IPS | 81 | 22.70 |
| LSP | 71 | 19.89 |
| LP | 70 | 19.61 |
| S | 32 | 8.96 |
| LIP | 26 | 7.28 |
| LI | 23 | 6.44 |
| SPS | 15 | 4.20 |
| RI | 8 | 2.24 |
| RA | 8 | 2.24 |
| RS | 7 | 1.96 |
| RSA | 5 | 1.40 |
| PH | 4 | 1.12 |
| LS | 4 | 1.12 |
| RIA | 3 | 0.84 |
| Total | 357 | 100.00 |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | |
| AP | IPS | LSP | LP | S | LIP | LI | SPS | RI | RA | RS | RSA | PH | LS | RIA |
| Total | 81 | 71 | 70 | 32 | 26 | 23 | 15 | 8 | 8 | 7 | 5 | 4 | 4 | 3 |
| Sensitivity | 0.9706 | 1.0000 | 0.9714 | 1.0000 | 0.69231 | 1.00000 | 0.85714 | 0.00000 | 1.00000 | 1.00000 | 0.00000 | 0.00000 | 0.00000 | 0.0000 |
| Specificity | 1.0000 | 0.9933 | 0.9929 | 1.0000 | 0.98765 | 0.99394 | 1.00000 | 1.00000 | 0.88304 | 1.00000 | 1.00000 | 1.00000 | 1.00000 | 1.0000 |
| Prevalence | 0.1943 | 0.1429 | 0.2000 | 0.1200 | 0.07429 | 0.05714 | 0.04000 | 0.02857 | 0.02286 | 0.01714 | 0.01143 | 0.01714 | 0.01143 | 0.0000 |
| Balanced Accuracy | 0.9853 | 0.9967 | 0.9821 | 1.0000 | 0.83998 | 0.99697 | 0.92857 | 0.50000 | 0.94152 | 1.00000 | 0.50000 | 0.50000 | 0.50000 | 0.5000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senoner, T.; Pfeifer, B.; Barbieri, F.; Adukauskaite, A.; Dichtl, W.; Bauer, A.; Hintringer, F. Identifying the Location of an Accessory Pathway in Pre-Excitation Syndromes Using an Artificial Intelligence-Based Algorithm. J. Clin. Med. 2021, 10, 4394. https://doi.org/10.3390/jcm10194394
Senoner T, Pfeifer B, Barbieri F, Adukauskaite A, Dichtl W, Bauer A, Hintringer F. Identifying the Location of an Accessory Pathway in Pre-Excitation Syndromes Using an Artificial Intelligence-Based Algorithm. Journal of Clinical Medicine. 2021; 10(19):4394. https://doi.org/10.3390/jcm10194394
Chicago/Turabian StyleSenoner, Thomas, Bernhard Pfeifer, Fabian Barbieri, Agne Adukauskaite, Wolfgang Dichtl, Axel Bauer, and Florian Hintringer. 2021. "Identifying the Location of an Accessory Pathway in Pre-Excitation Syndromes Using an Artificial Intelligence-Based Algorithm" Journal of Clinical Medicine 10, no. 19: 4394. https://doi.org/10.3390/jcm10194394






