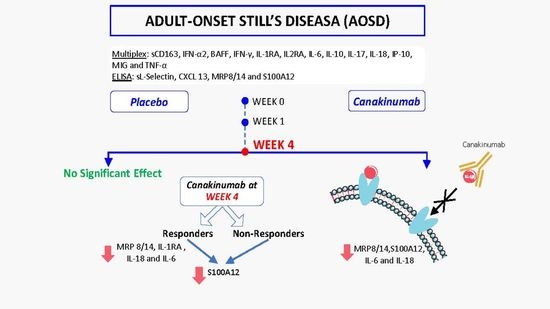
Distinct Effects of Interleukin-1β Inhibition upon Cytokine Profile in Patients with Adult-Onset Still’s Disease and Active Articular Manifestation Responding to Canakinumab
Abstract
1. Introduction
2. Patients
3. Methods
4. Statistics
5. Results
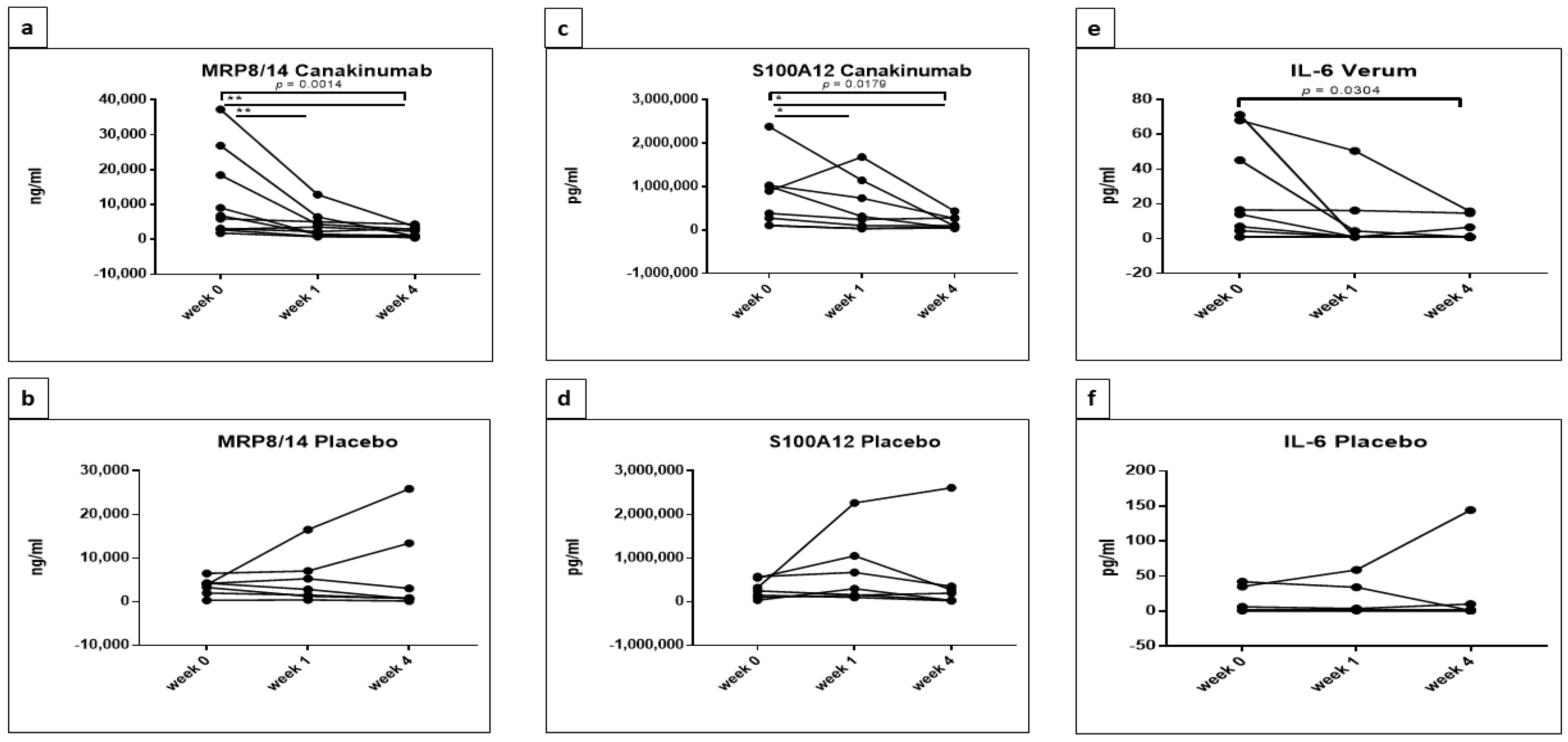
5.1. Statistically Significant Differences in the Concentrations of MRP8/14 (Calprotectin), S100A12, IL-2RA, IL-6, IL-18 and sL-Selectin between Placebo and Canakinumab Groups
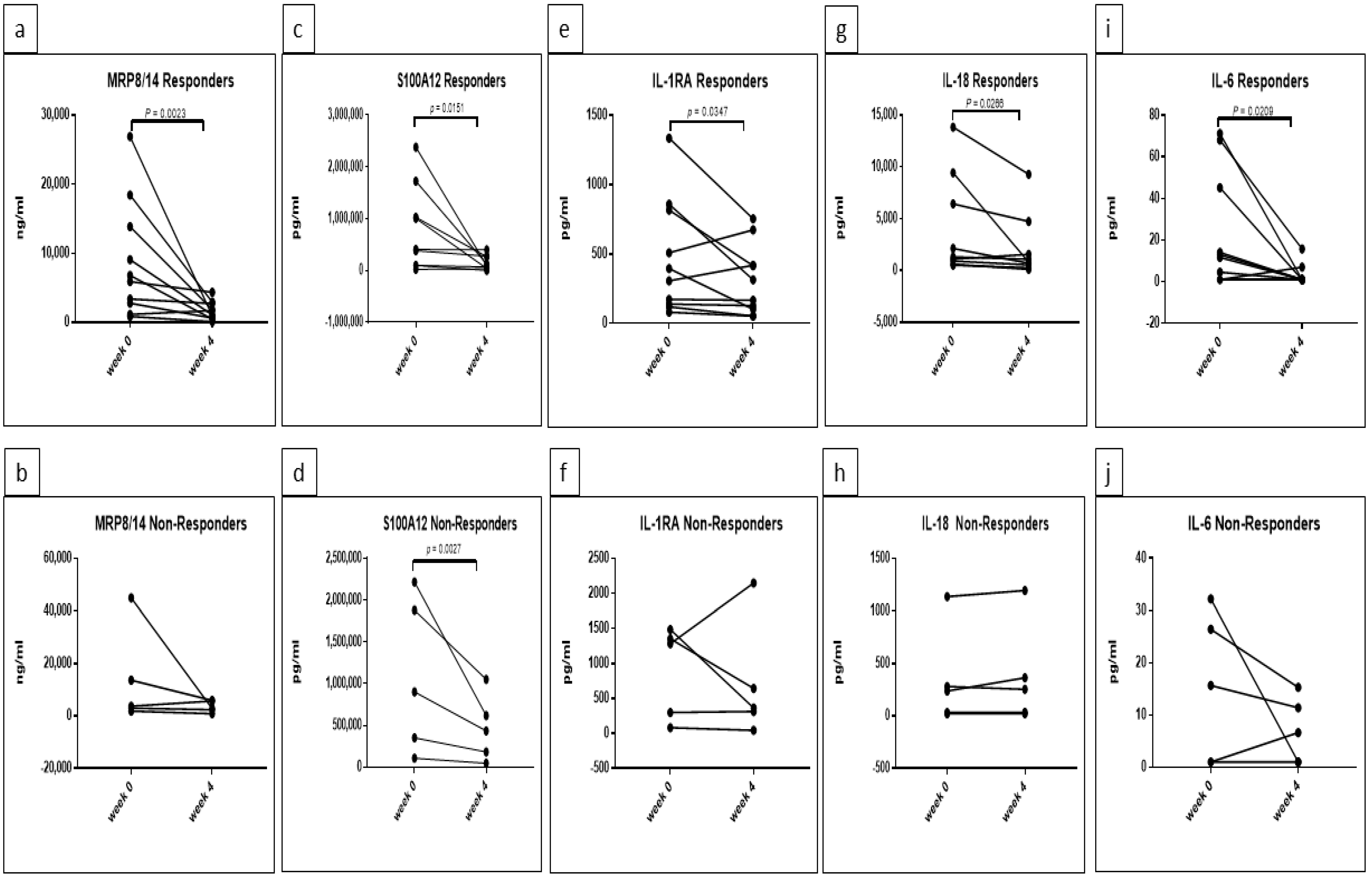
5.2. Response Rate Has an Effect on the Concentrations of MRP8/14, S100A12, IL-1RA, IL-18 and IL-6 in Canakinumab Group
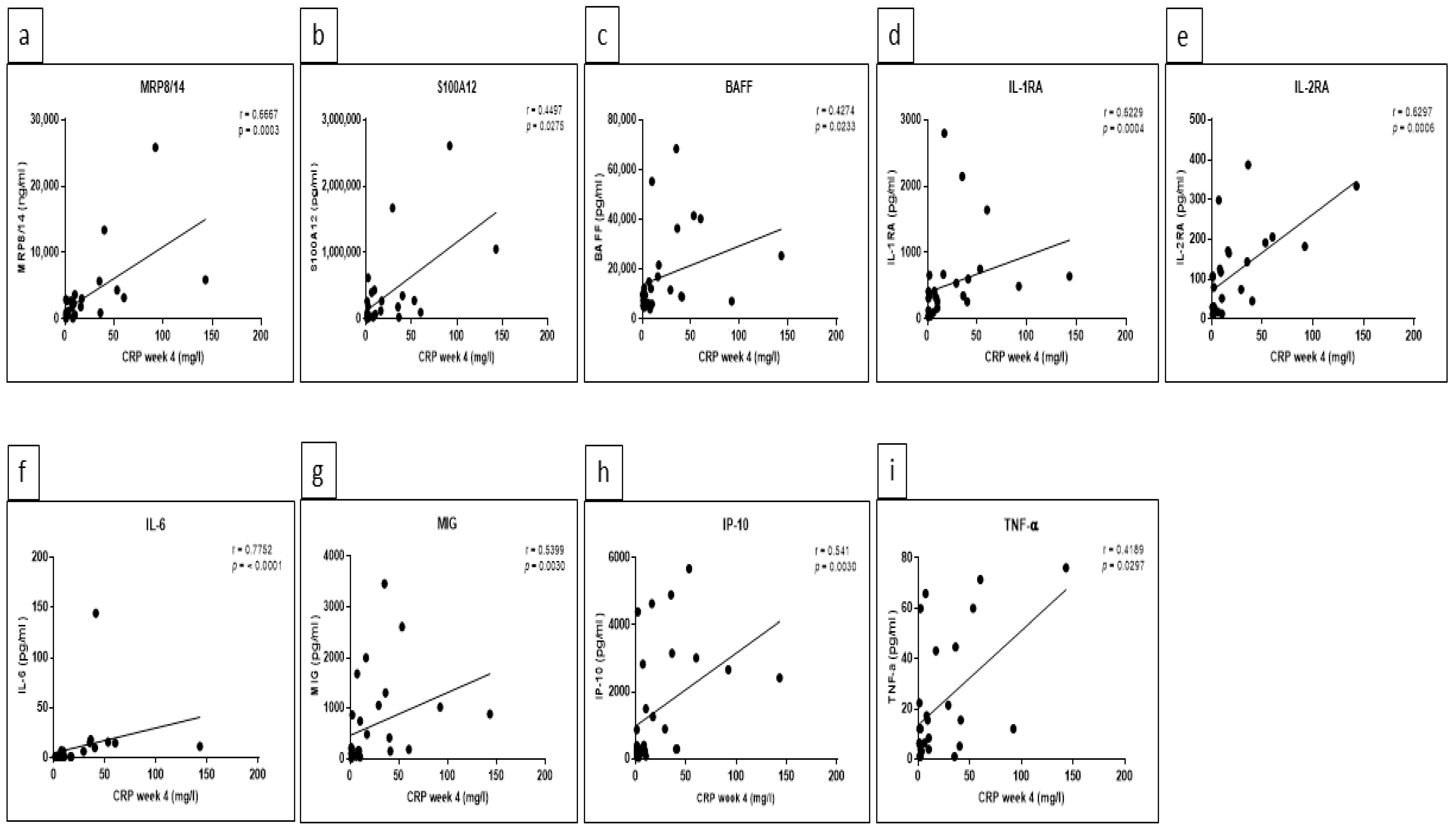
5.3. Significant Correlations between Certain Cytokine/Chemokine Levels and CRP as well as Ferritin Were Detected at Week 4
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mahroum, N.; Mahagna, H.; Amital, H. Diagnosis and classification of adult Still’s disease. J. Autoimmun. 2014, 48–49, 34–37. [Google Scholar] [CrossRef]
- Bywaters, E.G. Still’s disease in the adult. Ann. Rheum. Dis. 1971, 30, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Still, G.F. On a form of chronic joint disease in children. Med.-Chir. Trans. 1897, 80, 47–60. [Google Scholar] [CrossRef]
- Terkeltaub, R.; Esdaile, J.M.; Décary, F.; Harth, M.; Lister, J.; Lapointe, N. HLA—Bw35 and Prognosis in Adult Still’s Disease. Arthritis Rheum. 1981, 24, 1469–1472. [Google Scholar] [CrossRef] [PubMed]
- Wouters, J.M.G.W.; Reekers, P.; van de Putte, L.B.A. Adult-onset still’s disease. Disease course and HLA associations. Arthritis Rheum. 1986, 29, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Pouchot, J.; Sampalis, J.S.; Beaudet, F.; Carette, S.; Décary, F.; Salusinsky-sternbach, M.; Hill, R.O.; Gutkowski, A.; Harth, M.; Myhal, D.; et al. Adult Still’s Disease: Manifestations, Disease Course, and Outcome in 62 Patients. Medicine 1991, 70, 118–136. [Google Scholar] [CrossRef]
- Perez, C.; Artola, V. Adult Still’s disease associated with Mycoplasma pneumoniae infection. Clin. Infect. Dis. 2001, 32, E105–E106. [Google Scholar] [CrossRef]
- Escudero, F.J.; Len, O.; Falcó, V.; de Sevilla, T.F.; Sellas, A. Rubella infection in adult onset Still’s disease. Ann. Rheum. Dis. 2000, 59, 490. [Google Scholar] [CrossRef]
- Mavragani, C.P.; Spyridakis, E.G.; Koutsilieris, M. Adult-Onset Still’s Disease: From Pathophysiology to Targeted Therapies. Int. J. Inflamm. 2012, 2012, 879020. [Google Scholar] [CrossRef]
- Wang, M.Y.; Jia, J.C.; Yang, C.D.; Hu, Q.Y. Pathogenesis, disease course, and prognosis of adult-onset Still’s disease: An update and review. Chin. Med. J. 2019, 132, 2856–2864. [Google Scholar] [CrossRef]
- Gerfaud-Valentin, M.; Jamilloux, Y.; Iwaz, J.; Seve, P. Adult-onset Still’s disease. Autoimmun. Rev. 2014, 13, 708–722. [Google Scholar] [CrossRef] [PubMed]
- Matsui, K.; Tsuchida, T.; Hiroishi, K.; Tominaga, K.; Hayashi, N.; Hada, T.; Higashino, K. High serum level of macrophage-colony stimulating factor (M-CSF) in adult-onset Still’s disease. Rheumatology 1999, 38, 477–478. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hoshino, T.; Ohta, A.; Yang, D.; Kawamoto, M.; Kikuchi, M.; Inoue, Y.; Kamizono, S.; Ota, T.; Itoh, K.; Oizumi, K. Elevated serum interleukin 6, interferon-gamma, and tumor necrosis factor-alpha levels in patients with adult Still’s disease. J. Rheumatol. 1998, 25, 396–398. [Google Scholar] [PubMed]
- Smalley, D.M.; Ley, K. L-selectin: Mechanisms and physiological significance of ectodomain cleavage. J. Cell. Mol. Med. 2005, 9, 255–266. [Google Scholar] [CrossRef]
- Jung, J.-Y.; Suh, C.-H.; Kim, H.-A. The role of damage-associated molecular pattern for pathogenesis and biomarkers in adult-onset Still’s disease. Expert Rev. Mol. Diagn. 2019, 19, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Feist, E.; Mitrovic, S.; Fautrel, B. Mechanisms, biomarkers and targets for adult-onset Still’s disease. Nat. Rev. Rheumatol. 2018, 14, 603–618. [Google Scholar] [CrossRef]
- Church, L.D.; Cook, G.P.; McDermott, M.F. Primer: Inflammasomes and interleukin 1beta in inflammatory disorders. Nat. Clin. Pract. Rheumatol. 2008, 4, 34–42. [Google Scholar] [CrossRef]
- Hsieh, C.-W.; Chen, Y.-M.; Lin, C.-C.; Tang, K.-T.; Chen, H.-H.; Hung, W.-T.; Lai, K.-L.; Chen, D.-Y. Elevated Expression of the NLRP3 Inflammasome and Its Correlation with Disease Activity in Adult-onset Still Disease. J. Rheumatol. 2017, 44, 1142–1150. [Google Scholar] [CrossRef]
- Cush, J.J. Autoinflammatory syndromes. Dermatol. Clin. 2013, 31, 471–480. [Google Scholar] [CrossRef][Green Version]
- Chen, D.Y.; Chen, Y.M.; Lan, J.L.; Lin, C.C.; Chen, H.H.; Hsieh, C.W. Potential role of Th17 cells in the pathogenesis of adult-onset Still’s disease. Rheumatology 2010, 49, 2305–2312. [Google Scholar] [CrossRef]
- Chen, D.Y.; Lin, C.C.; Chen, Y.M.; Lan, J.L.; Hung, W.T.; Chen, H.H.; Lai, K.L.; Hsieh, C.W. Involvement of TLR7 MyD88-dependent signaling pathway in the pathogenesis of adult-onset Still’s disease. Arthritis Res. Ther. 2013, 15, R39. [Google Scholar] [CrossRef]
- Choi, J.H.; Suh, C.H.; Lee, Y.M.; Suh, Y.J.; Lee, S.K.; Kim, S.S.; Nahm, D.H.; Park, H.S. Serum cytokine profiles in patients with adult onset Still’s disease. J. Rheumatol. 2003, 30, 2422–2427. [Google Scholar]
- Fujii, T.; Nojima, T.; Yasuoka, H.; Satoh, S.; Nakamura, K.; Kuwana, M.; Suwa, A.; Hirakata, M.; Mimori, T. Cytokine and immunogenetic profiles in Japanese patients with adult Still’s disease. Association with chronic articular disease. Rheumatology 2001, 40, 1398–1404. [Google Scholar] [CrossRef]
- Chen, D.Y.; Chen, Y.M.; Chen, H.H.; Hsieh, C.W.; Lin, C.C.; Lan, J.L. The associations of circulating CD4+CD25 high regulatory T cells and TGF-β with disease activity and clinical course in patients with adult-onset Still’s disease. Connect. Tissue Res. 2010, 51, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, Z.; Chi, H.; Hu, Q.; Ye, J.; Liu, H.; Cheng, X.; Shi, H.; Zhou, Z.; Teng, J.; et al. Elevated serum levels of interleukin-10 in adult-onset Still’s disease are associated with disease activity. Clin. Rheumatol. 2019, 38, 3205–3210. [Google Scholar] [CrossRef] [PubMed]
- Castañeda, S.; Blanco, R.; González-Gay, M.A. Adult-onset Still’s disease: Advances in the treatment. Best Pract. Res. Clin. Rheumatol. 2016, 30, 222–238. [Google Scholar] [CrossRef] [PubMed]
- Castañeda, S.; Atienza-Mateo, B.; Martín-Varillas, J.L.; López-Matencio, J.M.S.; González-Gay, M.A. Anakinra for the treatment of adult-onset Still’s disease. Expert Rev. Clin. Immunol. 2018, 14, 979–992. [Google Scholar] [CrossRef]
- Junge, G.; Mason, J.; Feist, E. Adult onset Still’s disease—The evidence that anti-interleukin-1 treatment is effective and well-tolerated (a comprehensive literature review). Semin. Arthritis Rheum. 2017, 47, 295–302. [Google Scholar] [CrossRef]
- Sfriso, P.; Bindoli, S.; Doria, A.; Feist, E.; Galozzi, P. Canakinumab for the treatment of adult-onset Still’s disease. Expert Rev. Clin. Immunol. 2020, 16, 129–138. [Google Scholar] [CrossRef]
- Kedor, C.; Listing, J.; Zernicke, J.; Weiß, A.; Behrens, F.; Blank, N.; Henes, J.C.; Kekow, J.; Rubbert-Roth, A.; Schulze-Koops, H.; et al. Canakinumab for Treatment of Adult-Onset Still’s Disease to Achieve Reduction of Arthritic Manifestation (CONSIDER): Phase II, randomised, double-blind, placebo-controlled, multicentre, investigator-initiated trial. Ann. Rheum. Dis. 2020, 79, 1090–1097. [Google Scholar] [CrossRef]
- Kedor, C.; Listing, J.; Zernicke, J.; Feist, E. Response to: ‘Changing the outcome measures, changing the results? The urgent need of a specific Disease Activity Score to adult-onset Still’s disease’ by Ruscitti et al. Ann. Rheum. Dis. 2020, 2020, 218143. [Google Scholar]
- van Gestel, A.M.; Haagsma, C.J.; van Riel, P.L. Validation of rheumatoid arthritis improvement criteria that include simplified joint counts. Arthritis Rheum. 1998, 41, 1845–1850. [Google Scholar] [CrossRef]
- Fransen, J.; Creemers, M.C.W.; Van Riel, P.L.C.M. Remission in rheumatoid arthritis: Agreement of the disease activity score (DAS28) with the ARA preliminary remission criteria. Rheumatology 2004, 43, 1252–1255. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.-Y.; Park, Y.-B.; Ha, Y.-J.; Lee, K.-H.; Lee, S.-K. Serum Calprotectin as a Marker for Disease Activity and Severity in Adult-onset Still’s Disease. J. Rheumatol. 2010, 37, 1029–1034. [Google Scholar] [CrossRef]
- Bae, C.-B.; Suh, C.-H.; An, J.-M.; Jung, J.-Y.; Jeon, J.-Y.; Nam, J.-Y.; Kim, H.-A. Serum S100A12 May Be a Useful Biomarker of Disease Activity in Adult-onset Still’s Disease. J. Rheumatol. 2014, 41, 2403–2408. [Google Scholar] [CrossRef]
- Perrier, S.; Darakhshan, F.; Hajduch, E. IL-1 receptor antagonist in metabolic diseases: Dr Jekyll or Mr Hyde? FEBS Lett. 2006, 580, 6289–6294. [Google Scholar] [CrossRef] [PubMed]
- Chikanza, I.C.; Roux-Lombard, P.; Dayer, J.-M.; Panayi, G.S. Dysregulation of the in vivo production of interleukin-1 receptor antagonist in patients with rheumatoid arthritis pathogenetic implications. Arthritis Rheum. 1995, 38, 642–648. [Google Scholar] [CrossRef]
- Suzuki, H.; Takemura, H.; Kashiwagi, H. Interleukin-1 receptor antagonist in patients with active systemic lupus erythematosus: Enhanced production by monocytes and correlation with disease activity. Arthritis Rheum. 1995, 38, 1055–1059. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, A.A.; LeClercq, S.A.; Yan, A.; Homik, J.E.; Dinarello, C.A. Rapid responses to anakinra in patients with refractory adult-onset Still’s disease. Arthritis Rheum. 2005, 52, 1794–1803. [Google Scholar] [CrossRef]
- Girard, C.; Rech, J.; Brown, M.; Allali, D.; Roux-Lombard, P.; Spertini, F.; Schiffrin, E.J.; Schett, G.; Manger, B.; Bas, S.; et al. Elevated serum levels of free interleukin-18 in adult-onset Still’s disease. Rheumatology 2016, 55, 2237–2247. [Google Scholar] [CrossRef]
- Ortiz-Sanjuán, F.; Blanco, R.; Riancho-Zarrabeitia, L.; Castañeda, S.; Olivé, A.; Riveros, A.; Velloso-Feijoo, M.L.; Narváez, J.; Jiménez-Moleón, I.; Maiz-Alonso, O.; et al. Efficacy of Anakinra in Refractory Adult-Onset Still’s Disease: Multicenter Study of 41 Patients and Literature Review. Medicine 2015, 94, e1554. [Google Scholar] [CrossRef] [PubMed]
- Gabay, C.; Smith, M.F.; Eidlen, D.; Arend, W.P. Interleukin 1 receptor antagonist (IL-1Ra) is an acute-phase protein. J. Clin. Investig. 1997, 99, 2930–2940. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.-Y.; Lan, J.-L.; Lin, F.-J.; Hsieh, T.-Y. Proinflammatory cytokine profiles in sera and pathological tissues of patients with active untreated adult onset Still’s disease. J. Rheumatol. 2004, 31, 2189–2198. [Google Scholar] [PubMed]
- Hinze, T.; Kessel, C.; Hinze, C.H.; Seibert, J.; Gram, H.; Foell, D. A dysregulated interleukin-18/interferon-γ/CXCL9 axis impacts treatment response to canakinumab in systemic juvenile idiopathic arthritis. Rheumatology 2021. online ahead of print. [Google Scholar] [CrossRef]
- Font, J.; Pizcueta, P.; Ramos-Casals, M.; Cervera, R.; García-Carrasco, M.; Navarro, M.; Ingelmo, M.; Engel, P. Increased serum levels of soluble l-selectin (CD62L) in patients with active systemic lupus erythematosus (SLE). Clin. Exp. Immunol. 2000, 119, 169–174. [Google Scholar] [CrossRef]
- Haznedaroglu, E.; Karaaslan, Y.; Büyükaşik, Y.; Koşar, A.; Ozcebe, O.; Haznedaroglu, I.C.; Kirazli, E.; Dündar, S.V. Selectin adhesion molecules in Behçet’s disease. Ann. Rheum. Dis. 2000, 59, 61–63. [Google Scholar] [CrossRef] [PubMed]
- Han, J.H.; Suh, C.-H.; Jung, J.-Y.; Nam, J.-Y.; Kwon, J.E.; Yim, H.; Kim, H.-A. Association of CXCL10 and CXCL13 levels with disease activity and cutaneous manifestation in active adult-onset Still’s disease. Arthritis Res. Ther. 2015, 17, 260. [Google Scholar] [CrossRef]
- Bracaglia, C.; De Graaf, K.; Marafon, D.P.; Guilhot, F.; Ferlin, W.; Prencipe, G.; Caiello, I.; Davì, S.; Schulert, G.; Ravelli, A.; et al. Elevated circulating levels of interferon-γ and interferon-γ-induced chemokines characterise patients with macrophage activation syndrome complicating systemic juvenile idiopathic arthritis. Ann. Rheum. Dis. 2017, 76, 166–172. [Google Scholar] [CrossRef]
- Colafrancesco, S.; Priori, R.; Alessandri, C.; Astorri, E.; Perricone, C.; Blank, M.; Agmon-Levin, N.; Shoenfeld, Y.; Valesini, G. sCD163 in AOSD: A biomarker for macrophage activation related to hyperferritinemia. Immunol. Res. 2014, 60, 177–183. [Google Scholar] [CrossRef]



| Patients | Placebo | Canakinumab | ||||||
|---|---|---|---|---|---|---|---|---|
| Men | Women | Total | Men | Women | Total | |||
| Number | 4 | 10 | 14 | 7 | 10 | 17 | ||
| Mean age in years (average) | 35 (28–40) | 46 (24–70) | 40 | 41 (22–63) | 45 (24–61) | 43 | ||
| CRP mg/L baseline mean | 48 | 37 | 39 | 33 | 37 | 35 | ||
| CRP mg/L week 12 mean | 21 | 25 | 24 | 14 | 25 | 21 | ||
| Ferritin ng/mL baseline mean | 883 | 718 | 751 | 514 | 811 | 689 | ||
| Ferritin ng/mL week 12 mean | 322 | 633 | 576 | 278 | 548 | 437 | ||
| Medication before study | c-DMARDs | 2 | 5 | 7 | 6 | 7 | 13 | |
| biological | Anti-IL1 (anakinra) | 2 | 8 | 10 | 6 | 5 | 11 | |
| Anti-TNF | 4 | 4 | 1 | 2 | 3 | |||
| Anti-IL6 | 2 | 2 | 2 | 2 | 4 | |||
| steroidal | Prednisolone | 2 | 9 | 11 | 7 | 10 | 17 | |
| NSAIDs | COXIB | 2 | 2 | 4 | ||||
| DAS28-ESR baseline | Remission | |||||||
| Low | 1 | 1 | ||||||
| Moderate | 1 | 3 | 4 | 5 | 6 | 11 | ||
| High | 1 | 5 | 6 | 2 | 2 | 4 | ||
| DAS28-ESR week 12 | Remission | 2 | 2 | 2 | 3 | 5 | ||
| Low | 3 | 3 | 1 | 2 | 3 | |||
| Moderate | 1 | 3 | 4 | 4 | 2 | 6 | ||
| High | 1 | 1 | 2 | 1 | 1 | |||
| DAS28-ESR Improvement by at least 1.2 | Yes | 5 | 5 | 4 | 6 | 10 | ||
| No | 2 | 4 | 6 | 3 | 2 | 5 | ||
| Cytokine | Source of Variation | p Value | Summary | F (DFn, DFd) | Tukey’s Multiple Comparisons Test | Adjusted p Value | Summary |
|---|---|---|---|---|---|---|---|
| MRP8/14 | Interaction | 0.0353 | * | F (2, 30) = 3.744 | Placebo | ns | |
| Treatment | 0.5932 | ns | Canakinumab | ||||
| Time | 0.0009 | *** | F (2, 30) = 8.983 | week 0 vs. week 1 | 0.0065 | ** | |
| week 0 vs. week 4 | <0.0001 | **** | |||||
| week 1 vs. week 4 | 0.157 | ns | |||||
| S100A12 | Interaction | 0.0757 | ns | Placebo | ns | ||
| Treatment | 0.8699 | ns | Canakinumab | ||||
| Time | 0.0061 | ** | F (2, 26) = 6.245 | week 0 vs. week 1 | 0.2236 | ns | |
| week 0 vs. week 4 | 0.0041 | ** | |||||
| week 1 vs. week 4 | 0.1746 | ns | |||||
| IL-2RA | Interaction | 0.0471 | * | F (2, 30) = 3.388 | Placebo | ||
| Treatment | 0.6039 | ns | week 0 vs. week 1 | 0.0972 | ns | ||
| Time | 0.086 | ns | week 0 vs. week 4 | 0.0429 | * | ||
| week 1 vs. week 4 | 0.9209 | ns | |||||
| Canakinumab | ns | ||||||
| IL-6 | Interaction | 0.1085 | ns | Placebo | ns | ||
| Treatment | 0.7974 | ns | Canakinumab | ||||
| Time | 0.0404 | * | F (2, 32) = 3.555 | week 0 vs. week 1 | 0.0133 | * | |
| week 0 vs. week 4 | 0.008 | ** | |||||
| week 1 vs. week 4 | 0.9779 | ns | |||||
| IL-18 | Interaction | 0.6183 | ns | Placebo | ns | ||
| Treatment | 0.9114 | ns | Canakinumab | ||||
| Time | 0.0041 | ** | F (2, 30) = 6.634 | week 0 vs. week 1 | 0.8451 | ns | |
| week 0 vs. week 4 | 0.0436 | * | |||||
| week 1 vs. week 4 | 0.0118 | * | |||||
| Sidak’s multiple comparisons test | Adjusted p Value | Summary | |||||
| sL-Selectin | Interaction | 0.2684 | ns | Placebo-Canakinumab | |||
| Treatment | 0.0492 | * | F (1, 15) = 4.578 | week 0 | 0.8361 | ns | |
| Time | 0.6646 | ns | week 1 | 0.9326 | ns | ||
| week 4 | 0.0361 | * |
| Cytokine | Group | p Value | Summary | Tukey’s Multiple Comparisons Test | Adjusted p Value | Summary |
|---|---|---|---|---|---|---|
| MRP8/14 | Placebo | 0.3967 | ns | Placebo | ns | |
| Canakinumab | 0.0014 | ** | Canakinumab | |||
| week 0 vs. week 1 | 0.0035 | ** | ||||
| week 0 vs. week 4 | 0.0064 | ** | ||||
| week 1 vs. week 4 | 0.0758 | ns | ||||
| S100A12 | Placebo | 0.1282 | ns | Placebo | ns | |
| Canakinumab | 0.0179 | * | Canakinumab | |||
| week 0 vs. week 1 | 0.0417 | * | ||||
| week 0 vs. week 4 | 0.0321 | * | ||||
| week 1 vs. week 4 | 0.2709 | ns | ||||
| IL-6 | Placebo | 0.6997 | ns | Placebo | ns | |
| Canakinumab | 0.0304 | * | Canakinumab | ns | ||
| Cytokine | Source of Variation | p Value | Summary | F (DFn, DFd) | Sidak’s Multiple Comparisons Test | Adjusted p Value | Summary |
|---|---|---|---|---|---|---|---|
| MRP8/14 | Interaction | 0.2892 | ns | week 0–week 4 | |||
| DAS28 improvement | 0.3022 | ns | Responder | 0.0023 | ** | ||
| Time | 0.0027 | ** | F (1, 13) = 13.61 | Non-Responder | 0.2562 | ns | |
| S100A12 | Interaction | 0.3104 | ns | week 0–week 4 | |||
| DAS28 improvement | 0.1944 | ns | Responder | 0.0056 | ** | ||
| Time | 0.0051 | ** | F (1, 12) = 11.65 | Non-Responder | 0.3075 | ns | |
| IL-1RA | Interaction | 0.9816 | ns | week 0–week 4 | |||
| DAS28 improvement | 0.3475 | ns | Responder | 0.0877 | ns | ||
| Time | 0.0226 | * | F (1, 13) = 6.688 | Non-Responder | 0.2501 | ns | |
| IL-18 | Interaction | 0.05 | * | F (1, 12) = 4.749 | week 0–week 4 | ||
| DAS28 improvement | 0.0111 | * | F (1, 12) = 8.989 | Responder | 0.0132 | * | |
| Time | 0.108 | ns | Non-Responder | 0.9548 | ns | ||
| Responder-Non-Responder | |||||||
| week 0 | 0.0042 | ** | |||||
| week 4 | 0.0523 | ns | |||||
| IL-6 | Interaction | 0.275 | ns | week 0–week 4 | |||
| DAS28 improvement | 0.5756 | ns | Responder | 0.0308 | * | ||
| Time | 0.0585 | ns | Non-Responder | 0.8209 | ns |
| Cytokine | Group | p Value | Summary |
|---|---|---|---|
| MRP8/14 | Responders | 0.0023 | ** |
| Non-Responders | 0.196 | ns | |
| S100A12 | Responders | 0.0151 | * |
| Non-Responders | 0.0027 | ** | |
| IL-1RA | Responders | 0.0347 | * |
| Non-Responders | 0.2558 | ns | |
| IL-18 | Responders | 0.0266 | * |
| Non-Responders | 0.3871 | ns | |
| IL-6 | Responders | 0.0209 | * |
| Non-Responders | 0.601 | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghannam, K.; Zernicke, J.; Kedor, C.; Listing, J.; Burmester, G.-R.; Foell, D.; Feist, E. Distinct Effects of Interleukin-1β Inhibition upon Cytokine Profile in Patients with Adult-Onset Still’s Disease and Active Articular Manifestation Responding to Canakinumab. J. Clin. Med. 2021, 10, 4400. https://doi.org/10.3390/jcm10194400
Ghannam K, Zernicke J, Kedor C, Listing J, Burmester G-R, Foell D, Feist E. Distinct Effects of Interleukin-1β Inhibition upon Cytokine Profile in Patients with Adult-Onset Still’s Disease and Active Articular Manifestation Responding to Canakinumab. Journal of Clinical Medicine. 2021; 10(19):4400. https://doi.org/10.3390/jcm10194400
Chicago/Turabian StyleGhannam, Khetam, Jan Zernicke, Claudia Kedor, Joachim Listing, Gerd-R. Burmester, Dirk Foell, and Eugen Feist. 2021. "Distinct Effects of Interleukin-1β Inhibition upon Cytokine Profile in Patients with Adult-Onset Still’s Disease and Active Articular Manifestation Responding to Canakinumab" Journal of Clinical Medicine 10, no. 19: 4400. https://doi.org/10.3390/jcm10194400
APA StyleGhannam, K., Zernicke, J., Kedor, C., Listing, J., Burmester, G.-R., Foell, D., & Feist, E. (2021). Distinct Effects of Interleukin-1β Inhibition upon Cytokine Profile in Patients with Adult-Onset Still’s Disease and Active Articular Manifestation Responding to Canakinumab. Journal of Clinical Medicine, 10(19), 4400. https://doi.org/10.3390/jcm10194400