XEN Glaucoma Implant for the Management of Glaucoma in Naïve Patients versus Patients with Previous Glaucoma Surgery
Abstract
:1. Introduction
2. Materials and Methods
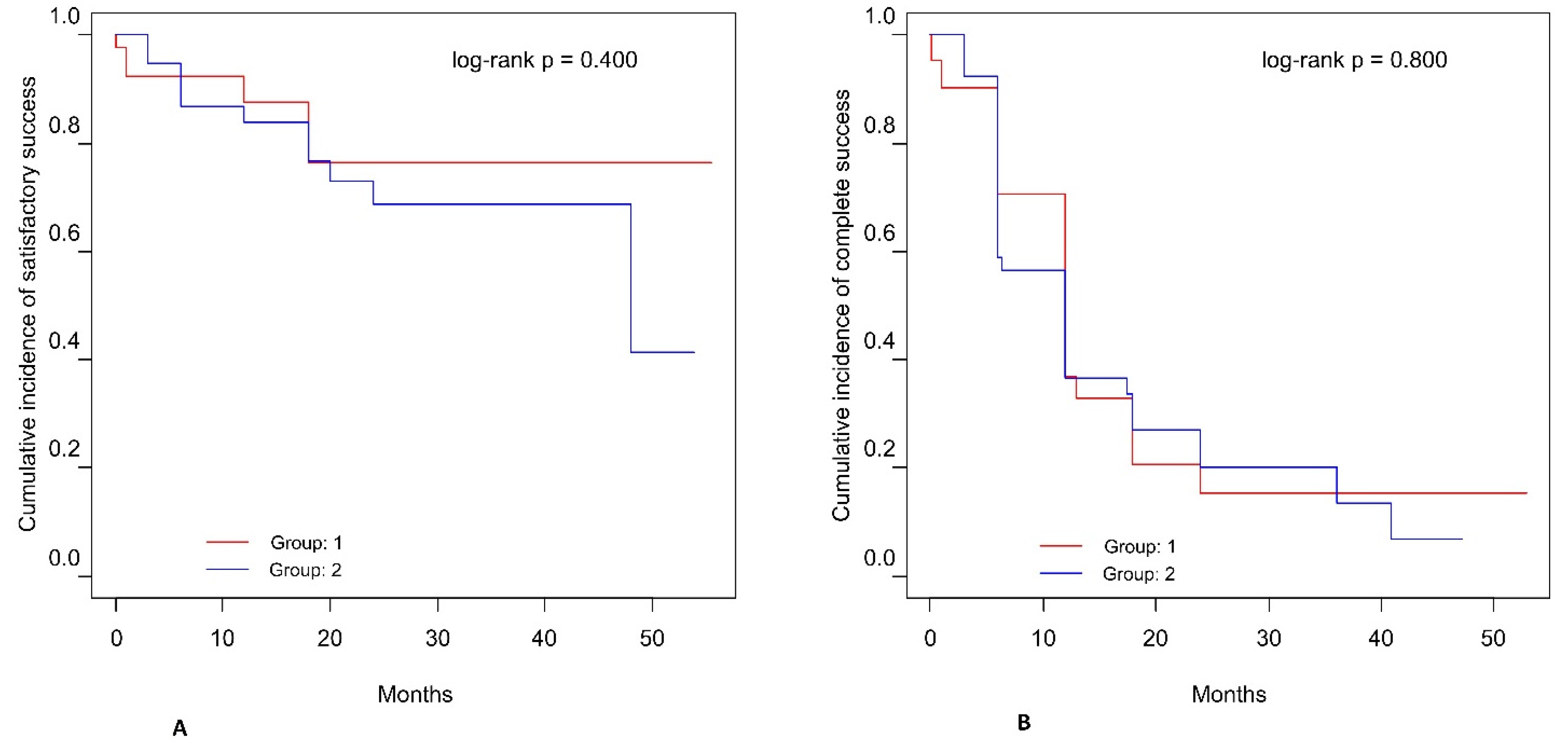
3. Results
3.1. Demographics and Glaucoma History
3.2. Additional Interventions and Procedures
3.3. Postoperative Complications
3.4. Visual Field
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, M.F.; Kim, C.H.; Coleman, A.L. Cyclodestructive procedures for refractory glaucoma. Cochrane Database Syst. Rev. 2019, 3, CD012223. [Google Scholar] [CrossRef]
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef]
- Ozdamar, A.; Aras, C.; Karacorlu, M. Suprachoroidal seton implantation in refractory glaucoma: A novel surgical technique. J. Glaucoma 2003, 12, 354–359. [Google Scholar] [CrossRef]
- Gessesse, G.W. The Ahmed Glaucoma Valve in refractory glaucoma: Experiences in Southwest Ethiopia. Ethiop. J. Health Sci. 2015, 25, 267–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jagannathan, J.; George, R.; Shantha, B.; Vijaya, L. Outcome of repeat trabeculectomy with mitomycin C in isolation or combined with phacoemulsification. Indian J. Ophthalmol. 2021, 69, 94–98. [Google Scholar]
- Gedde, S.J.; Schiffman, J.C.; Feuer, W.J.; Herndon, L.W.; Brandt, J.D.; Budenz, D.L. Tube versus Trabeculectomy Study Group. Treatment outcomes in the Tube Versus Trabeculectomy (TVT) study after five years of follow-up. Am. J. Ophthalmol. 2012, 5, 789–803.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Law, S.K.; Shih, K.; Tran, D.H.; Coleman, A.L.; Caprioli, J. Long-term outcomes of repeat vs. initial trabeculectomy in open-angle glaucoma. Am. J. Ophthalmol. 2009, 148, 685–695. [Google Scholar] [CrossRef]
- Panarelli, J.F.; Yan, D.B.; Francis, B.; Craven, E.R. XEN gel stents open conjunctiva technique: A practical approach paper. Adv. Ther. 2020, 37, 2538–2549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhingra, D.; Bhartiya, S. Evaluating glaucoma surgeries in the MIGS context. Rom. J. Ophthalmol. 2020, 64, 85–95. [Google Scholar] [CrossRef]
- Soltau, J.B.; Rothman, R.F.; Budenz, D.L.; Greenfield, D.S.; Feuer, W.; Liebmann, J.M.; Ritch, R. Risk factors for glaucoma filtering bleb infections. Arch. Ophthalmol. 2000, 118, 338–342. [Google Scholar] [CrossRef] [Green Version]
- Marcos Parra, M.T.; Salinas López, J.A.; López Grau, N.S.; Ceausescu, A.M.; Pérez Santonja, J.J. XEN implant device versus trabeculectomy, either alone or in combination with phacoemulsification, in open-angle glaucoma patients. Graefes Arch. Clin. Exp. Ophthalmol. 2019, 257, 1741–1750. [Google Scholar] [CrossRef]
- Olgun, A.; Duzgun, E.; Yildiz, A.M.; Atmaca, F.; Yildiz, A.A.; Sendul, S.Y. XEN gel stent versus trabeculectomy: Short-term effects on corneal endothelial cells. Eur. J. Ophthalmol. 2021, 31, 346–353. [Google Scholar] [CrossRef]
- Sacchi, M.; Agnifili, L.; Brescia, L.; Oddone, F.; Villani, E.; Nucci, P.; Mastropasqua, L. Structural imaging of conjunctival filtering blebs in XEN gel implantation and trabeculectomy: A confocal and anterior segment optical coherence tomography study. Graefes Arch. Clin. Exp. Ophthalmol. 2020, 258, 1763–1770. [Google Scholar] [CrossRef]
- Gillmann, K.; Bravetti, G.E.; Mermoud, A.; Rao, H.L.; Mansouri, K. XEN gel stent in pseudoexfoliative glaucoma: 2-year results of a prospective evaluation. J. Glaucoma 2019, 28, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, K.; Bravetti, G.E.; Gillmann, K.; Rao, H.L.; Ch’ng, T.W.; Mermoud, A. Two-year outcomes of XEN gel stent surgery in patients with open-angle glaucoma. Ophthalmol. Glaucoma 2019, 2, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Sheybani, A.; Dick, H.B.; Ahmed, I.I. Early clinical results of a novel Ab interno gel stent for the surgical treatment of open-angle glaucoma. J. Glaucoma 2016, 25, e691–e696. [Google Scholar] [CrossRef] [PubMed]
- Cankaya, A.B.; Elgin, U. Comparison of the outcome of repeat trabeculectomy with adjunctive mitomycin C and initial trabeculectomy. Korean J. Ophthalmol. 2011, 25, 401–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, K.; Lind, J.; Sheybani, A. Safety and efficacy outcomes of the Xen45 gel stent use for refractory glaucoma: A surgery series from surgeon trainees at a tertiary teaching hospital. Eye Vis. 2020, 7, 5. [Google Scholar] [CrossRef]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M.; STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. PLoS Med. 2007, 4, e297. [Google Scholar] [CrossRef] [Green Version]
- Fea, A.M.; Durr, G.M.; Marolo, P.; Malinverni, L.; Economou, M.A.; Ahmed, I. XEN® gel stent: A comprehensive review on its use as a treatment option for refractory glaucoma. Clin. Ophthalmol. 2020, 14, 1805–1832. [Google Scholar] [CrossRef]
- Kozera, M.; Konopinska, J.; Mariak, Z.; Rekas, M. Effectiveness of iStent trabecular micro-bypass system combined with phacoemulsification vs. phacoemulsification alone in patients with glaucoma and cataract depending on the initial intraocular pressure. Ophthalmic Res. 2020, 64, 327–336. [Google Scholar] [CrossRef]
- Tan, N.E.; Tracer, N.; Terraciano, A.; Parikh, H.A.; Panarelli, J.F.; Radcliffe, N.M. Comparison of safety and efficacy between ab interno and ab externo approaches to XEN Gel Stent placement. Clin. Ophthalmol. 2021, 26, 299–305. [Google Scholar] [CrossRef] [PubMed]
- AGIS Investigators. The advanced glaucoma intervention study, 6: Effect of cataract on visual field and visual acuity. Arch. Ophthalmol. 2000, 118, 1639–1652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konopińska, J.; Deniziak, M.; Saeed, E.; Bartczak, A.; Zalewska, R.; Mariak, Z.; Rękas, M. Prospective randomized study comparing combined phaco-express and phacotrabeculectomy in open angle glaucoma treatment: 12-month follow-up. J. Ophthalmol. 2015, 2015, 720109. [Google Scholar] [CrossRef] [PubMed]
- Dhand, N.K.; Khatkar, M.S. Statulator: An Online Statistical Calculator. Sample Size Calculator for Comparing Two Paired Means. 2014. Available online: http://statulator.com/SampleSize/ss2PM.html (accessed on 23 October 2020).
- Little, R.J.A.; Rubin, D.B. Statistical Analysis with the Missing Data; John Wiley and Sons: New York, NY, USA, 1987; p. 278. [Google Scholar]
- Karimi, A.; Hopes, M.; Martin, K.R.; Lindfield, D. Efficacy and safety of the ab-interno Xen gel stent after failed trabeculectomy. J. Glaucoma. 2018, 27, 864–868. [Google Scholar] [CrossRef]
- Costa, V.P.; Spaeth, G.L.; Eiferman, R.A.; Orengo-Nania, S. Wound healing modulation in glaucoma filtration surgery. Ophthalmic Surg. 1993, 24, 152–170. [Google Scholar] [PubMed]
- Hengerer, F.H.; Kohnen, T.; Mueller, M.; Conrad-Hengerer, I. Ab interno gel implant for the treatment of glaucoma patients with or without prior glaucoma surgery: 1-year results. J. Glaucoma 2017, 26, 1130–1136. [Google Scholar] [CrossRef] [PubMed]
- Grover, D.S.; Flynn, W.J.; Bashford, K.P.; Lewis, R.A.; Duh, Y.J.; Nangia, R.S.; Niksch, B. Performance and Safety of a New Ab Interno Gelatin Stent in Refractory Glaucoma at 12 Months. Am. J. Ophthalmol. 2017, 183, 25–36. [Google Scholar] [CrossRef] [Green Version]
| Group 1 No Previous Surgery (n = 43) | Group 2 1 or More Previous Surgeries (n = 43) | p | |||
|---|---|---|---|---|---|
| n | %/Mean (SD)/Median (Range) | n | %/Mean (SD)/Median (Range) | ||
| Sex, n (%) | |||||
| Female | 22 | 51.2 | 26 | 60.5 | 0.515 |
| Male | 21 | 48.8 | 17 | 39.5 | |
| Age at surgery, years, mean (SD) | 36 | 57.42 (15.78) | 34 | 60.04 (19.20) | 0.603 |
| Duration of glaucoma, years, mean (SD) | 41 | 10.03 (10.86) | 37 | 18.18 (14.88) | 0.008 |
| Number of previous surgeries, median (range) | 42 | 0.00 | 40 | 2.00 (1.00–6.00) | <0.001 1 |
| Naïve Patients (n = 43) | Patients with Previous Surgery (n = 43) | MD (95% CI) 2 | p 3 | |||
|---|---|---|---|---|---|---|
| Mean (SD) | Median (Range) | Mean (SD) | Median (Range) | |||
| Visual Acuity (CDVA) | ||||||
| Pre–op | 0.37 (0.28) | 0.30 (0.00–0.90) | 0.38 (0.31) | 0.30 (0.01–1.00) | 0.01(−0.12–0.14) | 0.877 |
| Final | 0.44 (0.36) | 0.40 (0.01–1.00) | 0.45 (0.36) | 0.40 (0.00–1.00) | 0.01(−0.14–0.18) | 0.840 |
| Final vs. Pre-op: | ||||||
| Change | 0.08 (0.19) | 0.03 (–0.34–0.50) | 0.07 (0.19) | 0.05 (−0.30–0.68) | −0.01(−0.09–0.08) | 0.902 |
| MD (95% CI) 1 | 0.08 (0.02–0.13) | 0.07 (0.01–0.14) | ||||
| p 4 | 0.011 | 0.024 | ||||
| Intraocular Pressure (IOP) | ||||||
| Pre–op | 25.00 (7.52) | 22.00 (18.00–45.00) | 25.35 (7.81) | 22.50 (18.00–45.00) | 0.35(−3.00–3.70) | 0.836 |
| Final | 16.83 (5.12) | 16.00 (9.00–35.00) | 17.54 (5.34) | 17.00 (10.00–35.00) | 0.71(−1.62–3.04) | 0.546 |
| Final vs. Pre-op: | ||||||
| Change | −6.59 (10.26) | −5.00(−36.00–28.00) | −8.00 (8.86) | −7.00(−34.00–3.00) | −1.41(−5.68–2.85) | 0.511 |
| Change (%) | −29.4% (20.1%) | –25.0%(−40.9–−16.7%) | −26.5% (25.4%) | –26.1%(−48.3–−7.8%) | 2.9(−7.3–13.2%) | 0.567 |
| MD (95% CI) 1 | −6.59(−10.96–−5.73) | −8.00(−10.87–−5.13) | ||||
| p 4 | <0.001 | <0.001 | ||||
| Characteristic | Naïve Patients (n = 43) | Patients with Previous Glaucoma Surgery (n = 43) | p | ||
|---|---|---|---|---|---|
| n | %/Median (Range) | n | %/Median (Range) | ||
| CDVA change, n (%) | |||||
| Decline | 7/42 | 16.7 | 8/39 | 20.5 | 0.878 |
| No change | 10/42 | 23.8 | 8/39 | 20.5 | |
| Increase | 25/42 | 59.5 | 23/39 | 59.0 | |
| IOP change, n (%) | |||||
| Decline | 36/41 | 87.8 | 31/39 | 79.5 | 0.383 1 |
| No change | 2/41 | 4.9 | 1/39 | 2.6 | |
| Increase | 3/41 | 7.3 | 7/39 | 17.9 | |
| Decline ≥20% | 27/41 | 65.9 | 22/39 | 56.4 | 0.492 |
| Decline <20% | 14/41 | 34.1 | 17/39 | 43.6 | |
| IOP final level <18 mmHg | 35/41 | 85.4 | 24/39 | 61.5 | 0.030 |
| Massage recommendation, n (%) | 15/43 | 34.9 | 9/43 | 20.9 | 0.229 |
| Needling, n (%) | 26/37 | 70.3 | 28/38 | 73.7 | 0.943 |
| Number of needlings | 37 | 1.00 (0.00;4.00) | 38 | 1.50 (0.00;12.00) | 0.117 2 |
| Number of drugs before surgery | 43 | 4.00 (0.00;5.00) | 43 | 4.00 (0.00;5.00) | 0.280 2 |
| Time to administration of first drug, days | 32 | 21.00 (0.00;429.00) | 33 | 42.00 (0.00;730.00) | 0.058 2 |
| Final number of drugs | 41 | 2.00 (0.00;4.00) | 41 | 2.00 (0.00;4.00) | 0.186 2 |
| Next procedures, n (%) | 15/43 | 34.9 | 10/43 | 23.3 | 0.342 |
| Complications, n (%) | 10/43 | 23.3 | 13/43 | 30.2 | 0.626 |
| Reoperations, n (%) | 3/18 | 16.7 | 8/25 | 32.0 | 0.309 1 |
| Group 1 n (%) | Group 2 n (%) | p * | |
|---|---|---|---|
| Intraoperative | |||
| Bleeding | - | 1 (2.5) | 0.662 |
| Postoperative | |||
| Hyphema | |||
| Blood level in AC | 2 (4.7) | 2 (5.0) | 0.877 |
| Erythrocytes in AC | - | - | - |
| Wound leakage | 1 (2.3) | 2 (5.0) | 0.383 |
| Fibrosis | 9 (21.4) | 9 (22.5) | 0.881 |
| Anterior chamber cells | 3 (7.1) | 3 (7.5) | 0.841 |
| Hypotony | |||
| Until 7 days | - | 2 (5.0) | 0.071 |
| Until 30 days | - | 1 (2.5) | 0.392 |
| Until 180 days | 1 (2.3) | 1 (2.5) | 0.901 |
| Choroid detachment | 1 (2.3) | 3 (7.5) | 0.219 |
| Macular edema | 1 (2.3) | - | 0.413 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lewczuk, K.; Konopińska, J.; Jabłońska, J.; Rudowicz, J.; Laszewicz, P.; Dmuchowska, D.A.; Mariak, Z.; Rękas, M. XEN Glaucoma Implant for the Management of Glaucoma in Naïve Patients versus Patients with Previous Glaucoma Surgery. J. Clin. Med. 2021, 10, 4417. https://doi.org/10.3390/jcm10194417
Lewczuk K, Konopińska J, Jabłońska J, Rudowicz J, Laszewicz P, Dmuchowska DA, Mariak Z, Rękas M. XEN Glaucoma Implant for the Management of Glaucoma in Naïve Patients versus Patients with Previous Glaucoma Surgery. Journal of Clinical Medicine. 2021; 10(19):4417. https://doi.org/10.3390/jcm10194417
Chicago/Turabian StyleLewczuk, Katarzyna, Joanna Konopińska, Joanna Jabłońska, Jacek Rudowicz, Patrycja Laszewicz, Diana Anna Dmuchowska, Zofia Mariak, and Marek Rękas. 2021. "XEN Glaucoma Implant for the Management of Glaucoma in Naïve Patients versus Patients with Previous Glaucoma Surgery" Journal of Clinical Medicine 10, no. 19: 4417. https://doi.org/10.3390/jcm10194417
APA StyleLewczuk, K., Konopińska, J., Jabłońska, J., Rudowicz, J., Laszewicz, P., Dmuchowska, D. A., Mariak, Z., & Rękas, M. (2021). XEN Glaucoma Implant for the Management of Glaucoma in Naïve Patients versus Patients with Previous Glaucoma Surgery. Journal of Clinical Medicine, 10(19), 4417. https://doi.org/10.3390/jcm10194417







