Endoscopic Resection for Gastric Subepithelial Tumor with Backup Laparoscopic Surgery: Description of a Single-Center Experience
Abstract
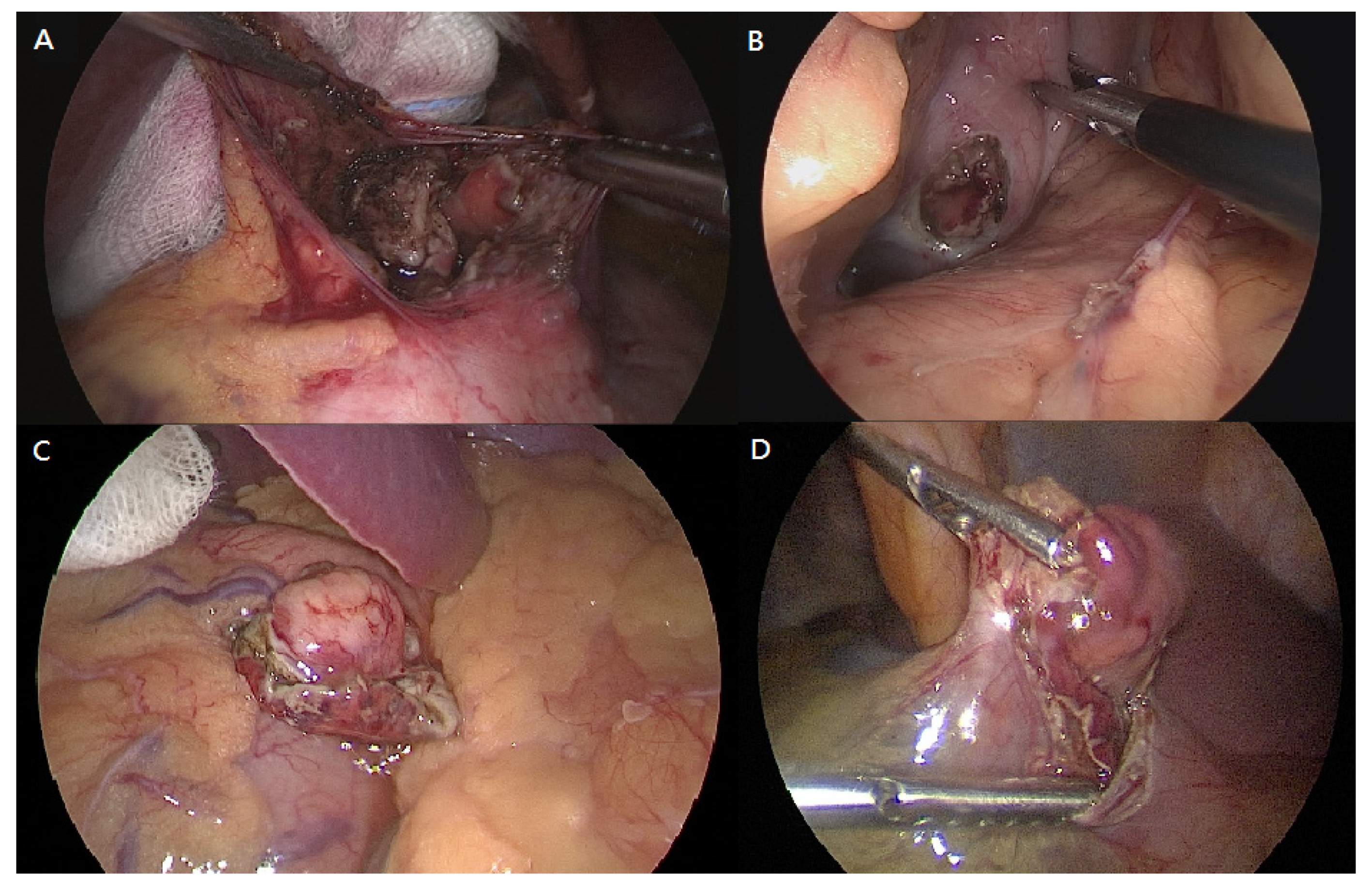
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Procedure
2.3. Clinicopathological Factors
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hwang, J.H.; Rulyak, S.D.; Kimmey, M.B. American Gastroenterological Association Institute technical review on the management of gastric subepithelial masses. Gastroenterology 2006, 7, 2217–2228. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.W.; Korean ESD Study Group. Current guidelines in the management of upper gastrointestinal subepithelial tumors. Clin. Endosc. 2016, 3, 235–240. [Google Scholar] [CrossRef]
- Miettinen, M.; Lasota, J. Gastrointestinal stromal tumors (GISTs): Definition, occurrence, pathology, differential diagnosis and molecular genetics. Pol. J. Pathol. 2003, 1, 3–24. [Google Scholar]
- Casali, P.G.; Abecassis, N.; Aro, H.T.; Bauer, S.; Biagini, R.; Bielack, S.; Bonvalot, S.; Boukovinas, I.; Bovee, J.V.M.G.; Brodowicz, T.; et al. Gastrointestinal stromal tumours: ESMO–EURACAN clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv68–iv78. [Google Scholar] [CrossRef] [PubMed]
- Demetri, G.D.; von Mehren, M.; Antonescu, C.R.; DeMatteo, R.P.; Ganjoo, K.N.; Maki, R.G.; Pisters, P.W.; Raut, C.P.; Riedel, R.F.; Schuetze, S.; et al. NCCN Task Force report: Update on the management of patients with gastrointestinal stromal tumors. J. Natl. Compr. Cancer Netw. 2010, 8, S1–S41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koo, D.H.; Ryu, M.H.; Kim, K.M.; Yang, H.K.; Sawaki, A.; Hirota, S.; Zheng, J.; Zhang, B.; Tzen, C.Y.; Yeh, C.N.; et al. Asian consensus guidelines for the diagnosis and management of gastrointestinal stromal tumor. Cancer Res. Treat. 2016, 4, 1155–1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ntourakis, D.; Mavrogenis, G. Cooperative laparoscopic endoscopic and hybrid laparoscopic surgery for upper gastrointestinal tumors: Current status. World J. Gastroenterol. 2015, 43, 12482–12497. [Google Scholar] [CrossRef]
- Hiki, N.; Yamamoto, Y.; Fukunaga, T.; Yamaguchi, T.; Nunobe, S.; Tokunaga, M.; Miki, A.; Ohyama, S.; Seto, Y. Laparoscopic and endoscopic cooperative surgery for gastrointestinal stromal tumor dissection. Surg. Endosc. 2008, 7, 1729–1735. [Google Scholar] [CrossRef]
- Matsuda, T.; Nunobe, S.; Ohashi, M.; Hiki, N. Laparoscopic endoscopic cooperative surgery (LECS) for the upper gastrointestinal tract. Transl. Gastroenterol. Hepatol. 2017, 5, 40. [Google Scholar] [CrossRef] [Green Version]
- Tsujimoto, H.; Yaguchi, Y.; Kumano, I.; Takahata, R.; Ono, S.; Hase, K. Successful gastric submucosal tumor resection using laparoscopic and endoscopic cooperative surgery. World J. Surg. 2012, 2, 327–330. [Google Scholar] [CrossRef]
- Nabeshima, K.; Tomioku, M.; Nakamura, K.; Yasuda, S. Combination of laparoscopic and endoscopic approaches to neoplasia with non-exposure technique (CLEAN-NET) for GIST with ulceration. Tokai J. Exp. Clin. Med. 2015, 40, 115–119. [Google Scholar] [PubMed]
- Kushnir, V.M.; Keswani, R.N.; Hollander, T.G.; Kohlmeier, C.; Mullady, D.K.; Azar, R.R.; Murad, F.M.; Komanduri, S.; Edmundowicz, S.A.; Early, D.S. Compliance with surveillance recommendations for foregut subepithelial tumors is poor: Results of a prospective multicenter study. Gastrointest. Endosc. 2015, 81, 1378–1384. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.H.; Yao, L.Q.; Qin, X.Y.; Cai, M.Y.; Xu, M.D.; Zhong, Y.S.; Chen, W.F.; Zhang, Y.Q.; Qin, W.Z.; Hu, J.W.; et al. Endoscopic full-thickness resection without laparoscopic assistance for gastric submucosal tumors originated from the muscularis propria. Surg. Endosc. 2011, 9, 2926–2931. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.R.; Song, J.T.; Qu, B.; Wen, J.F.; Yin, J.B.; Liu, W. Endoscopic muscularis dissection for upper gastrointestinal subepithelial tumors originating from the muscularis propria. Surg. Endosc. 2012, 11, 3141–3148. [Google Scholar] [CrossRef] [PubMed]
- Abe, N.; Takeuchi, H.; Ooki, A.; Nagao, G.; Masaki, T.; Mori, T.; Sugiyama, M. Recent developments in gastric endoscopic submucosal dissection: Towards the era of endoscopic resection of layers deeper than the submucosa. Dig. Endosc. 2013, 25, 64–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.H. Endoscopic treatment for gastrointestinal stromal tumor: Advantages and hurdles. World J. Gastrointest. Endosc. 2015, 3, 192. [Google Scholar] [CrossRef]
- Lu, J.; Jaio, T.; Li, Y.; Zheng, M.; Lu, X. Facilitating retroflexed endoscopic full-thickness resection through loop-mediated or rope-mediated countertraction (with videos). Gastrointest. Endosc. 2016, 1, 223–228. [Google Scholar] [CrossRef]
- Shi, D.; Li, R.; Chen, W.; Zhang, D.; Zhang, L.; Guo, R.; Yao, P.; Wu, X. Application of novel endoloops to close the defects resulted from endoscopic full-thickness resection with single-channel gastroscope: A multicenter study. Surg. Endosc. 2017, 2, 837–842. [Google Scholar] [CrossRef]
- Yen, H.H.; Hsu, H.T. Gastric subcentimeter subepithelial tumor: Successful resection with an over-the-scope padlock clip. Endoscopy 2021. [Google Scholar] [CrossRef]
- Chiu, P.W.Y.; Yip, H.C.; Teoh, A.Y.B.; Wong, V.W.; Chan, S.M.; Wong, S.K.; Ng, E.K.W. Per oral endoscopic tumor (POET) resection for treatment of upper gastrointestinal subepithelial tumors. Surg. Endosc. 2019, 4, 1326–1333. [Google Scholar] [CrossRef]
- Białek, A.; Wiechowska-Kozłowska, A.; Pertkiewicz, J.; Polkowski, M.; Milkiewicz, P.; Karpińska, K.; Ławniczak, M.; Starzyńska, T. Endoscopic submucosal dissection for treatment of gastric subepithelial tumors (with video). Gastrointest. Endosc. 2012, 2, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.Y.; Lien, J.M.; Tsai, M.H.; Chiu, C.T.; Chen, T.C.; Yang, K.C.; Ng, S.C. Modified endoscopic submucosal dissection with enucleation for treatment of gastric subepithelial tumors originating from the muscularis propria layer. BMC Gastroenterol. 2012, 12, 124. [Google Scholar] [CrossRef] [Green Version]
- Chun, S.Y.; Kim, K.O.; Park, D.S.; Lee, I.J.; Park, J.W.; Moon, S.H.; Baek, I.H.; Kim, J.H.; Park, C.K.; Kwon, M.J. Endoscopic submucosal dissection as a treatment for gastric subepithelial tumors that originate from the muscularis propria layer: A preliminary analysis of appropriate indications. Surg. Endosc. 2013, 9, 3271–3279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.S.; Kim, G.H.; Park, D.Y.; Yoon, J.M.; Kim, T.W.; Seo, J.H.; Lee, B.E.; Song, G.A. Endoscopic submucosal dissection for gastric subepithelial tumors: A single-center experience. Gastroenterol. Res. Pract. 2015, 2015, 425469. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, K.O. Management of gastric subepithelial tumors: The role of endoscopy. World J. Gastrointest. Endosc. 2016, 8, 418–424. [Google Scholar] [CrossRef]
- Tan, Y.; Tan, L.; Lu, J.; Huo, J.; Liu, D. Endoscopic resection of gastric gastrointestinal stromal tumors. Transl. Gastroenterol. Hepatol. 2017, 2, 115. [Google Scholar] [CrossRef]
- Oda, I.; Suzuki, H.; Nonaka, S.; Yoshinaga, S. Complications of gastric endoscopic submucosal dissection. Dig. Endosc. 2013, 25, 71–78. [Google Scholar] [CrossRef]
- Saito, I.; Tsuji, Y.; Sakaguchi, Y.; Niimi, K.; Ono, S.; Kodashima, S.; Yamamichi, N.; Fujishiro, M.; Koike, K. Complications related to gastric endoscopic submucosal dissection and their managements. Clin. Endosc. 2014, 5, 398–403. [Google Scholar] [CrossRef]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 2, 187–196. [Google Scholar] [CrossRef] [Green Version]
- Fletcher, C.D.; Berman, J.J.; Corless, C.; Gorstein, F.; Lasota, J.; Longley, B.J.; Miettinen, M.; O’Leary, T.J.; Remotti, H.; Rubin, B.P.; et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum. Pathol. 2002, 5, 459–465. [Google Scholar] [CrossRef] [Green Version]
- Matsuda, T.; Hiki, N.; Nunobe, S.; Aikou, S.; Hirasawa, T.; Yamamoto, Y.; Kumagai, K.; Ohashi, M.; Sano, T.; Yamaguchi, T. Feasibility of laparoscopic and endoscopic cooperative surgery for gastric submucosal tumors (with video). Gastrointest. Endosc. 2016, 1, 47–52. [Google Scholar] [CrossRef]
- Liao, G.Q.; Chen, T.; Qi, X.L.; Hu, Y.F.; Liu, H.; Yu, J.; Li, G.X. Laparoscopic management of gastric gastrointestinal stromal tumors: A retrospective 10-year single-center experience. World J. Gastroenterol. 2017, 19, 3522–3529. [Google Scholar] [CrossRef]
- Lamm, S.H.; Steinemann, D.C.; Linke, G.R.; Eucker, D.; Simon, T.; Zerz, A.; Stoll, R. Total inverse transgastric resection with transoral specimen removal. Surg. Endosc. 2015, 11, 3363–3366. [Google Scholar] [CrossRef]
- Mazer, L.; Worth, P.; Visser, B. Minimally invasive options for gastrointestinal stromal tumors of the stomach. Surg. Endosc. 2021, 3, 1324–1330. [Google Scholar] [CrossRef]
- Matsuda, T.; Nunobe, S.; Kosuga, T.; Kawahira, H.; Inaki, N.; Kitashiro, S.; Abe, N.; Miyashiro, I.; Nagao, S.; Nishizaki, M.; et al. Laparoscopic and luminal endoscopic cooperative surgery can be a standard treatment for submucosal tumors of the stomach: A retrospective multicenter study. Endoscopy 2017, 49, 476–483. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Kikuchi, D.; Nagami, Y.; Nonaka, K.; Tsuji, Y.; Fujimoto, A.; Sanomura, Y.; Tanaka, K.; Abe, S.; Zhang, S.; et al. Management of adverse events related to endoscopic resection of upper gastrointestinal neoplasms: Review of the literature and recommendations from experts. Dig. Endosc. 2019, 31 (Suppl. 1), 4–20. [Google Scholar] [CrossRef]
- Hanaoka, N.; Uedo, N.; Ishihara, R.; Higashino, K.; Takeuchi, Y.; Inoue, T.; Chatani, R.; Hanafusa, M.; Tsujii, Y.; Kanzaki, H.; et al. Clinical features and outcomes of delayed perforation after endoscopic submucosal dissection for early gastric cancer. Endoscopy 2010, 12, 1112–1115. [Google Scholar] [CrossRef]
- Kato, M.; Nishida, T.; Tsutsui, S.; Komori, M.; Michida, T.; Yamamoto, K.; Kawai, N.; Kitamura, S.; Zushi, S.; Nishihara, A.; et al. Endoscopic submucosal dissection as a treatment for gastric noninvasive neoplasia: A multicenter study by Osaka University ESD Study Group. J. Gastroenterol. 2011, 3, 325–331. [Google Scholar] [CrossRef]
- Yoo, J.H.; Shin, S.J.; Lee, K.M.; Choi, J.M.; Wi, J.O.; Kim, D.H.; Lim, S.G.; Hwang, J.C.; Cheong, J.Y.; Yoo, B.M.; et al. Risk factors for perforations associated with endoscopic submucosal dissection in gastric lesions: Emphasis on perforation type. Surg. Endosc. 2012, 26, 2456–2464. [Google Scholar] [CrossRef]
- Ohta, T.; Ishihara, R.; Uedo, N.; Takeuchi, Y.; Nagai, K.; Matsui, F.; Kawada, N.; Yamashina, T.; Kanzaki, H.; Hanafusa, M.; et al. Factors predicting perforation during endoscopic submucosal dissection for gastric cancer. Gastrointest. Endosc. 2012, 6, 1159–1165. [Google Scholar] [CrossRef]
- Suzuki, H.; Oda, I.; Sekiguchi, M.; Abe, S.; Nonaka, S.; Yoshinaga, S.; Nakajima, T.; Saito, Y. Management and associated factors of delayed perforation after gastric endoscopic submucosal dissection. World J. Gastroenterol. 2015, 44, 12635–12643. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Nishisaki, H.; Sakai, H.; Tokuyama, N.; Sawai, H.; Sakai, A.; Mimura, T.; Kushida, S.; Tsumura, H.; Sakamoto, T.; et al. Clinical factors of delayed perforation after endoscopic submucosal dissection for gastric neoplasms. Gastroenterol. Res. Pract. 2017, 2017, 7404613. [Google Scholar] [CrossRef] [Green Version]
- Onogi, F.; Araki, H.; Ibuka, T.; Manabe, Y.; Yamazaki, K.; Nishiwaki, S.; Moriwaki, H. “Transmural air leak”: A computed tomographic finding following endoscopic submucosal dissection of gastric tumors. Endoscopy 2010, 6, 441–447. [Google Scholar] [CrossRef]
- Dong, X.; Chen, W.; Cui, Z.; Chen, T.; Liu, X.; Chen, D.; Jiang, W.; Li, K.; Dong, S.; Feng, M.; et al. Laparoscopic resection is better than endoscopic dissection for gastric gastrointestinal stromal tumor between 2 and 5 cm in size: A case-matched study in a gastrointestinal center. Surg. Endosc. 2020, 11, 5098–5106. [Google Scholar] [CrossRef]
- Dai, W.J.; Liu, G.; Wang, M.; Liu, W.J.; Song, W.; Yang, X.Z.; Wang, Q.L.; Zhang, X.Y.; Fan, Z.N. Endoscopic versus laparoscopic resection of gastric gastrointestinal stromal tumors: A multicenter study. Oncotarget 2017, 7, 11259–11267. [Google Scholar] [CrossRef] [Green Version]
- Andalib, I.; Yeoun, D.; Reddy, R.; Xie, S.; Iqbal, S. Endoscopic resection of gastric gastrointestinal stromal tumors originating from the muscularis propria layer in North America: Methods and feasibility data. Surg. Endosc. 2018, 4, 1787–1792. [Google Scholar] [CrossRef] [PubMed]
- Pang, T.; Zhao, Y.; Fan, T.; Hu, Q.; Raymond, D.; Cao, S.; Zhang, W.; Wang, Y.; Zhang, B.; Lv, Y.; et al. Comparison of safety and outcomes between endoscopic and surgical resections of small (≤5 cm) primary gastric gastrointestinal stromal tumors. J. Cancer 2019, 17, 4132–4141. [Google Scholar] [CrossRef] [PubMed]
- Corless, C.L.; McGreevey, L.; Haley, A.; Town, A.; Heinrich, M.C. KIT mutations are common in incidental gastrointestinal stromal tumors one centimeter or less in size. Am. J. Pathol. 2002, 5, 1567–1572. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, J.; Oshima, T.; Hori, K.; Tomita, T.; Kim, Y.; Watari, J.; Oh, K.; Hirota, S.; Matsumoto, T.; Miwa, H. Small gastrointestinal stromal tumor of the stomach showing rapid growth and early metastasis to the liver. Dig. Endosc. 2010, 4, 354–356. [Google Scholar] [CrossRef]
- Fang, Y.J.; Cheng, T.Y.; Sun, M.S.; Yang, C.S.; Chen, J.H.; Liao, W.C.; Wang, H.P. Suggested cutoff tumor size for management of small EUS-suspected gastric gastrointestinal stromal tumors. J. Formos. Med. Assoc. 2012, 111, 88–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, L.; Khan, S.; Hui, Y.; Zhao, J.; Li, B.; Ma, S.; Guo, J.; Chen, X.; Wang, B. Treatment recommendations for small gastric gastrointestinal stromal tumors: Positive endoscopic resection. Scand. J. Gastroenterol. 2019, 54, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.H.; Wu, P.Y.; Su, P.Y.; Yang, C.W.; Chen, Y.Y.; Chen, M.F.; Lin, W.C.; Tsai, C.L.; Lin, K.P. Performance Comparison of the Deep Learning and the Human Endoscopist for Bleeding Peptic Ulcer Disease. J. Med Biol. Eng. 2021. [Google Scholar] [CrossRef]
- Yen, H.H.; Wu, P.Y.; Chen, M.F.; Lin, W.C.; Tsai, C.L.; Lin, K.P. Current Status and Future Perspective of Artificial Intelligence in the Management of Peptic Ulcer Bleeding: A Review of Recent Literature. J. Clin. Med. 2021, 10, 3527. [Google Scholar] [CrossRef] [PubMed]




| ESD Only n = 56 | Backup Surgery n = 27 | p Value | |||
|---|---|---|---|---|---|
| Age, years | 54.5 | (48.0–63.0) | 56.0 | (50.5–63.3) | 0.4222 |
| Male gender | 26 | (46.4) | 9 | (33.3) | 0.2606 |
| Tumor location | 0.8634 | ||||
| Upper | 45 | (80.4) | 23 | (85.2) | |
| Middle | 3 | (5.4) | 1 | (3.7) | |
| Low | 8 | (14.2) | 3 | (11.1) | |
| Tumor size | 0.1455 | ||||
| ≤2 cm | 42 | (75.0) | 16 | (59.3) | |
| >2 cm | 14 | (25.0) | 11 | (40.7) | |
| Layer of tumor depth | 0.0792 | ||||
| Submucosa | 6 | (10.7) | 0 | (0.0) | |
| Muscularis propria | 50 | (89.3) | 27 | (100.0) | |
| Exophytic growth | 0 | (0.0) | 10 | (37.0) | <0.0001 * |
| Procedure time, mins | 60.0 | (40.0–90.0) | 185.0 | (152.0–236.8) | <0.0001 * |
| Length of stay, days | 5 | (4–6) | 7 | (7–8) | <0.0001 * |
| Clavien ≥ III complication | 4 | (7.1) | 0 | (0.0) | 0.1571 |
| Pathology | 0.4387 | ||||
| Malignant/malignant potential | 26 | (46.4) | 15 | (55.6) | |
| Benign | 30 | (53.6) | 12 | (44.4) | |
| Case | Age | Sex | Tumor Location | Size (cm) | Depth | ESD Time (minutes) | Time to Diagnosis (hours) | Symptom/Signs | Image Survey | Severity | Management | LOS (days) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 56 | F | U, les | 1.0 | SM | 60 | 6 | Fever | CXR: Bil. subphrenic air | Grade I | Conservative care | 8 |
| 2 | 43 | F | L, ant | 2.6 | SM | 90 | 8 | Localized abd. pain | CT: Pneumoperitoneum with few ascites | Grade I | Conservative care | 8 |
| 3 | 69 | F | U, ant | 0.4 | MP | 33 | 47 | Localized abd. pain | CXR: Bil subphrenic air | Grade III | Sono-guided aspiration | 8 |
| 4 | 42 | M | U, ant | 0.5 | MP | 20 | 6 | Hematemesis | CT: Pneumoperitoneum with internal bleeding | Grade IIIb | Laparoscopic gastrorrhaphy | 8 |
| 5 | 52 | M | U, post | 0.5 | MP | 40 | 24 | Fever with peritonitis | CT: pneumoperitoneum with few ascites | Grade IIIb | Laparoscopic gastrorrhaphy | 9 |
| Total n = 83 | ESD Only n = 56 | Backup Surgery n = 27 | |
|---|---|---|---|
| Malignant or malignant potential | |||
| GIST (%) | 39 (47) | 23 | 16 |
| 22 (26.5) | 16 | 7 |
| 12 (14.5) | 3 | 8 |
| 5 (6) | 4 | 1 |
| Neuroendocrine tumor | 1 (1.2) | 1 | 0 |
| Hyperplastic polyp with focal high-grade dysplasia | 1 (1.2) | 1 | 0 |
| Benign | |||
| Leiomyoma (%) | 31 (37.4) | 25 | 6 |
| Ectopic pancreas | 3 (3.6) | 1 | 2 |
| Calcifying fibrous tumor | 2 (2.4) | 1 | 1 |
| Lipoma | 1 (1.2) | 1 | 0 |
| Inflammatory fibroid polyp | 1 (1.2) | 1 | 0 |
| Gastritis cystica profunda | 1 (1.2) | 1 | 0 |
| Plexiform fibromyxoma | 1 (1.2) | 0 | 1 |
| Elastofibroma | 1 (1.2) | 1 | 0 |
| Pyloric gland adenoma | 1 (1.2) | 0 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, W.-J.; Tsao, L.-C.; Yen, H.-H.; Yang, C.-W.; Lin, J.; Lin, K.-H. Endoscopic Resection for Gastric Subepithelial Tumor with Backup Laparoscopic Surgery: Description of a Single-Center Experience. J. Clin. Med. 2021, 10, 4423. https://doi.org/10.3390/jcm10194423
Chang W-J, Tsao L-C, Yen H-H, Yang C-W, Lin J, Lin K-H. Endoscopic Resection for Gastric Subepithelial Tumor with Backup Laparoscopic Surgery: Description of a Single-Center Experience. Journal of Clinical Medicine. 2021; 10(19):4423. https://doi.org/10.3390/jcm10194423
Chicago/Turabian StyleChang, Wei-Jung, Lien-Cheng Tsao, Hsu-Heng Yen, Chia-Wei Yang, Joseph Lin, and Kuo-Hua Lin. 2021. "Endoscopic Resection for Gastric Subepithelial Tumor with Backup Laparoscopic Surgery: Description of a Single-Center Experience" Journal of Clinical Medicine 10, no. 19: 4423. https://doi.org/10.3390/jcm10194423
APA StyleChang, W.-J., Tsao, L.-C., Yen, H.-H., Yang, C.-W., Lin, J., & Lin, K.-H. (2021). Endoscopic Resection for Gastric Subepithelial Tumor with Backup Laparoscopic Surgery: Description of a Single-Center Experience. Journal of Clinical Medicine, 10(19), 4423. https://doi.org/10.3390/jcm10194423






