Hypoalgesic and Motor Effects of Neural Mobilisation versus Soft-Tissue Interventions in Experimental Craniofacial Hyperalgesia: A Single-Blinded Randomised Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
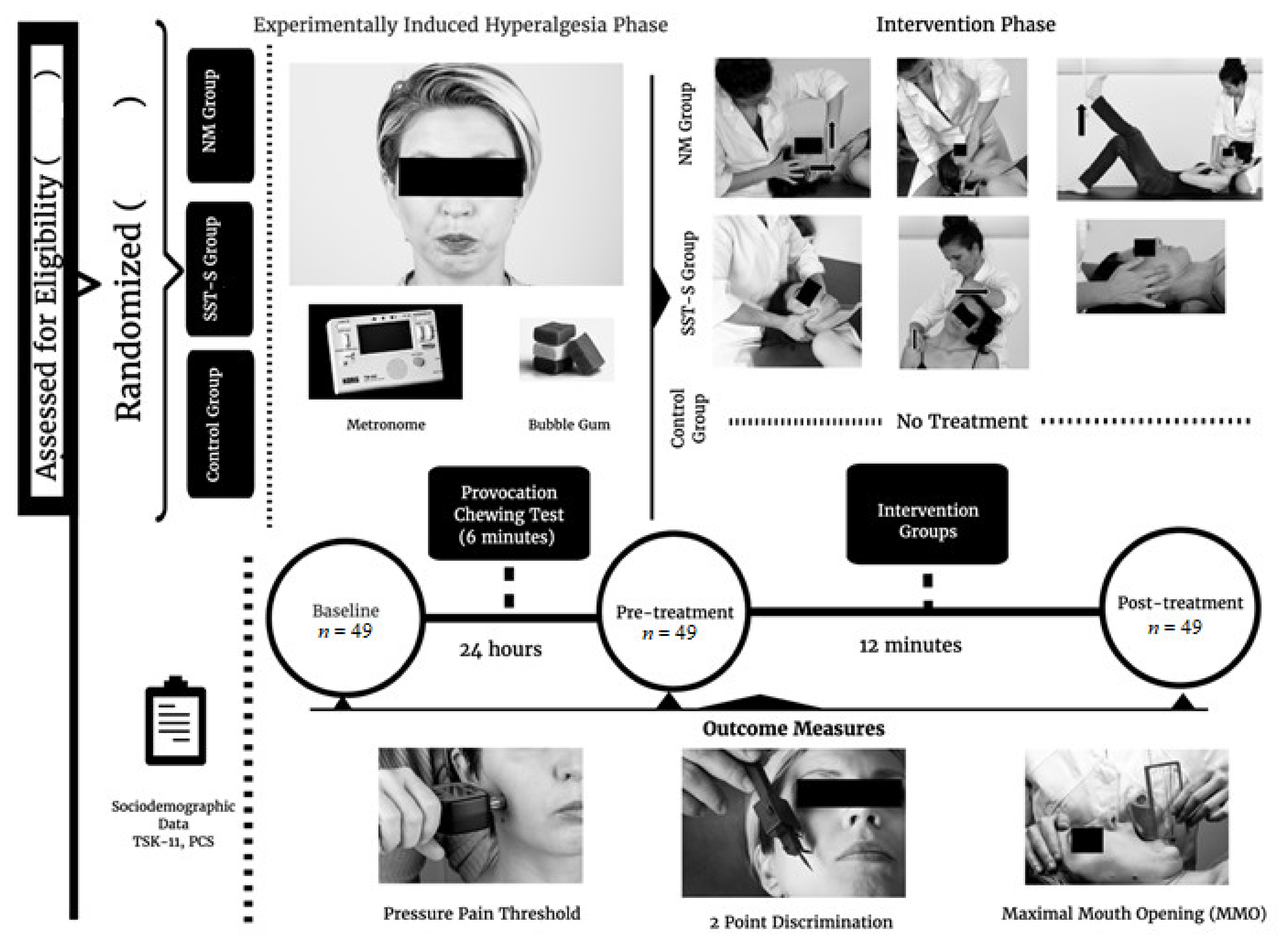
2.1. Study Design
2.2. Randomisation and Blinding
2.3. Selection and Description of Participants
2.4. Interventions
2.4.1. Neural Mobilisation Group
2.4.2. Soft-Tissue Techniques and Stretching Group
2.4.3. Control Group
2.5. Outcomes and Measurements
2.5.1. Primary Outcome Measurements
2.5.2. Secondary Outcome Measurements
2.5.3. Psychological Variables
2.6. Provocation Chewing Test
2.7. Procedure
2.8. Sample Size Calculation
2.9. Statistical Analysis
3. Results
3.1. Demographic and Clinical Data on Participants
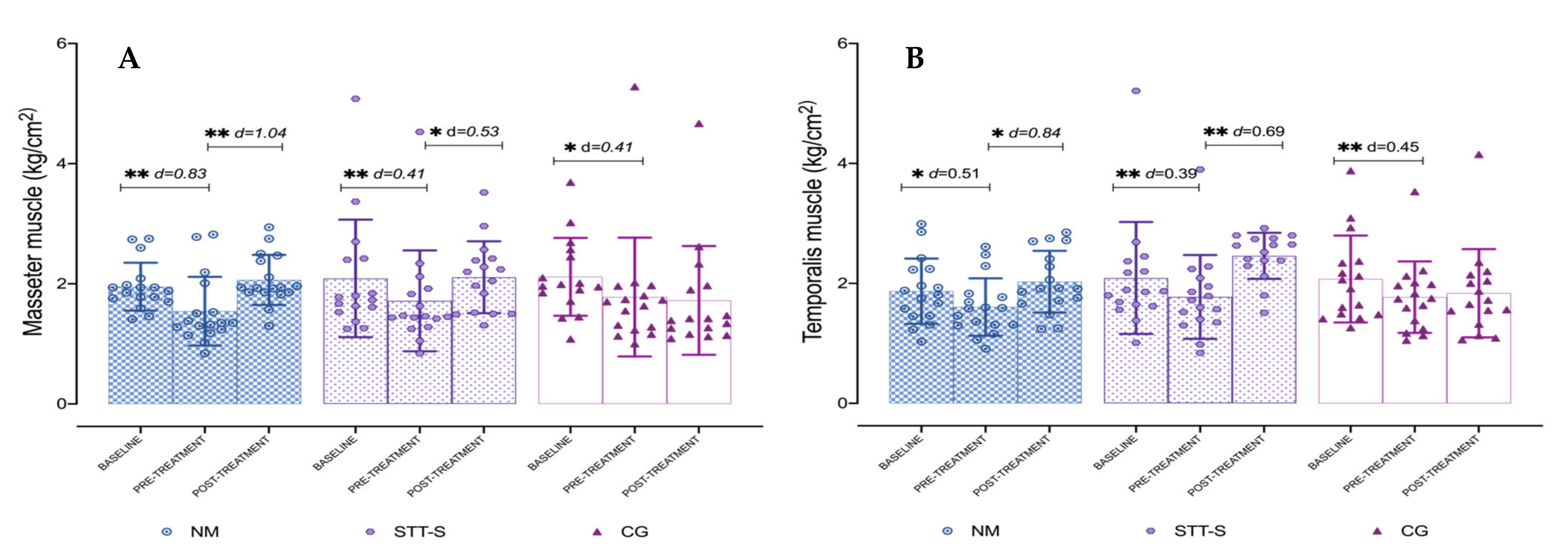
3.2. Pressure Pain Thresholds over Craniofacial and Cervical Muscles
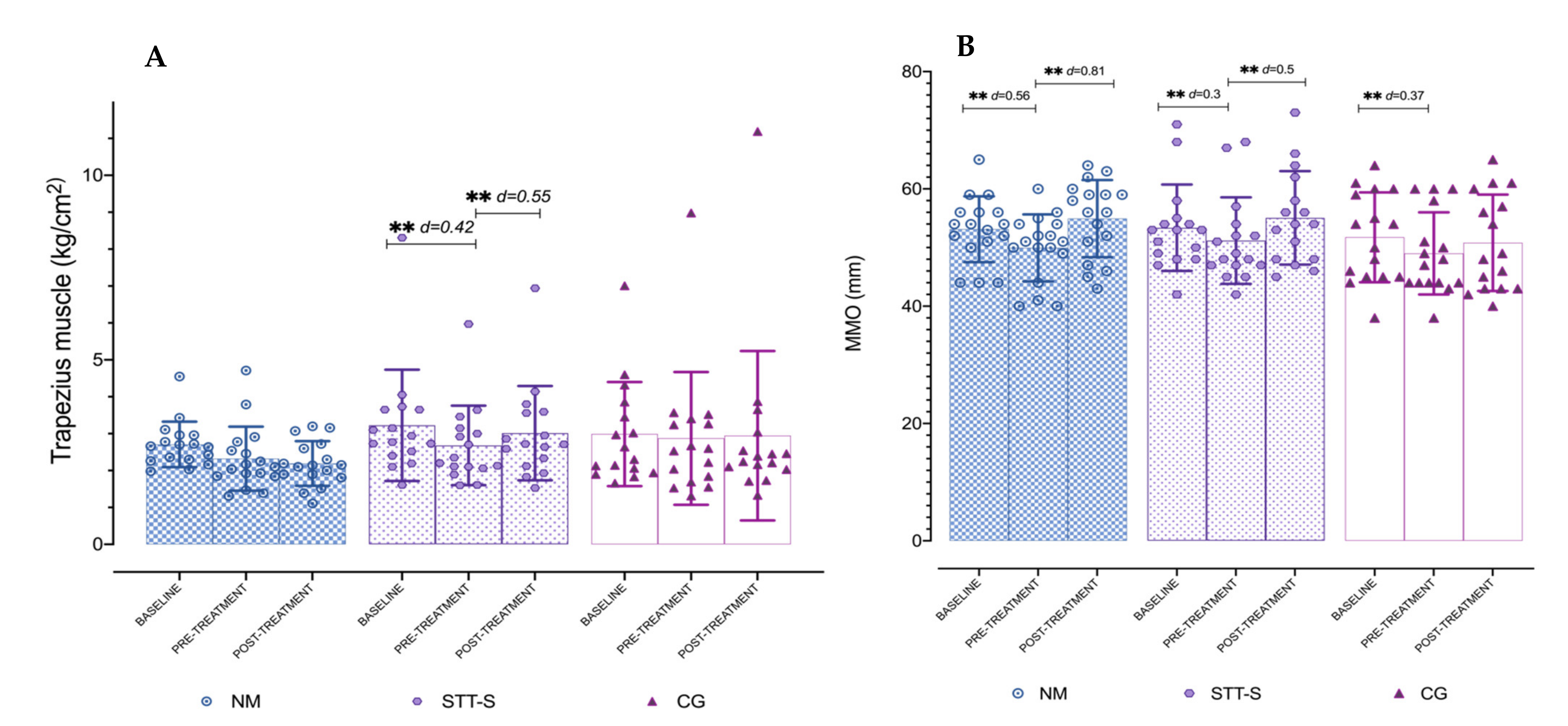
3.3. Maximum Mouth Opening
3.4. Two-Point Discrimination over Trigeminal and Cervical Areas
4. Discussion
4.1. Limitations
4.2. Clinical Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CG | control group |
| CI | confidence interval |
| DN | dry needling |
| DOMS | delayed onset muscle soreness |
| IC | ischemic compression |
| LMTrPs | latent myofascial trigger points |
| MDC | minimal detectable change |
| MMO | maximum mouth opening |
| MTrPs | myofascial trigger points |
| NM | neural mobilisation |
| NS | nervous system |
| OMPT | orthopaedic manual therapy |
| PCS | pain catastrophising scale |
| PPT | pressure pain threshold |
| ROM | range of motion |
| SD | standard deviation |
| STT | soft-tissue technique |
| STT-S | soft-tissues techniques and stretching |
| TPD | two-point discrimination |
| TSK-11 | Tampa scale for kinesiophobia |
| US | ultrasound |
Appendix A. Summary of Techniques for Neural Mobilisation Group
| Neural Thoracic Mobilisation with Wedge | ||
| Description | Illustration | |
| Patient position | Supine, with crossed legs, the right leg on top, and the left leg with knee at 90° of flexion and foot flat on the table. A wedge is placed under the upper thoracic spine. | 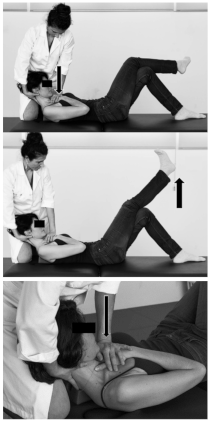 |
| Therapist position | Stands on one side of the table next to the patient. | |
| Mobilisation technique | One hand holds the patient’s occiput and maintains a craniocervical and cervical flexion, and the other hand placed over the patient’s sternum. The mobilisation is performed alternating the following positions: (1) Pressure over the sternum, performing craniocervical and neck flexion, with flexed right knee; (2) Straighten the knee, releasing pressure over sternum. Technique performed for about 4 min. | |
| Trigeminocervical Neural Mobilisation with Wedge | ||
| Description | Illustration | |
| Patient position | Supine, with bent hips and knees. The wedge is placed under the middle cervical region. |  |
| Therapist position | Stands on one side of the table next to the patient. | |
| Mobilisation technique | One hand placed at the occiput and the other hand with the web space against the maxilla. The mobilisation has two alternating positions: (1) Neck flexion, craniocervical extension, and mouth opening; (2) Neutral neck position, craniocervical flexion and mouth closed. Technique performed for about 4 min. | |
| Auriculotemporal Neural Mobilisation | ||
| Description | Illustration | |
| Patient position | Supine on the table. | 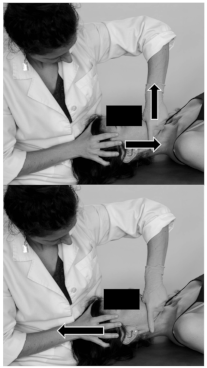 |
| Therapist position | Situated at the opposite side of the joint to mobilise. | |
| Mobilisation technique | The cranial hand located at the patient’s forehead stabilising the head and with the fingers placed over the anterior temporal muscle. The other hand with the thumb placed over the first and second molars, and the second finger reaching the mandibular angle, and the other fingers gently folded around the body of the mandible. Alternating positions to perform the mobilisation: (1) First a distraction of the joint followed by an anterior glide; (2) Rest position of the joint and a cranial traction of the soft tissue over the temporal muscle with the hand placed over it. Technique performed for about 4 min. | |
Appendix B. Summary of Techniques for Soft-Tissue Techniques and Stretching Group
| Intermittent Compression in Latent Trigger Points | ||
| Description | Illustration | |
| Patient position | Supine on the table. | 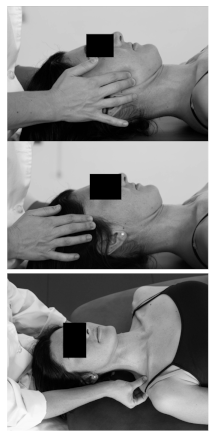 |
| Therapist position | Stands at the head of the table. | |
| Soft-tissue technique | Therapist performs an intermittent compression over the masseter, anterior temporalis and upper trapezius muscles of the right side. The therapist performs 5 cycles of 5 s compression and relaxation of 5 s. | |
| Dynamic Soft-Tissue Mobilisation of Masseter Muscle | ||
| Description | Illustration | |
| Patient position | Supine on the table. | 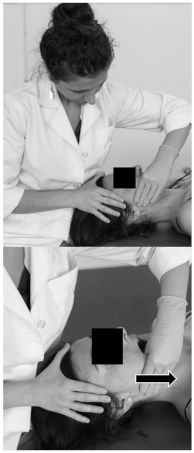 |
| Therapist position | Situated at the opposite side to treat. | |
| Soft-tissue technique | One hand placed over the frontal bone stabilising the head, the other hand placed with a pinch grip over the masseter muscle. A caudal glide movement is performed over the masseter fibres while the patient performs an active mouth opening. The technique is performed for 3.5 min, on the right side. | |
| Dynamic Soft-Tissue Mobilisation of CERVICO-Craniomandibular Muscle | ||
| Description | Illustration | |
| Patient position | Supine on the table. |  |
| Therapist position | Situated at the head of the table. | |
| Soft-tissue technique | Both hands are placed with the thenar eminence and thumb above the mandibular branch and masseter to perform a posterior and caudal soft-tissue mobilisation, while the other fingers are placed backwards reaching the occiput performing an anterior craniocervical roll at the same time. The technique is performed for 3.5 min. | |
| Passive Stretching | ||
| Description | Illustration | |
| Patient position | Sitting in a chair with backrest and feet flat on the floor. |  |
| Therapist position | The therapist stands behind the chair. | |
| Stretching technique | Upper trapezius and scalene: the therapist stretches down towards muscle insertion with the thenar of one hand while using the other grip to bend the head and cervical spine to the opposite side (left). Levator scapulae: the therapist rotates left and lateral flexes facet joints at level C1–4 to the same side by moving the head forward while with the other hand presses the shoulder. The therapist stretches for 30 s each muscle and repeats this technique two times. | |
References
- Lucas, K.R.; Rich, P.A.; Polus, B.I. How Common Are Latent Myofascial Trigger Points in the Scapular Positioning Muscles? J. Musculoskelet. Pain 2008, 16, 279–286. [Google Scholar] [CrossRef]
- Ge, H.-Y.; Arendt-Nielsen, L. Latent Myofascial Trigger Points. Curr. Pain Headache Rep. 2011, 15, 386–392. [Google Scholar] [CrossRef]
- Li, L.-T.; Ge, H.-Y.; Yue, S.-W.; Arendt-Nielsen, L. Nociceptive and Non-nociceptive Hypersensitivity at Latent Myofascial Trigger Points. Clin. J. Pain 2009, 25, 132–137. [Google Scholar] [CrossRef]
- Celik, D.; Yeldan, I. The relationship between latent trigger point and muscle strength in healthy subjects: A double-blind study. J. Back Musculoskelet. Rehabil. 2011, 24, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.-Y.; Monterde, S.; Graven-Nielsen, T.; Arendt-Nielsen, L. Latent My-ofascial Trigger Points Are Associated with an Increased Intramuscular Electromyographic Activity during Synergistic Muscle Activation. J. Pain 2014, 15, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Simons, D.G. Review of enigmatic MTrPs as a common cause of enigmatic musculoskeletal pain and dysfunction. J. Electromyogr. Kinesiol. 2004, 14, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Trampas, A.; Kitsios, A.; Sykaras, E.; Symeonidis, S.; Lazarou, L. Clinical massage and modified Proprioceptive Neuromuscular Facilitation stretching in males with latent myofascial trigger points. Phys. Ther. Sport 2010, 11, 91–98. [Google Scholar] [CrossRef]
- Aguilera, F.J.M.; Pecos-Martin, D.; Masanet, R.A.; Botella, A.C.; Soler, L.B.; Morell, F.B. Immediate Effect of Ultrasound and Ischemic Compression Techniques for the Treatment of Trapezius Latent Myofascial Trigger Points in Healthy Subjects: A Randomized Controlled Study. J. Manip. Physiol. Ther. 2009, 32, 515–520. [Google Scholar] [CrossRef]
- Grieve, R.; Clark, J.; Pearson, E.; Bullock, S.; Boyer, C.; Jarrett, A. The immediate effect of soleus trigger point pressure release on restricted ankle joint dorsiflexion: A pilot randomised controlled trial. J. Bodyw. Mov. Ther. 2011, 15, 42–49. [Google Scholar] [CrossRef]
- Simons, D.G.; Travell, J. Myofascial Pain and Dysfunction. The Trigger Point Manual; Williams & Wilkins: Baltimore, ML, USA, 1999; Volume 1, ISBN 0683083635. [Google Scholar]
- Ge, H.-Y.; Arendt-Nielsen, L.; Madeleine, P. Accelerated Muscle Fatigability of Latent Myofascial Trigger Points in Humans. Pain Med. 2012, 13, 957–964. [Google Scholar] [CrossRef]
- Ge, H.-Y.; Zhang, Y.; Boudreau, S.; Yue, S.-W.; Arendt-Nielsen, L. Induction of muscle cramps by nociceptive stimulation of latent myofascial trigger points. Exp. Brain Res. 2008, 187, 623–629. [Google Scholar] [CrossRef]
- Xu, Y.-M.; Ge, H.-Y.; Arendt-Nielsen, L. Sustained Nociceptive Mechanical Stimulation of Latent Myofascial Trigger Point Induces Central Sensitization in Healthy Subjects. J. Pain 2010, 11, 1348–1355. [Google Scholar] [CrossRef]
- Lucas, K.R.; Rich, P.A.; Polus, B.I. Muscle activation patterns in the scapular positioning muscles during loaded scapular plane elevation: The effects of Latent Myofascial Trigger Points. Clin. Biomech. 2010, 25, 765–770. [Google Scholar] [CrossRef]
- Hong, C.-Z.; Simons, D.G. Pathophysiologic and electrophysiologic mechanisms of myofascial trigger points. Arch. Phys. Med. Rehabil. 1998, 79, 863–872. [Google Scholar] [CrossRef]
- Celik, D.; Mutlu, E.K. Clinical Implication of Latent Myofascial Trigger Point. Curr. Pain Headache Rep. 2013, 17, 353. [Google Scholar] [CrossRef] [PubMed]
- De la Llave, A.I.; Alonso-Blanco, M.C.; Gil-Crujera, A.; Quesada, S.A.; Svensson, P.; Fernández-De-Las-Peñas, C. Myofascial Trigger Points in the Masticatory Muscles in Patients with and without Chronic Mechanical Neck Pain. J. Manip. Physiol. Ther. 2012, 35, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Campelo, N.M.; Rubens-Rebelatto, J.; Martín-Vallejo, F.J.; Alburquerque-Sendín, F.; Fernádez-De-Las-Peñas, C. The Immediate Effects of Atlanto-occipital Joint Manipulation and Suboccipital Muscle Inhibition Technique on Active Mouth Opening and Pressure Pain Sensitivity Over Latent Myofascial Trigger Points in the Masticatory Muscles. J. Orthop. Sports Phys. Ther. 2010, 40, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Kojidi, M.M.; Okhovatian, F.; Rahimi, A.; Baghban, A.A.; Azimi, H. Comparison Between the Effects of Passive and Active Soft Tissue Therapies on Latent Trigger Points of Upper Trapezius Muscle in Women: Single-Blind, Randomized Clinical Trial. J. Chiropr. Med. 2016, 15, 235–242. [Google Scholar] [CrossRef]
- Coppieters, M.W.; Hough, A.D.; Dilley, A. Different Nerve-Gliding Exercises Induce Different Magnitudes of Median Nerve Longitudinal Excursion: An In Vivo Study Using Dynamic Ultrasound Imaging. J. Orthop. Sports Phys. Ther. 2009, 39, 164–171. [Google Scholar] [CrossRef]
- Schmid, A.; Elliott, J.M.; Strudwick, M.W.; Little, M.; Coppieters, M.W. Effect of splinting and exercise on intraneural edema of the median nerve in carpal tunnel syndrome-an MRI study to reveal therapeutic mechanisms. J. Orthop. Res. 2012, 30, 1343–1350. [Google Scholar] [CrossRef]
- Boyd, B.S.; Wanek, L.; Gray, A.T.; Topp, K.S. Mechanosensitivity of the Lower Extremity Nervous System During Straight-Leg Raise Neurodynamic Testing in Healthy Individuals. J. Orthop. Sports Phys. Ther. 2009, 39, 780–790. [Google Scholar] [CrossRef] [PubMed]
- Pesonen, J.; Shacklock, M.; Rantanen, P.; Mäki, J.; Karttunen, L.; Kankaanpää, M.; Airaksinen, O.; Rade, M. Extending the straight leg raise test for improved clinical evaluation of sciatica: Reliability of hip internal rotation or ankle dorsiflexion. BMC Musculoskelet. Disord. 2021, 22, 1–8. [Google Scholar] [CrossRef]
- Coppieters, M.W.; Butler, D.S. Do ‘sliders’ slide and ‘tensioners’ tension? An analysis of neurodynamic techniques and considerations regarding their application. Man. Ther. 2008, 13, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Bialosky, J.E.; Bishop, M.D.; Price, N.D.; Robinson, M.E.; Vincent, K.R.; George, S. A Randomized Sham-Controlled Trial of a Neurodynamic Technique in the Treatment of Carpal Tunnel Syndrome. J. Orthop. Sports Phys. Ther. 2009, 39, 709–723. [Google Scholar] [CrossRef]
- Aksoy, C.C.; Kurt, V.; Okur, I.; Taspınar, F.; Taspinar, B. The immediate effect of neurodynamic techniques on jumping performance: A randomised double-blind study. J. Back Musculoskelet. Rehabil. 2020, 33, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Shafique, S.; Ahmad, S.; Rehman, S. Effect of Mulligan spinal mobilization with arm movement along with neurodynamics and manual traction in cervical radiculopathy patients: A randomized controlled trial. J. Pak. Med. Assoc. 2019, 69, 1601–1604. [Google Scholar] [CrossRef] [PubMed]
- Wolny, T.; Saulicz, E.; Linek, P.; Shacklock, M.; Myśliwiec, A. Efficacy of Manual Therapy Including Neurodynamic Techniques for the Treatment of Carpal Tunnel Syndrome: A Randomized Controlled Trial. J. Manip. Physiol. Ther. 2017, 40, 263–272. [Google Scholar] [CrossRef]
- Beneciuk, J.M.; Bishop, M.D.; George, S. Effects of Upper Extremity Neural Mobilization on Thermal Pain Sensitivity: A Sham-Controlled Study in Asymptomatic Participants. J. Orthop. Sports Phys. Ther. 2009, 39, 428–438. [Google Scholar] [CrossRef]
- Satkunskiene, D.; Ardekani, M.M.Z.; Khair, R.M.; Kutraite, G.; Venckuniene, K.; Snieckus, A.; Kamandulis, S. Effects of Warm-up on Hamstring Stiffness, Stress–Relaxation, Flexibility and Knee Proprioception in Young Soccer Players. J. Athl. Train. 2021. [Google Scholar] [CrossRef]
- Armijo-Olivo, S.; Pitance, L.; Singh, V.; Neto, F.; Thie, N.; Michelotti, A. Effectiveness of Manual Therapy and Therapeutic Exercise for Temporomandibular Disorders: Systematic Review and Meta-Analysis. Phys. Ther. 2016, 96, 9–25. [Google Scholar] [CrossRef]
- Ellis, R.F.; Hing, W. Neural Mobilization: A Systematic Review of Randomized Controlled Trials with an Analysis of Therapeutic Efficacy. J. Man. Manip. Ther. 2008, 16, 8–22. [Google Scholar] [CrossRef] [PubMed]
- La Touche, R.; Paris-Alemany, A.; Gil-Martínez, A.; Pardo-Montero, J.; Angulo-Díaz-Parreño, S.; Fernández-Carnero, J. Masticatory sensory-motor changes after an experimental chewing test influenced by pain catastrophizing and neck-pain-related disability in patients with headache attributed to temporomandibular disorders. J. Headache Pain 2015, 16, 1–14. [Google Scholar] [CrossRef]
- Conti, P.C.R.; Silva, R.D.S.; Araujo, C.D.R.P.D.; Rosseti, L.M.N.; Yassuda, S.; da Silva, R.O.F.; Pegoraro, L.F. Effect of experimental chewing on masticatory muscle pain onset. J. Appl. Oral Sci. 2011, 19, 34–40. [Google Scholar] [CrossRef][Green Version]
- Koutris, M.; Türker, K.S.; Lobbezoo, F.; Van Selms, M.K.A. Delayed-onset muscle soreness in human masticatory muscles increases inhibitory jaw reflex responses. J. Oral Rehabil. 2018, 45, 430–435. [Google Scholar] [CrossRef]
- Dawson, A.; List, T.; Ernberg, M.; Svensson, P. Assessment of proprioceptive allodynia after tooth-clenching exercises. J. Orofac. Pain 2012, 26, 39–48. [Google Scholar]
- Dawson, A. Experimental tooth clenching. A model for studying mechanisms of muscle pain. Swed. Dent. J. Suppl. 2013, 228, 9–94. [Google Scholar]
- Moher, D.; Schulz, K.F.; Altman, D.G.; CONSORT GROUP (Consolidated Standards of Reporting Trials). The CONSORT Statement: Revised Recommendations for Improving the Quality of Reports of Parallel-Group Randomized Trials. Ann. Intern. Med. 2001, 134, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Maquet, D.; Croisier, J.-L.; Demoulin, C.; Crielaard, J.-M. Pressure pain thresholds of tender point sites in patients with fibromyalgia and in healthy controls. Eur. J. Pain 2004, 8, 111–117. [Google Scholar] [CrossRef]
- Kinser, A.M.; A Sands, W.; Stone, M.H. Reliability and Validity of a Pressure Algometer. J. Strength Cond. Res. 2009, 23, 312–314. [Google Scholar] [CrossRef]
- Walton, D.M.; MacDermid, J.C.; Nielson, W.; Teasell, R.W.; Chiasson, M.; Brown, L. Reliability, Standard Error, and Minimum Detectable Change of Clinical Pressure Pain Threshold Testing in People with and without Acute Neck Pain. J. Orthop. Sports Phys. Ther. 2011, 41, 644–650. [Google Scholar] [CrossRef]
- La Touche, R.; París-Alemany, A.; von Piekartz, H.; Mannheimer, J.S.; Fernández-Carnero, J.; Rocabado, M. The Influence of Cranio-cervical Posture on Maximal Mouth Opening and Pressure Pain Threshold in Patients with Myofascial Temporomandibular Pain Disorders. Clin. J. Pain 2011, 27, 48–55. [Google Scholar] [CrossRef]
- Beltran-Alacreu, H.; López-De-Uralde-Villanueva, I.; Calvo-Lobo, C.; Fernandez-Carnero, J.; La Touche, R. Clinical features of patients with chronic non-specific neck pain per disability level: A novel observational study. Rev. Assoc. Médica Bras. 2018, 64, 700–709. [Google Scholar] [CrossRef]
- Chesterton, L.S.; Sim, J.; Wright, C.C.; Foster, N.E. Interrater Reliability of Algometry in Measuring Pressure Pain Thresholds in Healthy Humans, Using Multiple Raters. Clin. J. Pain 2007, 23, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Beltran-Alacreu, H.; López-De-Uralde-Villanueva, I.; Paris-Alemany, A.; Angulo-Díaz-Parreño, S.; La Touche, R. Intra-rater and Inter-rater Reliability of Mandibular Range of Motion Measures Considering a Neutral Craniocervical Position. J. Phys. Ther. Sci. 2014, 26, 915–920. [Google Scholar] [CrossRef] [PubMed][Green Version]
- La Touche, R.; Cuenca-Martínez, F.; Suso-Martí, L.; García-Vicente, A.; Navarro-Morales, B.; Paris-Alemany, A. Tactile trigeminal region acuity in temporomandibular disorders: A reliability and cross-sectional study. J. Oral Rehabil. 2019, 47, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Catley, M.; Tabor, A.; Wand, B.M.; Moseley, L. Assessing tactile acuity in rheumatology and musculoskeletal medicine—How reliable are two-point discrimination tests at the neck, hand, back and foot? Rheumatology 2013, 52, 1454–1461. [Google Scholar] [CrossRef]
- Martorella, G.; Côté, J.; Choinière, M. Pain catastrophizing: A dimensional concept analysis. J. Adv. Nurs. 2008, 63, 417–426. [Google Scholar] [CrossRef]
- García Campayo, J.; Rodero, B.; Alda, M.; Sobradiel, N.; Montero, J.; Moreno, S. Validation of the Spanish Version of the Pain Catastrophizing Scale in Fibromyalgia. Med. Clínica 2008, 131, 487–492. [Google Scholar] [CrossRef]
- Nijs, J.; Vanherberghen, K.; Duquet, W.; De Meirleir, K. Chronic Fatigue Syndrome: Lack of Association between Pain-Related Fear of Movement and Exercise Capacity and Disability. Phys. Ther. 2004, 84, 696–705. [Google Scholar] [CrossRef][Green Version]
- Gómez-Pérez, L.; López-Martínez, A.E.; Ruiz-Párraga, G.T. Psychometric Properties of the Spanish Version of the Tampa Scale for Kinesiophobia (TSK). J. Pain 2011, 12, 425–435. [Google Scholar] [CrossRef]
- Karibe, H.; Goddard, G.; Gear, R. Sex differences in masticatory muscle pain after chewing. J. Dent. Res. 2003, 82, 112–116. [Google Scholar] [CrossRef]
- Farella, M.; Bakke, M.; Michelotti, A.; Martina, R. Effects of prolonged gum chewing on pain and fatigue in human jaw muscles. Eur. J. Oral Sci. 2001, 109, 81–85. [Google Scholar] [CrossRef]
- Bennett, R. Myofascial pain syndromes and their evaluation. Best Pract. Res. Clin. Rheumatol. 2007, 21, 427–445. [Google Scholar] [CrossRef]
- Cohen, J. Eta-Squared and Partial Eta-Squared in Fixed Factor Anova Designs. Educ. Psychol. Meas. 1973, 33, 107–112. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988; p. 567. [Google Scholar]
- Binderup, A.T.; Arendt-Nielsen, L.; Madeleine, P. Pressure pain threshold mapping of the trapezius muscle reveals heterogeneity in the distribution of muscular hyperalgesia after eccentric exercise. Eur. J. Pain 2010, 14, 705–712. [Google Scholar] [CrossRef]
- Sonkodi, B.; Berkes, I.; Koltai, E. Have We Looked in the Wrong Direction for More Than 100 Years? Delayed Onset Muscle Soreness Is, in Fact, Neural Microdamage Rather Than Muscle Damage. Antioxidants 2020, 9, 212. [Google Scholar] [CrossRef]
- Ruiz-Sáez, M.; Fernández-De-Las-Peñas, C.; Blanco, C.R.; Martínez-Segura, R.; García-León, R. Changes in Pressure Pain Sensitivity in Latent Myofascial Trigger Points in the Upper Trapezius Muscle After a Cervical Spine Manipulation in Pain-Free Subjects. J. Manip. Physiol. Ther. 2007, 30, 578–583. [Google Scholar] [CrossRef]
- Sarrafzadeh, J.; Ahmadi, A.; Yassin, M. The Effects of Pressure Release, Phonophoresis of Hydrocortisone, and Ultrasound on Upper Trapezius Latent Myofascial Trigger Point. Arch. Phys. Med. Rehabil. 2012, 93, 72–77. [Google Scholar] [CrossRef]
- Cagnie, B.; Dewitte, V.; Coppieters, I.; Van Oosterwijck, J.; Cools, A.; Danneels, L. Effect of Ischemic Compression on Trigger Points in the Neck and Shoulder Muscles in Office Workers: A Cohort Study. J. Manip. Physiol. Ther. 2013, 36, 482–489. [Google Scholar] [CrossRef]
- Campa-Moran, I.; Rey-Gudin, E.; Fernandez-Carnero, J.; Paris-Alemany, A.; Gil-Martínez, A.; Lara, S.L.; Prieto-Baquero, A.; Alonso-Perez, J.L.; La Touche, R. Comparison of Dry Needling versus Orthopedic Manual Therapy in Patients with Myofascial Chronic Neck Pain: A Single-Blind, Randomized Pilot Study. Pain Res. Treat. 2015, 2015, 1–15. [Google Scholar] [CrossRef]
- Romero-Moraleda, B.; La Touche, R.; Lerma-Lara, S.; Ferrer-Peña, R.; Paredes, V.; Peinado, A.B.; Muñoz-García, D. Neurodynamic mobilization and foam rolling improved delayed-onset muscle soreness in a healthy adult population: A randomized controlled clinical trial. PeerJ 2017, 5, e3908. [Google Scholar] [CrossRef]
- Basson, A.; Olivier, B.; Ellis, R.; Coppieters, M.; Stewart, A.; Mudzi, W. The Effectiveness of Neural Mobilization for Neuromusculoskeletal Conditions: A Systematic Review and Meta-analysis. J. Orthop. Sports Phys. Ther. 2017, 47, 593–615. [Google Scholar] [CrossRef]
- Marcos-Martín, F.; González-Ferrero, L.; Martín-Alcocer, N.; Paris-Alemany, A.; La Touche, R. Multimodal physiotherapy treatment based on a biobehavioral approach for patients with chronic cervico-craniofacial pain: A prospective case series. Physiother. Theory Pract. 2018, 34, 671–681. [Google Scholar] [CrossRef]
- Kurt, V.; Aras, O.; Buker, N. Comparison of conservative treatment with and without neural mobilization for patients with low back pain: A prospective, randomized clinical trial. J. Back Musculoskelet. Rehabil. 2020, 33, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Ayub, A.; Osama, M.; Rehman, S.-U.; Ahmad, S. Effects of active versus passive upper extremity neural mobilization combined with mechanical traction and joint mobilization in females with cervical radiculopathy: A randomized controlled trial. J. Back Musculoskelet. Rehabil. 2019, 32, 725–730. [Google Scholar] [CrossRef]
- Bialosky, J.E.; Bishop, M.D.; Price, D.D.; Robinson, M.E.; George, S.Z. The mechanisms of manual therapy in the treatment of musculoskeletal pain: A comprehensive model. Man. Ther. 2009, 14, 531–538. [Google Scholar] [CrossRef]
- Brown, C.L.; Gilbert, K.K.; Brismee, J.-M.; Sizer, P.S.; James, C.R.; Smith, M.P. The effects of neurodynamic mobilization on fluid dispersion within the tibial nerve at the ankle: An unembalmed cadaveric study. J. Man. Manip. Ther. 2011, 19, 26–34. [Google Scholar] [CrossRef]
- Gilbert, K.K.; James, C.R.; Apte, G.; Brown, C.; Sizer, P.S.; Brismee, J.-M.; Smith, M.P. Effects of simulated neural mobilization on fluid movement in cadaveric peripheral nerve sections: Implications for the treatment of neuropathic pain and dysfunction. J. Man. Manip. Ther. 2014, 23, 219–225. [Google Scholar] [CrossRef]
- Boudier-Revéret, M.; Gilbert, K.; Allégue, D.R.; Moussadyk, M.; Brismée, J.; Sizer, P.; Feipel, V.; Dugailly, P.; Sobczak, S. Effect of neurodynamic mobilization on fluid dispersion in median nerve at the level of the carpal tunnel: A cadaveric study. Musculoskelet. Sci. Pract. 2017, 31, 45–51. [Google Scholar] [CrossRef]
- Costa, Y.M.; Araújo-Júnior, E.; Fiedler, L.S.; De Souza, P.; Silva, L.L.C.P.; Ferreira, D.M.A.O.; Conti, P.C.R.; Bonjardim, L.R.; De Araújo-Júnior, E.N.S.; Jara, P.R.S. Reproducibility of quantitative sensory testing applied to musculoskeletal orofacial region: Site and sex differences. Eur. J. Pain 2019, 23, 81–90. [Google Scholar] [CrossRef]
| Measure | NM (n = 17) | STT-S (n = 16) | CG (n = 16) | 95% CI | p Value |
|---|---|---|---|---|---|
| Age | 41.7 ± 15.0 | 34.9 ± 16.0 | 44.0 ± 3.5 | (34.75 to 43.37) | 0.39 |
| Gender | 0.16 | ||||
| 4 (23.5%) | 9 (56.3%) | 6 (37.5%) | ||
| 13 (76.5%) | 7 (43.8%) | 10 (62.5%) | ||
| Level of studies | 0.54 | ||||
| 1 (5.9%) | 0 (0.0%) | 0 (0.0%) | ||
| 0 (0.0%) | 1 (6.3%) | 0 (0.0%) | ||
| 2 (11.8%) | 1 (6.3%) | 3 (18.8%) | ||
| 14 (82.4%) | 14 (87.5%) | 13 (81.3%) | ||
| Marital status | 0.78 | ||||
| 6 (35.3%) | 8 (50.0%) | 4 (25.0%) | ||
| 8 (47.1%) | 4 (25.0%) | 8 (50.0%) | ||
| 0 (0.0%) | 1 (6.3%) | 1 (6.3%) | ||
| 1 (5.9%) | 2 (12.5%) | 1 (6.3%) | ||
| 2 (11.8%) | 1 (6.3%) | 2 (12.5%) | ||
| Employment situation | 0.19 | ||||
| 11 (64.7%) | 7 (43.8%) | 13 (81.3%) | ||
| 6 (35.3%) | 8 (50.0%) | 3 (18.8%) | ||
| 0 (0.0%) | 1 (6.3%) | 0 (0.0%) | ||
| PCS (0–54 points) | 9 (4, 13) | 6 (5, 18.5) | 7.5 (4, 13.75) | 0.88 | |
| TSK-11 (0–44 points) | 20 (18–25) | 26 (19.25–30.75) | 21.5 (17.25–27.75) | 0.35 |
| Measure | Groups | Mean ± SD | Mean Difference (95% CI); p -Value; Effect Size (d) | ||
|---|---|---|---|---|---|
| Baseline | Pre-Treatment | Post-Treatment | (a) Baseline vs. Pre-Treatment (b) Baseline vs. Post-Treatment (c) Pre-Treatment vs. Post-Treatment | ||
| PPT Masseter | NM | 1.95 ± 0.40 | 1.54 ± 0.57 | 2.06 ± 0.42 | (a) 0.41 (0.13 to 0.69); p = 0.002; d = 0.83 (b) −0.11 (−0.50 to 0.28); p > 0.05; d = 0.27 (c) −0.52 (−0.86 to −0.19); p = 0.01; d = 1.04 |
| STT-S | 2.09 ± 0.98 | 1.72 ± 0.84 | 2.11 ± 0.60 | (a) 0.37 (0.08 to 0.66); p = 0.008; d = 0.41 (b) −0.02 ( −0.42 to 0.38); p > 0.05; d = 0.02 (c) −0.39 (−0.74 to −0.05); p = 0.022; d = 0.53 | |
| CG | 2.12 ± 0.65 | 1.78 ± 0.99 | 1.72 ± 0.91 | (a) 0.34 (0.05 to 0.63); p = 0.018; d = 0.41 (b) 0.39 (−0.01 to 0.80); p > 0.05; d = 0.51 (c) 0.06 (−0.29 to 0.40); p > 0.05; d = 0.06 | |
| PPT Temporalis | NM | 1.87 ± 0.54 | 1.61 ± 0.48 | 2.03 ± 0.52 | (a) 0.26 (0.05 to 0.47); p = 0.01; d = 0.51 (b) −0.16 (−0.61 to 0.30); p > 0.05; d = 0.30 (c) −0.42 (−0.81 to −0.03); p = 0.029; d = 0.84 |
| STT-S | 2.09 ± 0.93 | 1.77 ± 0.70 | 2.16 ± 0.38 | (a) 0.32 (0.10 to 0.53); p = 0.002; d = 0.39 (b) −0.37 (−0.84 to 0.10); p > 0.05; d = 0.52 (c) −0.39 (−1.08 to −0.29); p < 0.001; d = 0.69 | |
| CG | 2.07 ± 0.72 | 1.77 ± 0.60 | 1.83 ± 0.74 | (a) 0.30 (0.09 to 0.52); p = 0.003; d = 0.45 (b) 0.24 (−0.23 to 0.71); p > 0.05; d = 0.33 (c) −0.07 (−0.46 to 0.33); p > 0.05; d = 0.09 | |
| Groups | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Measure | NM | STT-S | CG | ||||||
| % of Change | d | >MDC | % of Change | d | >MDC | % of Change | d | >MDC | |
| PPT Masseter | 33.8 | 1.04 | no | 22.8 | 0.53 | no | −0.01 | 0.06 | no |
| PPT Temporalis | 26.9 | 0.84 | 39 | 0.69 | 1.7 | 0.09 | |||
| PPT Trapezius | 9.9 | 0.36 | no | 23.1 | 0.55 | no | −6.9 | 0.09 | no |
| MMO | 10 | 0.81 | yes | 7.6 | 0.5 | yes | 3.7 | 0.23 | yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Sáez, M.; Sáenz-Jiménez, C.; Villafañe, J.H.; Paris-Alemany, A.; La Touche, R. Hypoalgesic and Motor Effects of Neural Mobilisation versus Soft-Tissue Interventions in Experimental Craniofacial Hyperalgesia: A Single-Blinded Randomised Controlled Trial. J. Clin. Med. 2021, 10, 4434. https://doi.org/10.3390/jcm10194434
Díaz-Sáez M, Sáenz-Jiménez C, Villafañe JH, Paris-Alemany A, La Touche R. Hypoalgesic and Motor Effects of Neural Mobilisation versus Soft-Tissue Interventions in Experimental Craniofacial Hyperalgesia: A Single-Blinded Randomised Controlled Trial. Journal of Clinical Medicine. 2021; 10(19):4434. https://doi.org/10.3390/jcm10194434
Chicago/Turabian StyleDíaz-Sáez, Marta, Cristina Sáenz-Jiménez, Jorge Hugo Villafañe, Alba Paris-Alemany, and Roy La Touche. 2021. "Hypoalgesic and Motor Effects of Neural Mobilisation versus Soft-Tissue Interventions in Experimental Craniofacial Hyperalgesia: A Single-Blinded Randomised Controlled Trial" Journal of Clinical Medicine 10, no. 19: 4434. https://doi.org/10.3390/jcm10194434
APA StyleDíaz-Sáez, M., Sáenz-Jiménez, C., Villafañe, J. H., Paris-Alemany, A., & La Touche, R. (2021). Hypoalgesic and Motor Effects of Neural Mobilisation versus Soft-Tissue Interventions in Experimental Craniofacial Hyperalgesia: A Single-Blinded Randomised Controlled Trial. Journal of Clinical Medicine, 10(19), 4434. https://doi.org/10.3390/jcm10194434








