Neutrophil-to-Lymphocyte Ratio as a Factor Predicting Radiotherapy Induced Oral Mucositis in Head Neck Cancer Patients Treated with Radiotherapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient and Clinical Data
2.2. NLR
2.3. RT
2.4. The Assessment of OM
2.5. Overall Survival
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
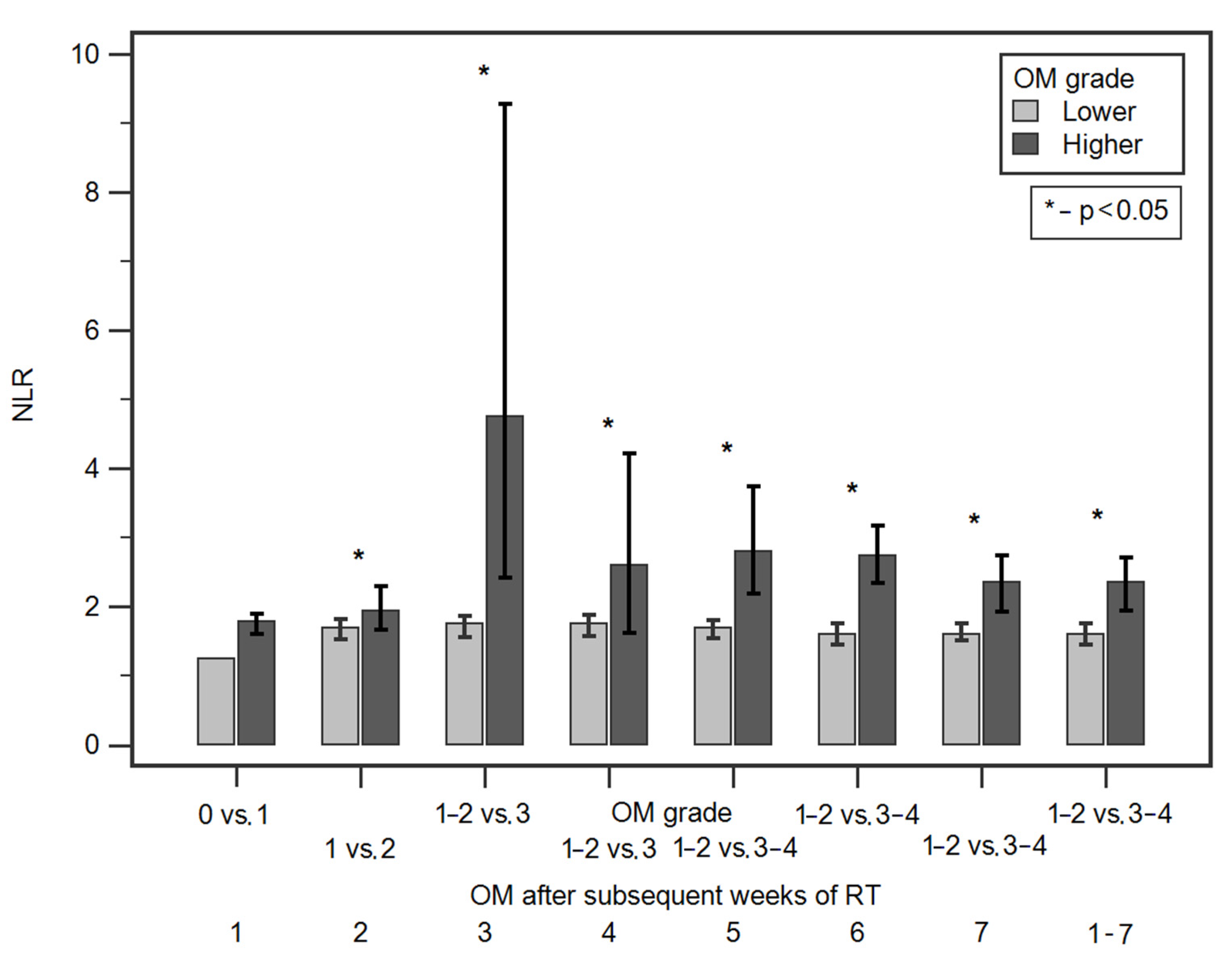
3.2. Comparison of NLR Values According to Demographic and Clinical Factors as Well as OM Grade after Subsequent Weeks of RT
3.3. Evaluation of Diagnostic Usefulness of NLR Values in Predicting the Occurrence of More Severe OM after Subsequent Weeks of RT (ROC Analysis)
3.4. Assessment of the Risk of More Severe OM after Subsequent Weeks of RT According to Demographic and Clinical Factors as Well as NLR Value
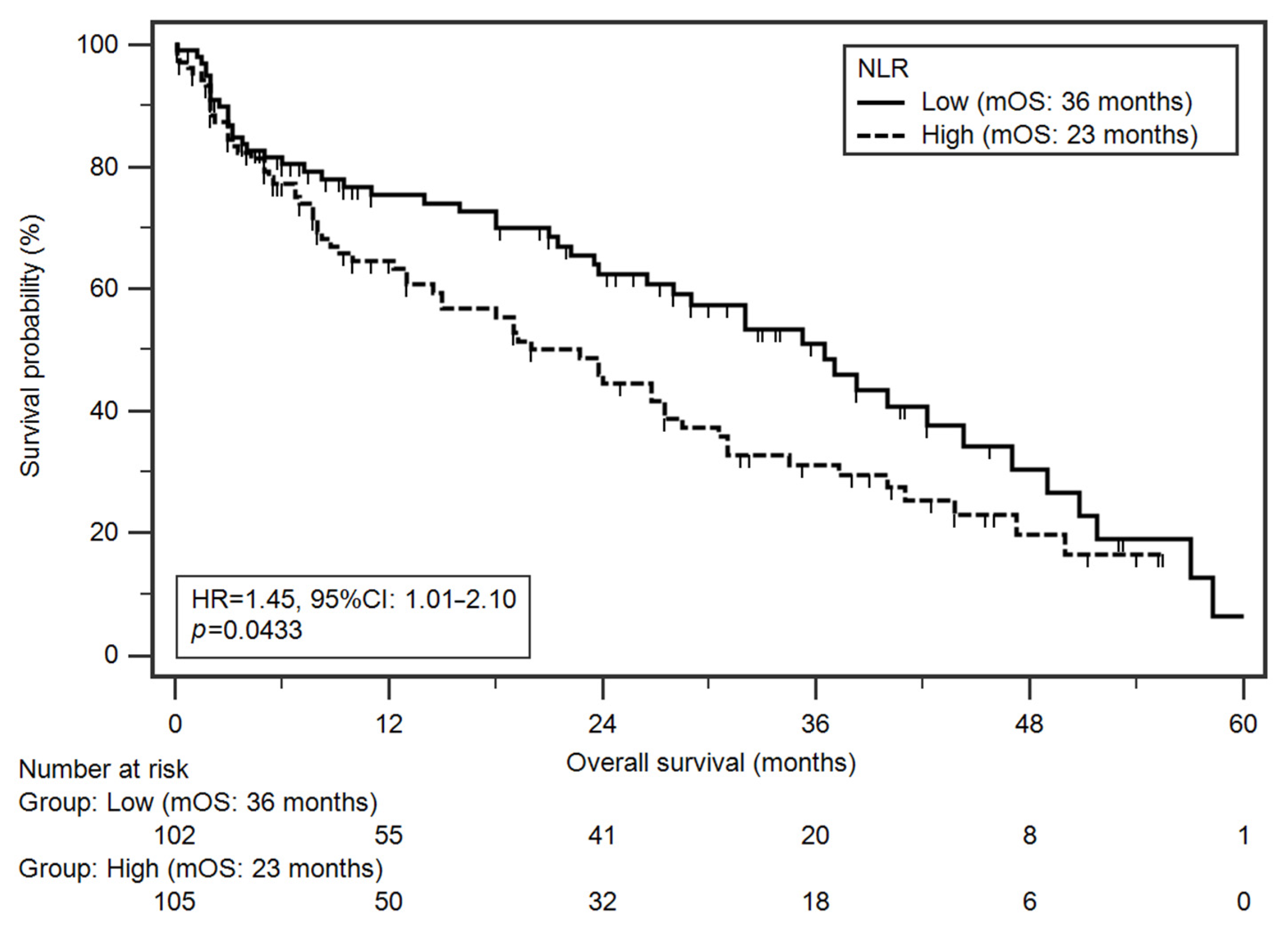
3.5. Survival Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [Green Version]
- Head and Neck Cancers Survival Statistics. Available online: http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/head-and-neck-cancers/survival#heading-Zero (accessed on 10 August 2021).
- Brockstein, B.; Haraf, D.J.; Rademaker, A.W.; Kies, M.S.; Stenson, K.M.; Rosen, F.; Mittal, B.B.; Pelzer, H.; Fung, B.B.; Witt, M.-E.; et al. Patterns of failure, prognostic factors and survival in locoregionally advanced head and neck cancer treated with concomitant chemoradiotherapy: A 9-year, 337-patient, multi-institutional experience. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2004, 15, 1179–1186. [Google Scholar] [CrossRef]
- Sonis, S.T. Oral mucositis in head and neck cancer: Risk, biology, and management. Am. Soc. Clin. Oncol. Educ. Book Am. Soc. Clin. Oncol. Annu. Meet. 2013. [Google Scholar] [CrossRef]
- Lalla, R.V.; Saunders, D.P.; Peterson, D.E. Chemotherapy or radiation-induced oral mucositis. Dent. Clin. N. Am. 2014, 58, 341–349. [Google Scholar] [CrossRef]
- Trotti, A.; Bellm, L.A.; Epstein, J.B.; Frame, D.; Fuchs, H.J.; Gwede, C.K.; Komaroff, E.; Nalysnyk, L.; Zilberberg, M.D. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: A systematic literature review. Radiother. Oncol. J. Eur. Soc. Radiol. Oncol. 2003, 66, 253–262. [Google Scholar] [CrossRef]
- Adelstein, D.J.; Li, Y.; Adams, G.L.; Wagner, H.J.; Kish, J.A.; Ensley, J.F.; Schuller, D.E.; Forastiere, A.A. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2003, 21, 92–98. [Google Scholar] [CrossRef] [Green Version]
- Langius, J.A.E.; van Dijk, A.M.; Doornaert, P.; Kruizenga, H.M.; Langendijk, J.A.; Leemans, C.R.; Weijs, P.J.M.; Verdonck-de Leeuw, I.M. More than 10% weight loss in head and neck cancer patients during radiotherapy is independently associated with deterioration in quality of life. Nutr. Cancer 2013, 65, 76–83. [Google Scholar] [CrossRef]
- Plevová, P. Prevention and treatment of chemotherapy- and radiotherapy-induced oral mucositis: A review. Oral Oncol. 1999, 35, 453–470. [Google Scholar] [CrossRef]
- Bishop, S.; Reed, W.M. The provision of enteral nutritional support during definitive chemoradiotherapy in head and neck cancer patients. J. Med. Radiat. Sci. 2015, 62, 267–276. [Google Scholar] [CrossRef]
- Deng, Z.; Kiyuna, A.; Hasegawa, M.; Nakasone, I.; Hosokawa, A.; Suzuki, M. Oral candidiasis in patients receiving radiation therapy for head and neck cancer. Otolaryngol. Neck Surg. Off. J. Am. Acad. Otolaryngol. Neck Surg. 2010, 143, 242–247. [Google Scholar] [CrossRef]
- Ruescher, T.J.; Sodeifi, A.; Scrivani, S.J.; Kaban, L.B.; Sonis, S.T. The impact of mucositis on alpha-hemolytic streptococcal infection in patients undergoing autologous bone marrow transplantation for hematologic malignancies. Cancer 1998, 82, 2275–2281. [Google Scholar] [CrossRef]
- Oronsky, B.; Goyal, S.; Kim, M.M.; Cabrales, P.; Lybeck, M.; Caroen, S.; Oronsky, N.; Burbano, E.; Carter, C.; Oronsky, A. A Review of Clinical Radioprotection and Chemoprotection for Oral Mucositis. Transl. Oncol. 2018, 11, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Sonis, S.T.; Elting, L.S.; Keefe, D.; Peterson, D.E.; Schubert, M.; Hauer-Jensen, M.; Bekele, B.N.; Raber-Durlacher, J.; Donnelly, J.P.; Rubenstein, E.B. Perspectives on cancer therapy-induced mucosal injury: Pathogenesis, measurement, epidemiology, and consequences for patients. Cancer 2004, 100, 1995–2025. [Google Scholar] [CrossRef] [PubMed]
- Sonis, S.T. Mucositis: The impact, biology and therapeutic opportunities of oral mucositis. Oral Oncol. 2009, 45, 1015–1020. [Google Scholar] [CrossRef] [PubMed]
- Fowler, J.F. Potential for increasing the differential response between tumors and normal tissues: Can proliferation rate be used? Int. J. Radiat. Oncol. Biol. Phys. 1986, 12, 641–645. [Google Scholar] [CrossRef]
- Withers, H.R.; Taylor, J.M.; Maciejewski, B. The hazard of accelerated tumor clonogen repopulation during radiotherapy. Acta Oncol. 1988, 27, 131–146. [Google Scholar] [CrossRef]
- Xiang, M.; Gensheimer, M.; Pollom, E.; Holsinger, C.; Colevas, A.; Le, Q.-T.; Beadle, B. Treatment Breaks During Definitive Head/Neck Radiotherapy: Survival Impact and Predisposing Factors. Int. J. Radiat. Oncol. 2020, 108, E39. [Google Scholar] [CrossRef]
- Gautam, A.P.; Fernandes, D.J.; Vidyasagar, M.S.; Maiya, A.G.; Vadhiraja, B.M. Low level laser therapy for concurrent chemoradiotherapy induced oral mucositis in head and neck cancer patients-a triple blinded randomized controlled trial. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2012, 104, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Tham, T.; Bardash, Y.; Herman, S.W.; Costantino, P.D. Neutrophil-to-lymphocyte ratio as a prognostic indicator in head and neck cancer: A systematic review and meta-analysis. Head Neck 2018, 40, 2546–2557. [Google Scholar] [CrossRef]
- Motomura, T.; Shirabe, K.; Mano, Y.; Muto, J.; Toshima, T.; Umemoto, Y.; Fukuhara, T.; Uchiyama, H.; Ikegami, T.; Yoshizumi, T.; et al. Neutrophil-lymphocyte ratio reflects hepatocellular carcinoma recurrence after liver transplantation via inflammatory microenvironment. J. Hepatol. 2013, 58, 58–64. [Google Scholar] [CrossRef]
- Kantola, T.; Klintrup, K.; Väyrynen, J.P.; Vornanen, J.; Bloigu, R.; Karhu, T.; Herzig, K.-H.; Näpänkangas, J.; Mäkelä, J.; Karttunen, T.J.; et al. Stage-dependent alterations of the serum cytokine pattern in colorectal carcinoma. Br. J. Cancer 2012, 107, 1729–1736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, M.; Jeong, C.W.; Kwak, C.; Kim, H.H.; Ku, J.H. The Prognostic Significance of the Early Postoperative Neutrophil-to-Lymphocyte Ratio in Patients with Urothelial Carcinoma of the Bladder Undergoing Radical Cystectomy. Ann. Surg. Oncol. 2016, 23, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Liu, Y.; Li, Q.; Li, Z.; Hou, H.; Wu, A. Pre-treatment neutrophil-to-lymphocyte ratio is associated with neutrophil and T-cell infiltration and predicts clinical outcome in patients with glioblastoma. BMC Cancer 2015, 15, 617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keizman, D.; Ish-Shalom, M.; Huang, P.; Eisenberger, M.A.; Pili, R.; Hammers, H.; Carducci, M.A. The association of pre-treatment neutrophil to lymphocyte ratio with response rate, progression free survival and overall survival of patients treated with sunitinib for metastatic renal cell carcinoma. Eur. J. Cancer 2012, 48, 202–208. [Google Scholar] [CrossRef] [Green Version]
- Cho, I.R.; Park, J.C.; Park, C.H.; Jo, J.H.; Lee, H.J.; Kim, S.; Shim, C.N.; Lee, H.; Shin, S.K.; Lee, S.K.; et al. Pre-treatment neutrophil to lymphocyte ratio as a prognostic marker to predict chemotherapeutic response and survival outcomes in metastatic advanced gastric cancer. Gastric Cancer Off. J. Int. Gastric Cancer Assoc. Jpn. Gastric Cancer Assoc. 2014, 17, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Yodying, H.; Matsuda, A.; Miyashita, M.; Matsumoto, S.; Sakurazawa, N.; Yamada, M.; Uchida, E. Prognostic Significance of Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio in Oncologic Outcomes of Esophageal Cancer: A Systematic Review and Meta-analysis. Ann. Surg. Oncol. 2016, 23, 646–654. [Google Scholar] [CrossRef]
- Keizman, D.; Gottfried, M.; Ish-Shalom, M.; Maimon, N.; Peer, A.; Neumann, A.; Rosenbaum, E.; Kovel, S.; Pili, R.; Sinibaldi, V.; et al. Pretreatment neutrophil-to-lymphocyte ratio in metastatic castration-resistant prostate cancer patients treated with ketoconazole: Association with outcome and predictive nomogram. Oncologist 2012, 17, 1508–1514. [Google Scholar] [CrossRef] [Green Version]
- Rosner, B. Fundamentals of Biostatistics; Thomson-Brooks/Cole: Belmont, CA, USA, 2006; ISBN 0534418201 9780534418205. [Google Scholar]
- Klein, J.P.; Moeschberger, M.L. Survival Analysis. Techniques for Censored and Truncated, 2nd ed.; Springer: New York, NY, USA, 2003; ISBN 978-0-387-95399-1. [Google Scholar]
- Lee, Y.H.; Choi, H.-S.; Jeong, H.; Kang, K.M.; Song, J.H.; Lee, W.S.; Lee, G.-W.; Song, H.-N.; Kim, H.-G.; Kang, M.H.; et al. Neutrophil-lymphocyte ratio and a dosimetric factor for predicting symptomatic radiation pneumonitis in non-small-cell lung cancer patients treated with concurrent chemoradiotherapy. Clin. Respir. J. 2018, 12, 1264–1273. [Google Scholar] [CrossRef]
- Masucci, M.T.; Minopoli, M.; Carriero, M.V. Tumor Associated Neutrophils. Their Role in Tumorigenesis, Metastasis, Prognosis and Therapy. Front. Oncol. 2019, 9, 1146. [Google Scholar] [CrossRef] [Green Version]
- Kowaliuk, M.; Bozsaky, E.; Gruber, S.; Kuess, P.; Dörr, W. Systemic administration of heparin ameliorates radiation-induced oral mucositis-preclinical studies in mice. Strahlenther. Onkol. 2018, 194, 686–692. [Google Scholar] [CrossRef] [Green Version]
- Gruber, S.; Schmidt, M.; Bozsaky, E.; Wolfram, K.; Haagen, J.; Habelt, B.; Puttrich, M.; Dörr, W. Modulation of radiation-induced oral mucositis by pentoxifylline: Preclinical studies. Strahlenther. Onkol. 2015, 191, 242–247. [Google Scholar] [CrossRef]
- Gruber, S.; Frings, K.; Kuess, P.; Dörr, W. Protective effects of systemic dermatan sulfate treatment in a preclinical model of radiation-induced oral mucositis. Strahlenther. Onkol. 2018, 194, 675–685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galdiero, M.R.; Varricchi, G.; Loffredo, S.; Mantovani, A.; Marone, G. Roles of neutrophils in cancer growth and progression. J. Leukoc. Biol. 2018, 103, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Jabłońska, E.; Kiluk, M.; Markiewicz, W.; Piotrowski, L.; Grabowska, Z.; Jabłoński, J. TNF-alpha, IL-6 and their soluble receptor serum levels and secretion by neutrophils in cancer patients. Arch. Immunol. Ther. Exp. 2001, 49, 63–69. [Google Scholar]
- Rawat, K.; Syeda, S.; Shrivastava, A. Neutrophil-derived granule cargoes: Paving the way for tumor growth and progression. Cancer Metastasis Rev. 2021, 40, 221–244. [Google Scholar] [CrossRef] [PubMed]
- Sonis, S.; Haddad, R.; Posner, M.; Watkins, B.; Fey, E.; Morgan, T.V.; Mookanamparambil, L.; Ramoni, M. Gene expression changes in peripheral blood cells provide insight into the biological mechanisms associated with regimen-related toxicities in patients being treated for head and neck cancers. Oral Oncol. 2007, 43, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Imtiaz, F.; Shafique, K.; Mirza, S.S.; Ayoob, Z.; Vart, P.; Rao, S. Neutrophil lymphocyte ratio as a measure of systemic inflammation in prevalent chronic diseases in Asian population. Int. Arch. Med. 2012, 5, 2. [Google Scholar] [CrossRef]
- Cupp, M.A.; Cariolou, M.; Tzoulaki, I.; Aune, D.; Evangelou, E.; Berlanga-Taylor, A.J. Neutrophil to lymphocyte ratio and cancer prognosis: An umbrella review of systematic reviews and meta-analyses of observational studies. BMC Med. 2020, 18, 360. [Google Scholar] [CrossRef]
- Günay, E.; Sarınç Ulaşlı, S.; Akar, O.; Ahsen, A.; Günay, S.; Koyuncu, T.; Unlü, M. Neutrophil-to-lymphocyte ratio in chronic obstructive pulmonary disease: A retrospective study. Inflammation 2014, 37, 374–380. [Google Scholar] [CrossRef]
- Kahramanca, S.; Ozgehan, G.; Seker, D.; Gökce, E.I.; Seker, G.; Tunç, G.; Küçükpınar, T.; Kargıcı, H. Neutrophil-to-lymphocyte ratio as a predictor of acute appendicitis. Ulus. Travma Acil Cerrahi Derg. Turk. J. Trauma Emerg. Surg. Tjtes 2014, 20, 19–22. [Google Scholar] [CrossRef]
- Kule, M.; Kara Polat, A.; Akın Belli, A.; Gökçen Kule, Z. Neutrophil to lymphocyte and platelet to lymphocyte ratios as an indicator of inflammation in patients with recurrent aphthous stomatitis. ENT Updates 2018, 8, 41–44. [Google Scholar] [CrossRef]
- Chung, K.F. Inflammatory mediators in chronic obstructive pulmonary disease. Curr. Drug Targets. Inflamm. Allergy 2005, 4, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.R.; Cook, E.J.; Goulder, F.; Justin, T.A.; Keeling, N.J. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J. Surg. Oncol. 2005, 91, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Ethier, J.-L.; Desautels, D.; Templeton, A.; Shah, P.S.; Amir, E. Prognostic role of neutrophil-to-lymphocyte ratio in breast cancer: A systematic review and meta-analysis. Breast Cancer Res. 2017, 19, 2. [Google Scholar] [CrossRef] [Green Version]
- Tazeh, N.N.; Canter, D.J.; Damodaran, S.; Rushmer, T.; Richards, K.A.; Abel, E.J.; Jarrard, D.F.; Downs, T.M. Neutrophil to Lymphocyte Ratio (NLR) at the Time of Transurethral Resection of Bladder Tumor: A Large Retrospective Study and Analysis of Racial Differences. Bladder Cancer 2017, 3, 89–94. [Google Scholar] [CrossRef] [Green Version]
- Templeton, A.J.; McNamara, M.G.; Šeruga, B.; Vera-Badillo, F.E.; Aneja, P.; Ocaña, A.; Leibowitz-Amit, R.; Sonpavde, G.; Knox, J.J.; Tran, B.; et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2014, 106, dju124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diakos, C.I.; Charles, K.A.; McMillan, D.C.; Clarke, S.J. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014, 15, e493–e503. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef]
- Bassani, B.; Baci, D.; Gallazzi, M.; Poggi, A.; Bruno, A.; Mortara, L. Natural Killer Cells as Key Players of Tumor Progression and Angiogenesis: Old and Novel Tools to Divert Their Pro-Tumor Activities into Potent Anti-Tumor Effects. Cancers 2019, 11, 461. [Google Scholar] [CrossRef] [Green Version]
- So, T.H.; Chan, S.K.; Chan, W.L.; Choi, H.; Chiang, C.L.; Lee, V.; Lam, T.C.; Wong, I.; Law, S.; Kwong, D.; et al. Lymphopenia and Radiation Dose to Circulating Lymphocytes With Neoadjuvant Chemoradiation in Esophageal Squamous Cell Carcinoma. Adv. Radiat. Oncol. 2020, 5, 880–888. [Google Scholar] [CrossRef]
- Ladbury, C.J.; Rusthoven, C.G.; Camidge, D.R.; Kavanagh, B.D.; Nath, S.K. Impact of Radiation Dose to the Host Immune System on Tumor Control and Survival for Stage III Non-Small Cell Lung Cancer Treated with Definitive Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 346–355. [Google Scholar] [CrossRef]
- Groutas, W.C.; Dou, D.; Alliston, K.R. Neutrophil elastase inhibitors. Expert Opin. Ther. Patents 2011, 21, 339–354. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, M.M.A.; El-Shimy, I.A.; Hadi, M.A. Neutrophil Elastase Inhibitors: A potential prophylactic treatment option for SARS-CoV-2-induced respiratory complications? Crit. Care 2020, 24, 311. [Google Scholar] [CrossRef]
- Jakimiuk, K.; Gesek, J.; Atanasov, A.G.; Tomczyk, M. Flavonoids as inhibitors of human neutrophil elastase. J. Enzym. Inhib. Med. Chem. 2021, 36, 1016–1028. [Google Scholar] [CrossRef] [PubMed]
- Cinausero, M.; Aprile, G.; Ermacora, P.; Basile, D.; Vitale, M.G.; Fanotto, V.; Parisi, G.; Calvetti, L.; Sonis, S.T. New Frontiers in the Pathobiology and Treatment of Cancer Regimen-Related Mucosal Injury. Front. Pharmacol. 2017, 8, 354. [Google Scholar] [CrossRef] [Green Version]
- Vera-Llonch, M.; Oster, G.; Hagiwara, M.; Sonis, S. Oral mucositis in patients undergoing radiation treatment for head and neck carcinoma. Cancer 2006, 106, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Adamsson Eryd, S.; Smith, J.G.; Melander, O.; Hedblad, B.; Engström, G. Incidence of coronary events and case fatality rate in relation to blood lymphocyte and neutrophil counts. Arter. Thromb. Vasc. Biol. 2012, 32, 533–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balta, S.; Cakar, M.; Demirkol, S.; Arslan, Z.; Akhan, M. Higher neutrophil to lymhocyte ratio in patients with metabolic syndrome. Clin. Appl. Thromb. Off. J. Int. Acad. Clin. Appl. Thromb. 2013, 19, 579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bese, N.S.; Hendry, J.; Jeremic, B. Effects of prolongation of overall treatment time due to unplanned interruptions during radiotherapy of different tumor sites and practical methods for compensation. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 654–661. [Google Scholar] [CrossRef]

| Variable | Study Group (n = 207) | ||
|---|---|---|---|
| Gender | Male | 191 (92.3%) | |
| Female | 16 (7.7%) | ||
| Age Median (range) | 62 (29–87) | ||
| Tumour location | Larynx | 90 (43.5%) | |
| Oropharynx or hypopharynx | 89 (43%) | ||
| Oral cavity | 28 (13.5%) | ||
| Histopathological diagnosis | Squamous cell carcinoma | 207 (100%) | |
| Grading | G1 | 39 (18.8%) | |
| G2 | 62 (30%) | ||
| G3 | 106 (51.2%) | ||
| TNM stage | III | 72(34.8%) | |
| IVA | 121 (58.4%) | ||
| IVB | 1 (0.5%) | ||
| IVC | 13 (6.3%) | ||
| Performance status | 1 | 142 (68.6%) | |
| 2 | 65 (31.4%) | ||
| Type of treatment | RT | Surgery + RT Surgry + RT-66 Gy/33 fx in 7 weeks Surgery + RT-60 Gy/30 fx in 6 weeks | 100 (48.3%) 23 (%) 77 (%) |
| RT alone 70 Gy/35 fx in 7 weeks | 28 (13.5%) | ||
| Induction CHTH + RT 70 Gy/35 fx in 7 weeks | 12 (5.8%) | ||
| C-RT | Surgery + C-RT Surgery + C-RT-66 Gy/33 fx in 7 weeks Surgery + C-RT-60 Gy/30 fx in 6 weeks | 49 (23.7%) 18 (%) 31 (%) | |
| Concurrent C-RT 70 Gy/35 fx in 7 weeks | 18 (8.7%) | ||
| Alcohol consumption | Yes | 44 (21.3%) | |
| No | 163 (78.7%) | ||
| Tobacco smoking (Currently) | Yes | 174 (84.1%) | |
| No | 33 (15.9%) | ||
| RT Week | OM Grade | Comparisons | ROC Curve Analysis | ||||
|---|---|---|---|---|---|---|---|
| NLR Median (Interquartile Range) | p | Cut-Off Value | Sensitivity | Specificity | AUC [95% CI] p | ||
| 1 | 0 | 1.24 (1.03–1.61) | 0.1029 | >1.61 | 56.7% | 100% | 0.74 [0.67–0.80] 0.0012 * |
| 1 | 1.79 (0.01–34.30) | ||||||
| 2 | 1 | 1.69 (0.01–15.90) | 0.0225 * | >2.49 | 37.7% | 79.2% | 0.59 [0.52–0.66] 0.0204 * |
| 2 | 1.94(0.66–34.30) | ||||||
| 3 | 1 or 2 | 1.75 (1.21–2.49) | 0.0005 * | >2.59 | 87.5% | 78.4% | 0.67 [0.60–0.73] <0.0001 * |
| 3 | 4.76 (2.70–7.69) | ||||||
| 4 | 1 or 2 | 1.72 (0.01–34.30) | 0.0159 * | >2.49 | 72.7% | 75.5% | 0.72 [0.65–0.78] 0.0041 * |
| 3 | 2.60 (1.30–8.00) | ||||||
| 5 | 1 or 2 | 1.70 (0.01–34.30) | <0.0001 * | >2.63 | 55.9% | 85% | 0.73 [0.66–0.79] <0.0001 * |
| 3 or 4 | 2.80 (1.02–9.60) | ||||||
| 6 | 1 or 2 | 1.60 (0.01–34.30) | <0.0001 * | >2.52 | 59.3% | 87.6% | 0.78 [0.72–0.83] <0.0001 * |
| 3 or 4 | 2.74 (1.11–12.40) | ||||||
| 7 | 1 or 2 | 1.60 (0.01–15.90) | <0.0001 * | >2.1 | 60.3% | 72.4% | 0.68 [0.61–0.74] <0.0001 * |
| 3 | 2.35 (0.80–34.30) | ||||||
| 1–7 | 0, 1 or 2 3 or 4 | 1.60 (1.17–2.10) 2.35 (1.39–3.31) | <0.0001 * | >2.06 | 59.8% | 75% | |
| RT Week | OM Grade | Odds Ratio | |||
|---|---|---|---|---|---|
| NLR * | Univariate | Multivariate a | |||
| <Cut-Off (%) r | ≥Cut-Off (%) p | OR [95% CI] p | OR [95% CI] p | ||
| 1 | 0 | 4 (4.3%) | - | 11.74 [0.62–221.05] 0.0999 | 96.4 [–] 0.9943 |
| 1 | 88 (95.7%) | 115 (100%) | |||
| 2 | 1 | 103 (68.2%) | 27 (48.2%) | 2.30 [2.33–4.31] 0.0090 * | 2.33 [1.23–4.41] 0.0093 * |
| 2 | 48 (31.8%) | 29 (51.8%) | |||
| 3 | 1 or 2 | 155 (99.4%) | 44 (86.3%) | 24.66 [2.95–205.83] 0.0031 * | 3.09 [1.67–5.70] 0.0003 |
| 3 | 1 (0.6%) | 7 (13.7%) | |||
| 4 | 1 or 2 | 148 (98%) | 48 (85.7%) | 8.22 [2.10–32.24] 0.0025 * | 8.45 [2.10–34.02] 0.0027 * |
| 3 | 3 (2%) | 8 (14.3%) | |||
| 5 | 1 or 2 | 147 (90.7%) | 15 (44.1%) | 12.41 [5.25–29.35] <0.0001 * | 7.09 [3.17–15.76] <0.0001 * |
| 3 or 4 | 15 (9.3%) | 19 (55.9%) | |||
| 6 | 1 or 2 | 134 (85.9%) | 19 (37.2%) | 10.26 [4.96–21.18] <0.0001 * | 11.66 [5.45–24.96] <0.0001 * |
| 3 or 4 | 22 (14.1%) | 32 (62.7%) | |||
| 7 | 1 or 2 | 97 (77%) | 37 (45.7%) | 3.98 [2.18–7.27] <0.0001 * | 4.46 [2.38–8.33] <0.0001 * |
| 3 | 29 (23%) | 44 (54.3%) | |||
| 1–7 | 0, 1 or 2 r | 89 (74.2%) | 35 (40.2%) | 4.26 [2.36–7.71] <0.0001 * | 4.55 [2.48–8.35] <0.0001 * |
| 3 or 4 | 31 (25.8%) | 52 (59.8%) | |||
| Variable | Univariate | Multivariate b | |||
|---|---|---|---|---|---|
| Median (Months) | HR (95% CI) | p | HR (95% CI) | p | |
| Sex Men r Women | 27 49 | 1.65 (0.85–3.20) | 0.2227 | 1.70 (0.75–3.87) | 0.2084 |
| Age [years] b ≥63 r <63 | 24 31 | 1.23 (0.85–1.78) | 0.2558 | 1.41 (0.97–2.05) | 0.0749 |
| Tumour location Larynx r Oral cavity, oropharynx, hypopharynx | 24 31 | 1.13 (0.78–1.64) | 0.4957 | 1.30 (0.89–1.89) | 0.1766 |
| TNM stage IVA–IVC r III | 24 38 | 1.78 (1.22–2.59) | 0.0041 * | 1.84 (1.21–2.78) | 0.0043 * |
| Performance status 1 r 2 | 32 24 | 0.76 (0.52–1.12) | 0.1888 | 0.74 (0.49–1.12) | 0.1604 |
| Alcohol consumption Yes r No | 21 28 | 1.33 (0.82–2.13) | 0.1963 | 1.29 (0.82–2.00) | 0.2701 |
| Tobacco smoking Yes r No | 27 31 | 1.51 (0.93–2.44) | 0.1443 | 1.44 (0.82–2.53) | 0.2074 |
| NLR c High (≥1.76) r Low (<1.76) | 23 36 | 1.45 (1.01–2.10) | 0.0433 * | 1.48 (1.02–2.15) | 0.0395 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Homa-Mlak, I.; Brzozowska, A.; Mlak, R.; Szudy-Szczyrek, A.; Małecka-Massalska, T. Neutrophil-to-Lymphocyte Ratio as a Factor Predicting Radiotherapy Induced Oral Mucositis in Head Neck Cancer Patients Treated with Radiotherapy. J. Clin. Med. 2021, 10, 4444. https://doi.org/10.3390/jcm10194444
Homa-Mlak I, Brzozowska A, Mlak R, Szudy-Szczyrek A, Małecka-Massalska T. Neutrophil-to-Lymphocyte Ratio as a Factor Predicting Radiotherapy Induced Oral Mucositis in Head Neck Cancer Patients Treated with Radiotherapy. Journal of Clinical Medicine. 2021; 10(19):4444. https://doi.org/10.3390/jcm10194444
Chicago/Turabian StyleHoma-Mlak, Iwona, Anna Brzozowska, Radosław Mlak, Aneta Szudy-Szczyrek, and Teresa Małecka-Massalska. 2021. "Neutrophil-to-Lymphocyte Ratio as a Factor Predicting Radiotherapy Induced Oral Mucositis in Head Neck Cancer Patients Treated with Radiotherapy" Journal of Clinical Medicine 10, no. 19: 4444. https://doi.org/10.3390/jcm10194444






