Effects of Immersive and Non-Immersive Virtual Reality on the Static and Dynamic Balance of Stroke Patients: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
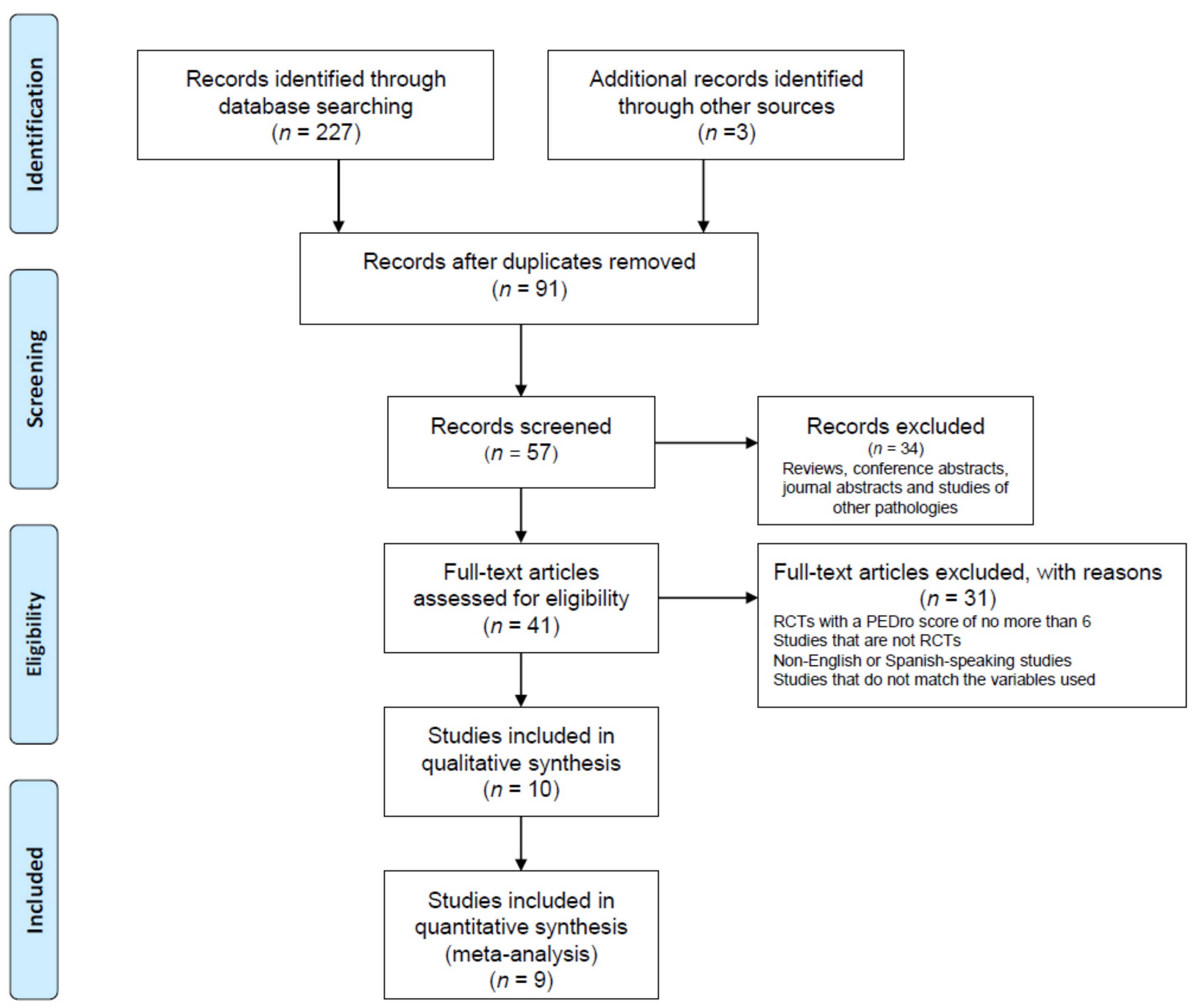
2. Methodology
2.1. Study Design
2.2. Information Sources
2.3. Search Strategy
- -
- “Physiotherapy” or “Physical therapy”;
- -
- “Virtual Reality”;
- -
- “Immersive Virtual Reality”;
- -
- “Non-immersive Virtual Reality”;
- -
- “Stroke”;
- -
- “Balance”;
- -
- “Static Balance”;
- -
- “Dynamic Balance”.
2.4. Eligibility Criteria
- -
- RCTs published in English and Spanish in the last ten years
- -
- RCTs developed with an adult population (>18 years old) with balance disorders as a consequence of suffering a stroke in the previous six months before therapeutic intervention
- -
- Studies that based their interventions on physiotherapeutic treatments using immersive or non-immersive virtual reality in isolation or compared to other forms of physiotherapeutic treatment
- -
- RCTs with scores equal to or greater than 6 on the PEDro scale, in order to improve the quality of the review
2.5. Variables/Outcomes
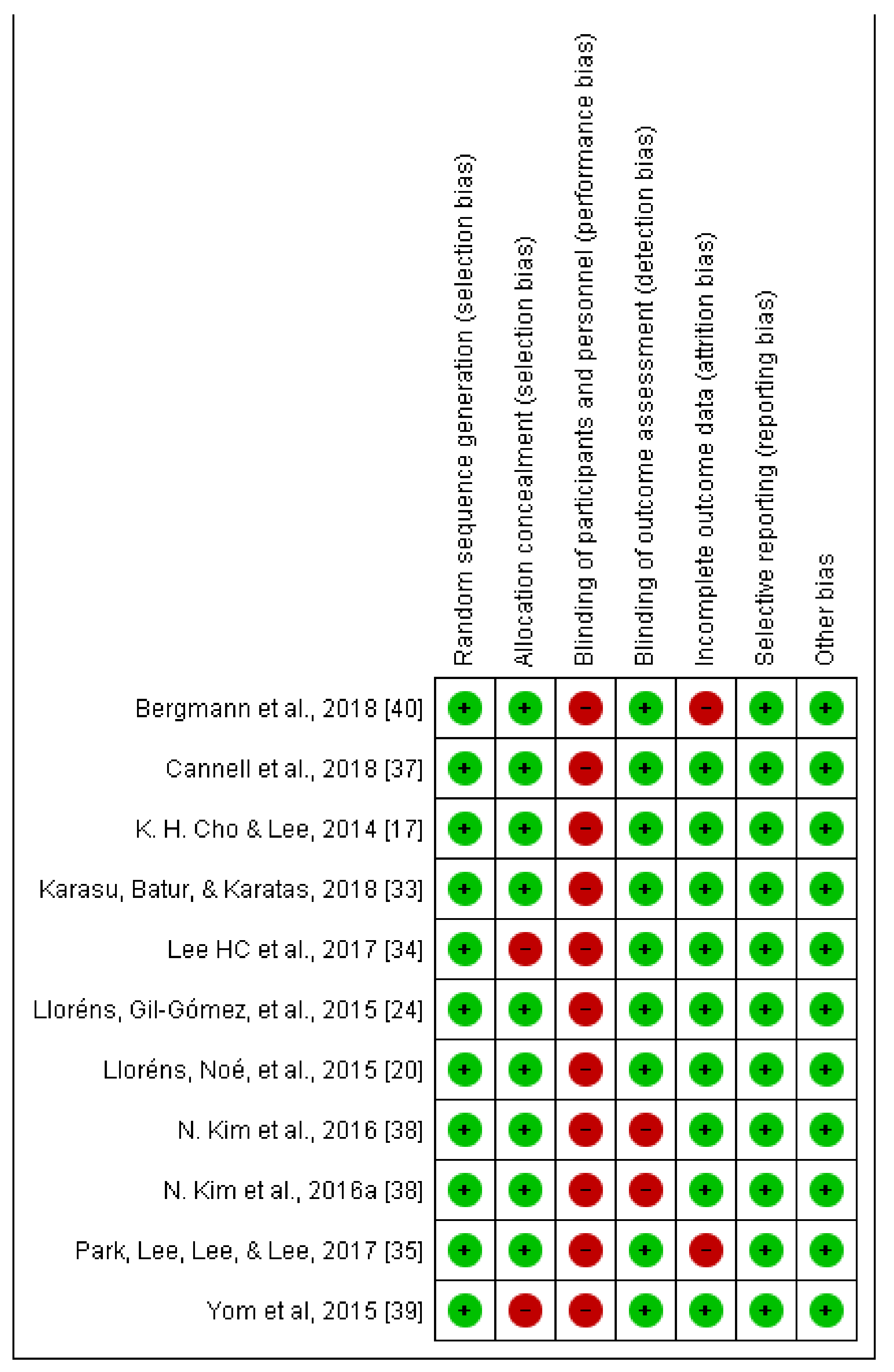
2.6. Assessment of the Methodological Quality of the Included Studies
2.7. Analysis of Data
3. Results
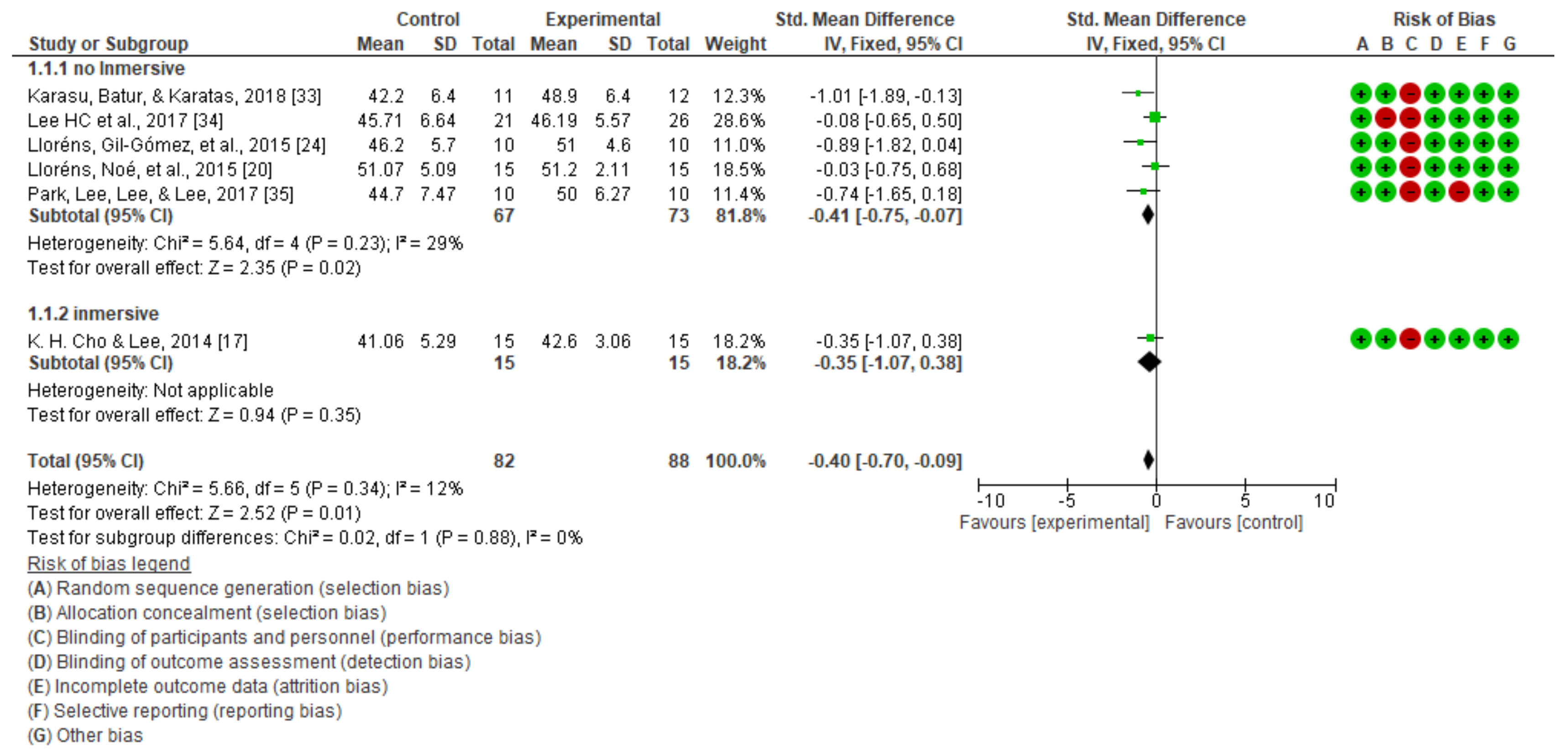
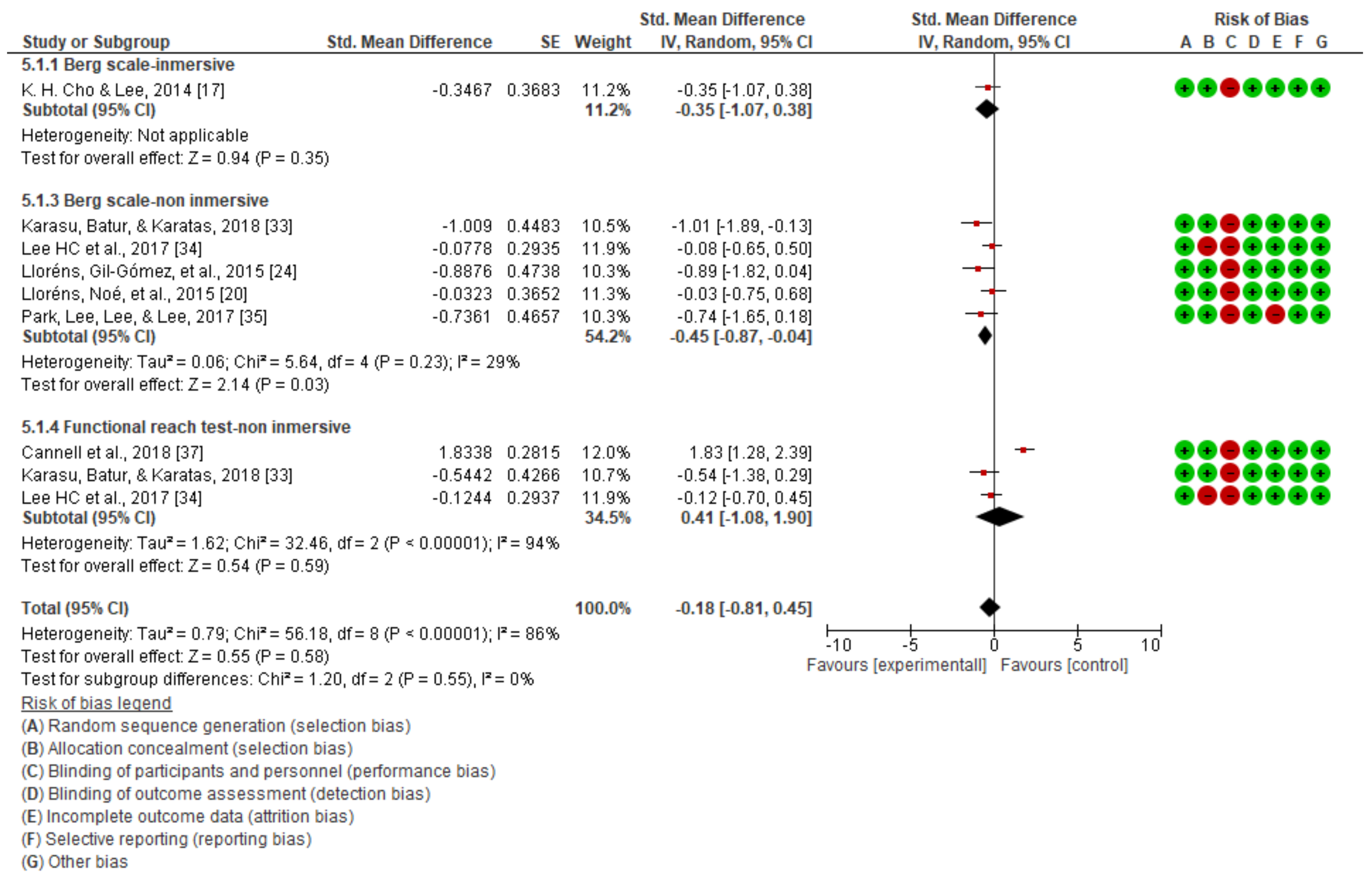
3.1. Static Balance
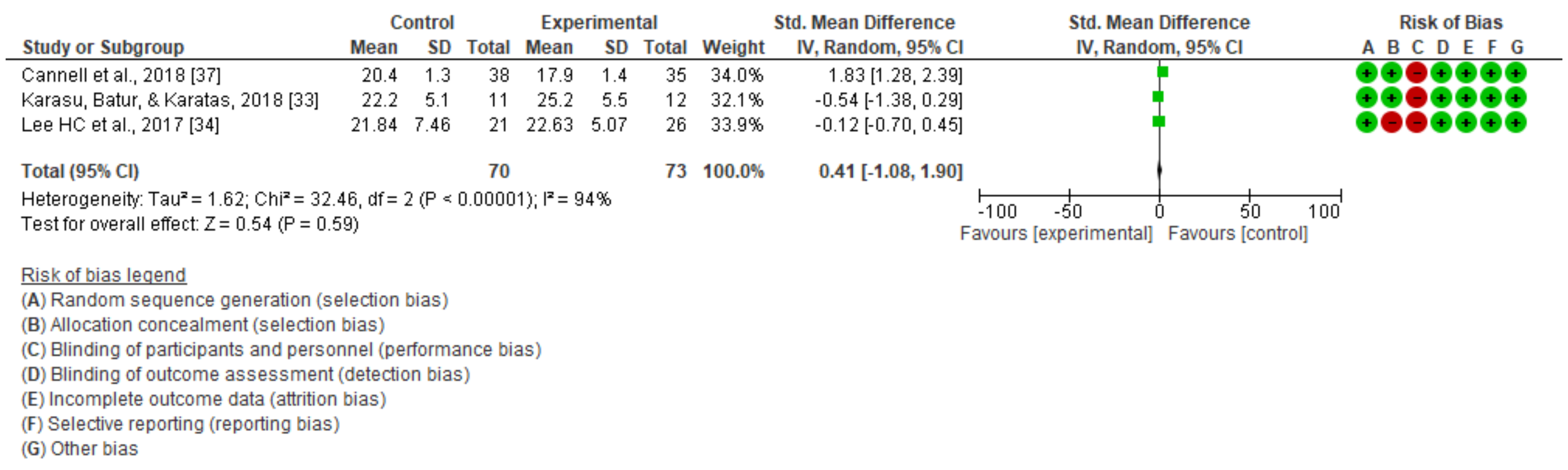
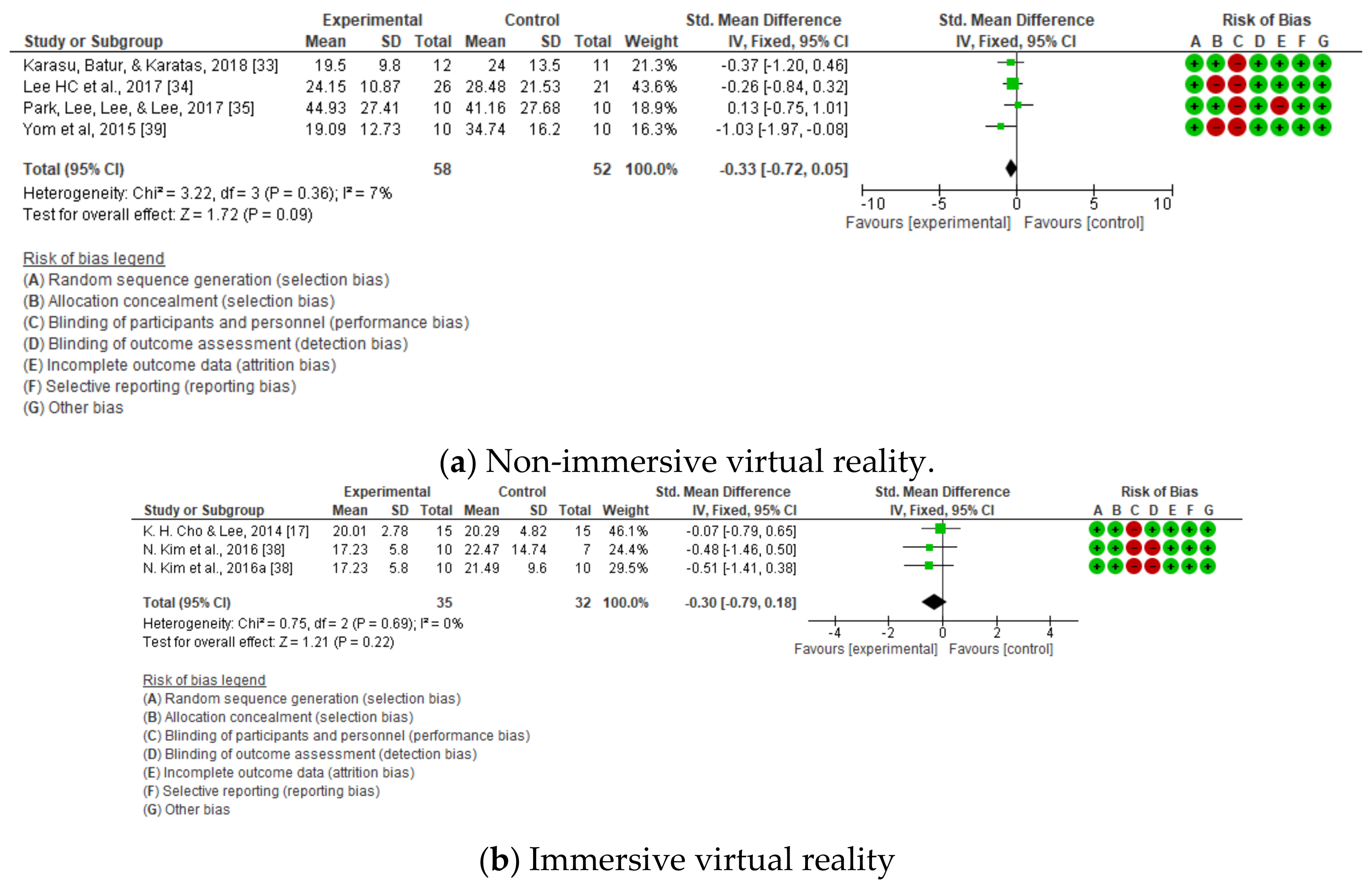
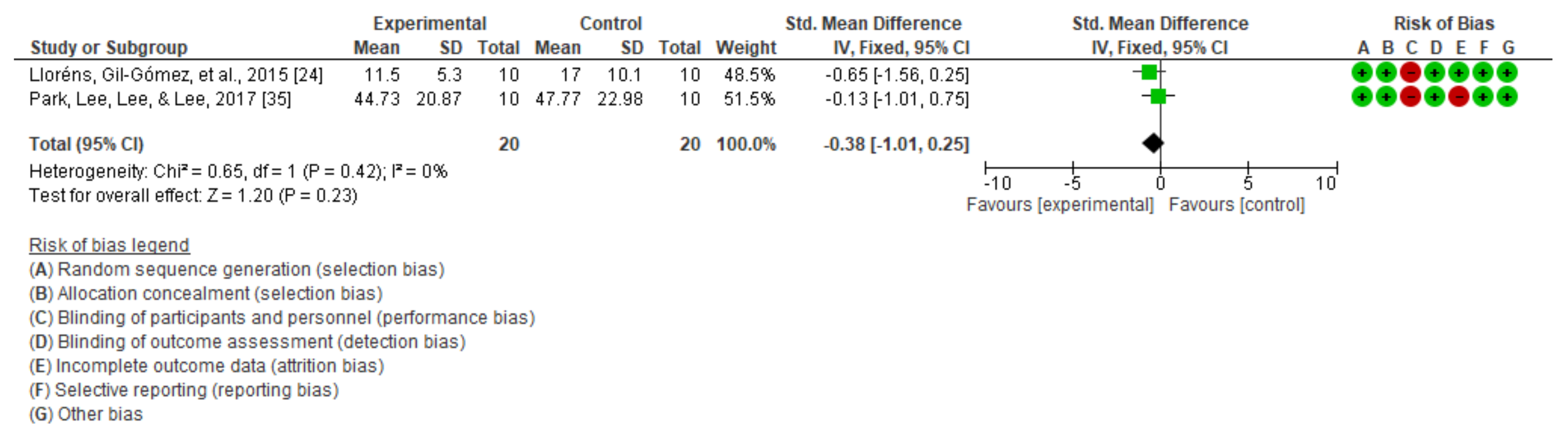
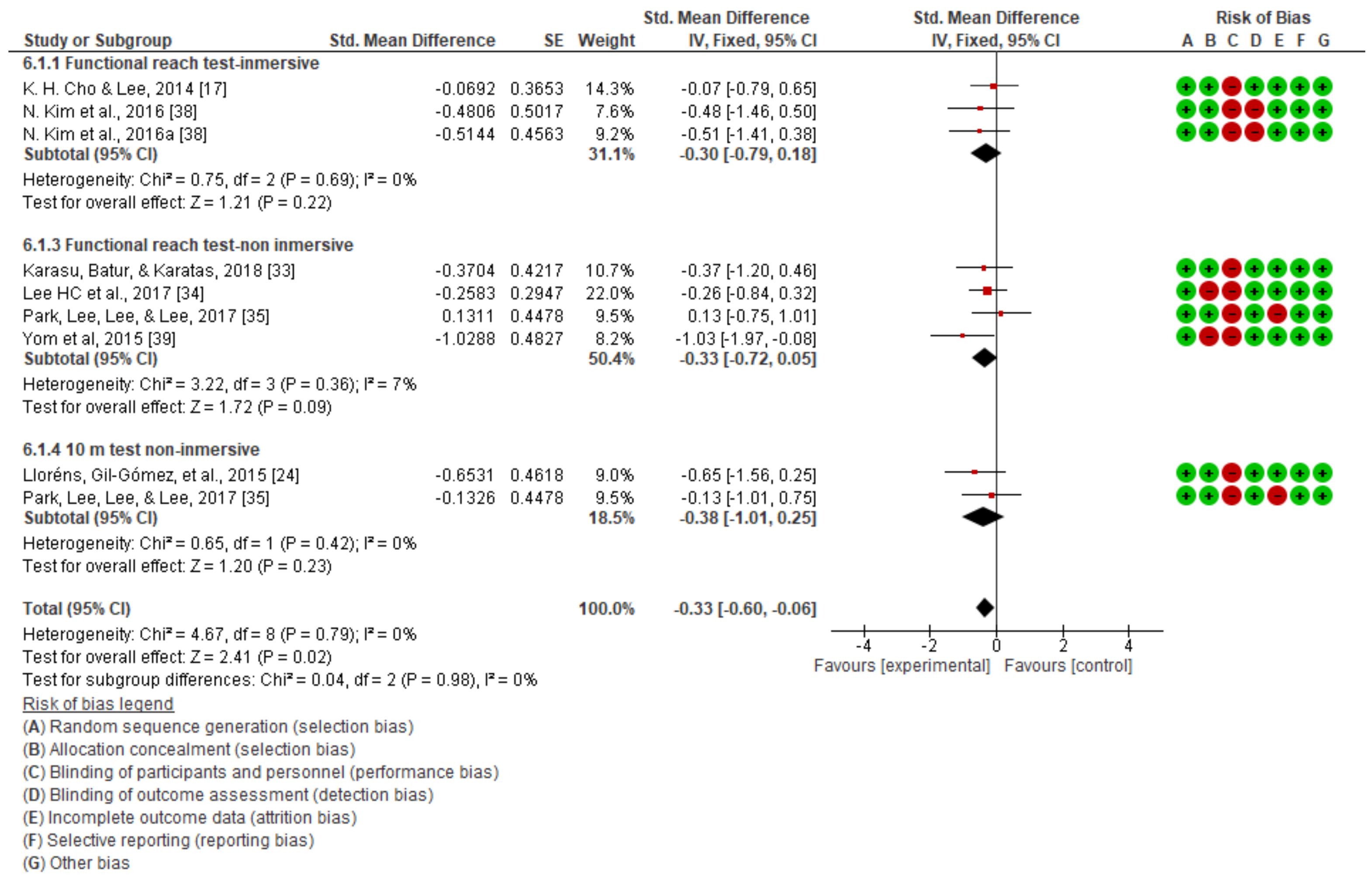
3.2. Dynamic Balance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO Launches List of Priority Medical Devices for Management of Cardiovascular Diseases and Diabetes. Available online: https://www.who.int/news/item/30-06-2021-who-launches-list-of-priority-medical-devices-for-management-of-cardiovascular-diseases-and-diabetes (accessed on 31 July 2021).
- Grefkes, C.; Fink, G.R. Connectivity-based approaches in stroke and recovery of function. Lancet Neurol. 2014, 13, 206–216. Available online: http://www.ncbi.nlm.nih.gov/pubmed/24457190 (accessed on 26 September 2021). [CrossRef]
- Grefkes, C.; Ward, N.S. Cortical reorganization after stroke: How much and how functional? Neuroscientist 2014, 20, 56–70. Available online: http://www.ncbi.nlm.nih.gov/pubmed/23774218 (accessed on 26 September 2021). [CrossRef] [PubMed]
- Rehme, A.K.; Grefkes, C. Cerebral network disorders after stroke: Evidence from imaging-based connectivity analyses of active and resting brain states in humans. J. Physiol. 2013, 59, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.T.; Wu, C.Y.; Liu, H.L.; Lin, K.C.; Wai, Y.Y.; Chen, Y.L. Neuroplastic changes in resting-state functional connectivity after stroke rehabilitation. Front. Hum. Neurosci. 2015, 9, 546. Available online: http://www.ncbi.nlm.nih.gov/pubmed/26557065 (accessed on 26 September 2021). [CrossRef] [PubMed] [Green Version]
- Takeuchi, N.; Izumi, S.I. Rehabilitation with poststroke motor recovery: A review with a focus on neural plasticity. Stroke Res. Treat. 2013, 2013, 128641. Available online: http://www.ncbi.nlm.nih.gov/pubmed/23738231 (accessed on 26 September 2021). [CrossRef] [PubMed] [Green Version]
- Bambirra, C.; de Betsan Rodrigues, M.C.; de Morais Faria, C.D.C.; de Paula, F.R. Clinical evaluation of balance in hemiparetic adults: A systematic review. Fisioter. Mov. 2015, 28, 187–200. [Google Scholar] [CrossRef] [Green Version]
- Hwang, H.S.; Kim, J.H.; Choi, B.R. Comparison of the effects of visual feedback training and unstable surface training on static and dynamic balance in patients with stroke. J. Phys. Ther. Sci. 2017, 29, 1720–1722. [Google Scholar] [CrossRef] [Green Version]
- Cho, H.Y.; Kim, K. Effects of Action Observation Training with Auditory Stimulation on Static and Dynamic Balance in Chronic Stroke Patients. J. Stroke Cerebrovasc. Dis. 2020, 29, 104775. Available online: http://www.ncbi.nlm.nih.gov/pubmed/32205026 (accessed on 26 September 2021).
- Kleim, J.A.; Jones, T.A. Principles of experience-dependent neural plasticity: Implications for rehabilitation after brain damage. J. Speech Lang. Hear. Res. 2008, 51, S225–S239. Available online: https://pubmed.ncbi.nlm.nih.gov/18230848/ (accessed on 26 September 2021). [CrossRef]
- Campfens, S.F.; Zandvliet, S.B.; Meskers, C.G.M.; Schouten, A.C.; van Putten, M.J.A.M.; van der Kooij, H. Poor motor function is associated with reduced sensory processing after stroke. Exp. Brain Res. 2015, 233, 1339–1349. Available online: http://www.ncbi.nlm.nih.gov/pubmed/25651979 (accessed on 26 September 2021).
- Jang, S.H.; Lee, J.-H. Impact of sensory integration training on balance among stroke patients: Sensory integration training on balance among stroke patients. Open Med. 2016, 11, 330–335. Available online: http://www.ncbi.nlm.nih.gov/pubmed/28352817 (accessed on 26 September 2021). [CrossRef]
- Freburger, J.K.; Li, D.; Johnson, A.M.; Fraher, E.P. Physical and Occupational Therapy From the Acute to Community Setting After Stroke: Predictors of Use, Continuity of Care, and Timeliness of Care. Arch. Phys. Med. Rehabil. 2018, 99, 1077–1089.e7. Available online: http://www.ncbi.nlm.nih.gov/pubmed/28389108 (accessed on 26 September 2021). [CrossRef]
- Hugues, A.; Di Marco, J.; Janiaud, P.; Xue, Y.; Pires, J.; Khademi, H.; Cucherat, M.; Bonan, I.; Gueyffier, F.; Rode, G. Efficiency of physical therapy on postural imbalance after stroke: Study protocol for a systematic review and meta-analysis. BMJ Open 2017, 7, e013348. Available online: http://www.ncbi.nlm.nih.gov/pubmed/28137928 (accessed on 26 September 2021).
- Li, S. Spasticity, motor recovery, and neural plasticity after stroke. Front. Neurol. 2017, 8, 120. Available online: http://www.ncbi.nlm.nih.gov/pubmed/28421032 (accessed on 26 September 2021). [CrossRef] [PubMed] [Green Version]
- Chen, L.; Lo, W.L.A.; Mao, Y.R.; Ding, M.H.; Lin, Q.; Li, H.; Zhao, J.L.; Xu, Z.Q.; Bian, R.H.; Huang, D.F. Effect of Virtual Reality on Postural and Balance Control in Patients with Stroke: A Systematic Literature Review. Biomed Res. Int. 2016, 2016, 7309272. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.H.; Lee, W.H. Effect of treadmill training based real-world video recording on balance and gait in chronic stroke patients: A randomized controlled trial. Gait Posture. 2014, 39, 523–528. [Google Scholar] [CrossRef] [PubMed]
- De Rooij, I.J.M.; van de Port, I.G.L.; Meijer, J.-W.G. Effect of Virtual Reality Training on Balance and Gait Ability in Patients with Stroke: Systematic Review and Meta-Analysis. Phys. Ther. 2016, 96, 1905–1918. [Google Scholar] [CrossRef]
- Laver, K.E.; Lange, B.; George, S.; Deutsch, J.E.; Saposnik, G.; Crotty, M. Virtual reality for stroke rehabilitation. Cochrane Database Syst. Rev. 2017, 11, CD008349. [Google Scholar] [CrossRef] [Green Version]
- Lloréns, R.; Noé, E.; Colomer, C.; Alcañiz, M. Effectiveness, usability, and cost-benefit of a virtual reality-based telerehabilitation program for balance recovery after stroke: A randomized controlled trial. Arch. Phys. Med. Rehabil. 2015, 96, 418–425.e2. [Google Scholar] [CrossRef] [Green Version]
- Lohse, K.R.; Hilderman, C.G.E.; Cheung, K.L.; Tatla, S.; Van Der Loos, H.F.M. Virtual reality therapy for adults post-stroke: A systematic review and meta-analysis exploring virtual environments and commercial games in therapy. PLoS ONE 2014, 9, e93318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasuda, K.; Muroi, D.; Ohira, M.; Iwata, H. Validation of an immersive virtual reality system for training near and far space neglect in individuals with stroke: A pilot study. Top. Stroke Rehabil. 2017, 24, 533–538. [Google Scholar] [CrossRef]
- Lee, M.; Son, J.; Kim, J.; Pyun, S.-B.; Eun, S.-D.; Yoon, B. Comparison of individualized virtual reality- and group-based rehabilitation in older adults with chronic stroke in community settings: A pilot randomized controlled trial. Eur. J. Integr. Med. 2016, 8, 738–746. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1876382016303225 (accessed on 26 September 2021). [CrossRef]
- Lloréns, R.; Gil-Gómez, J.A.; Alcañiz, M.; Colomer, C.; Noé, E. Improvement in balance using a virtual reality-based stepping exercise: A randomized controlled trial involving individuals with chronic stroke. Clin. Rehabil. 2015, 29, 261–268. Available online: https://pubmed.ncbi.nlm.nih.gov/25056999/ (accessed on 26 September 2021). [CrossRef] [Green Version]
- McEwen, D.; Taillon-Hobson, A.; Bilodeau, M.; Sveistrup, H.; Finestone, H. Virtual reality exercise improves mobility after stroke: An inpatient randomized controlled trial. Stroke 2014, 45, 1853–1855. Available online: http://www.ncbi.nlm.nih.gov/pubmed/24763929 (accessed on 26 September 2021). [CrossRef] [PubMed]
- Sheehy, L.; Taillon-Hobson, A.; Sveistrup, H.; Bilodeau, M.; Fergusson, D.; Levac, D.; Finestone, H. Does the addition of virtual reality training to a standard program of inpatient rehabilitation improve sitting balance ability and function after stroke? Protocol for a single-blind randomized controlled trial. BMC Neurol. 2016, 16, 42. Available online: http://www.ncbi.nlm.nih.gov/pubmed/27036515 (accessed on 26 September 2021). [CrossRef] [PubMed] [Green Version]
- Saposnik, G.; Cohen, L.G.; Mamdani, M.; Pooyania, S.; Ploughman, M.; Cheung, D.; Shaw, J.; Hall, J.; Nord, P.; Dukelow, S.; et al. Efficacy and safety of non-immersive virtual reality exercising in stroke rehabilitation (EVREST): A randomised, multicentre, single-blind, controlled trial. Lancet Neurol. 2016, 15, 1019. Available online: http://www.ncbi.nlm.nih.gov/pubmed/27365261 (accessed on 26 September 2021). [CrossRef] [Green Version]
- Ögün, M.N.; Kurul, R.; Yaşar, M.F.; Turkoglu, S.A.; Avci, Ş.; Yildiz, N. Effect of leap motion-based 3D immersive virtual reality usage on upper extremity function in ischemic stroke patients. Arq. Neuropsiquiatr. 2019, 77, 681–688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. Available online: https://www.bmj.com/content/372/bmj.n71 (accessed on 26 September 2021).
- Eriksen, M.B.; Frandsen, T.F. The impact of patient, intervention, comparison, outcome (Pico) as a search strategy tool on literature search quality: A systematic review. J. Med. Libr. Assoc. 2018, 106, 420–431. Available online: https://pubmed.ncbi.nlm.nih.gov/30271283/ (accessed on 26 September 2021). [CrossRef] [PubMed]
- Veerbeek, J.; van Wegen, E.; Peppen, R.P.S.; Hendriks, E.; Rietberg, M.B.; Wees, P.J.; Heijblom, K.; Goos, A.A.G.; Hanssen, W.O.; Harmeling-van der Wel, B.C.; et al. Clinical Practice Guideline for Physical Therapy after Stroke (Dutch: KNGF-Richtlijn Beroerte). 2014. Available online: https://www.researchgate.net/publication/282247781_Clinical_Practice_Guideline_for_Physical_Therapy_after_Stroke_Dutch_KNGF-richtlijn_Beroerte (accessed on 26 September 2021).
- Sullivan, J.E.; Crowner, B.E.; Kluding, P.M.; Nichols, D.; Rose, D.K.; Yoshida, R.; Pinto Zipp, G. Outcome measures for individuals with stroke: Process and recommendations from the American Physical Therapy Association neurology section task force. Phys. Ther. 2013, 93, 1383–1396. Available online: https://pubmed.ncbi.nlm.nih.gov/23704035/ (accessed on 26 September 2021). [CrossRef]
- Karasu, A.U.; Batur, E.B.; Karatas, G.K. Effectiveness of WII-based rehabilitation in stroke: A randomized controlled study. J. Rehabil. Med. 2018, 50, 406–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.C.; Huang, C.L.; Ho, S.H.; Sung, W.H. The Effect of a Virtual Reality Game Intervention on Balance for Patients with Stroke: A Randomized Controlled Trial. Games Health J. 2017, 6, 303–311. Available online: https://pubmed.ncbi.nlm.nih.gov/28771379/ (accessed on 26 September 2021). [CrossRef]
- Park, D.S.; Lee, D.G.; Lee, K.; Lee, G.C. Effects of Virtual Reality Training using Xbox Kinect on Motor Function in Stroke Survivors: A Preliminary Study. J. Stroke Cerebrovasc. Dis. 2017, 26, 2313–2319. [Google Scholar] [CrossRef]
- Llorens, R.; Noé, E.; Alcañiz, M.; Deutsch, J.E. Time since injury limits but does not prevent improvement and maintenance of gains in balance in chronic stroke. Brain Inj. 2018, 32, 303–309. Available online: http://www.ncbi.nlm.nih.gov/pubmed/29278927 (accessed on 26 September 2021). [CrossRef]
- Cannell, J.; Jovic, E.; Rathjen, A.; Lane, K.; Tyson, A.M.; Callisaya, M.L.; Smith, S.T.; Ahuja, K.D.; Bird, M.-L. The efficacy of interactive, motion capture-based rehabilitation on functional outcomes in an inpatient stroke population: A randomized controlled trial. Clin. Rehabil. 2018, 32, 191–200. Available online: http://www.ncbi.nlm.nih.gov/pubmed/28719977 (accessed on 26 September 2021). [CrossRef] [Green Version]
- Kim, N.; Lee, B.H.; Kim, Y.; Min, W. Effects of virtual reality treadmill training on community balance confidence and gait in people post-stroke: A randomized controlled trial. J. Exp. Stroke Transl. Med. 2016, 9, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Yom, C.; Cho, H.Y.; Lee, B.H. Effects of virtual reality-based ankle exercise on the dynamic balance, muscle tone, and gait of stroke patients. J. Phys. Ther. Sci. 2015, 27, 845–849. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4395728/ (accessed on 26 September 2021). [CrossRef] [Green Version]
- Bergmann, J.; Krewer, C.; Bauer, P.; Koenig, A.; Riener, R.; Müller, F. Virtual reality to augment robot-assisted gait training in non-ambulatory patients with a subacute stroke: A pilot randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2018, 54, 397–407. Available online: https://pubmed.ncbi.nlm.nih.gov/29265791/ (accessed on 26 September 2021). [CrossRef]
- De Morton, N.A. The PEDro scale is a valid measure of the methodological quality of clinical trials: A demographic study. Aust. J. Physiother. 2009, 55, 129–133. [Google Scholar] [CrossRef] [Green Version]
- Yamato, T.P.; Maher, C.; Koes, B.; Moseley, A. The PEDro scale had acceptably high convergent validity, construct validity, and interrater reliability in evaluating methodological quality of pharmaceutical trials. J. Clin. Epidemiol. 2017, 86, 176–181. Available online: http://www.ncbi.nlm.nih.gov/pubmed/28288916 (accessed on 26 September 2021). [CrossRef]
- Flansbjer, U.B.; Holmbäck, A.M.; Downham, D.; Patten, C.; Lexell, J. Reliability of gait performance tests in men and women with hemiparesis after stroke. J. Rehabil. Med. 2005, 37, 75–82. [Google Scholar]
- Harris, D.M.; Rantalainen, T.; Muthalib, M.; Johnson, L.; Teo, W.-P. Exergaming as a Viable Therapeutic Tool to Improve Static and Dynamic Balance among Older Adults and People with Idiopathic Parkinson’s Disease: A Systematic Review and Meta-Analysis. Front. Aging Neurosci. 2015, 7, 167. Available online: http://www.ncbi.nlm.nih.gov/pubmed/26441634 (accessed on 26 September 2021). [CrossRef]
- Perez-Marcos, D.; Bieler-Aeschlimann, M.; Serino, A. Virtual Reality as a Vehicle to Empower Motor-Cognitive Neurorehabilitation. Front. Psychol. 2018, 9, 2120. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6224455 (accessed on 26 September 2021). [CrossRef]
- Keshner, E.A.; Fung, J. The quest to apply VR technology to rehabilitation: Tribulations and treasures. J. Vestib. Res. 2017, 27, 1–5. Available online: https://pubmed.ncbi.nlm.nih.gov/28387695/ (accessed on 26 September 2021). [CrossRef] [Green Version]
- Porras, D.C.; Sharon, H.; Inzelberg, R.; Ziv-Ner, Y.; Zeilig, G.; Plotnik, M. Advanced virtual reality-based rehabilitation of balance and gait in clinical practice. Ther. Adv. Chronic. Dis. 2019, 10, 2040622319868379. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6710712 (accessed on 26 September 2021). [CrossRef]
- Kim, N.; Park, Y.; Lee, B.H. Effects of community-based virtual reality treadmill training on balance ability in patients with chronic stroke. J. Phys. Ther. Sci. 2015, 27, 655–658. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4395685/?report=abstract (accessed on 26 September 2021). [CrossRef] [Green Version]
- Gallagher, K.M. Helping Older Adults Sustain Their Physical Therapy Gains: A Theory-Based Intervention to Promote Adherence to Home Exercise Following Rehabilitation. J. Geriatr. Phys. Ther. 2016, 39, 20–29. Available online: https://pubmed.ncbi.nlm.nih.gov/25695468/ (accessed on 26 September 2021). [CrossRef]
- Schiza, E.; Matsangidou, M.; Neokleous, K.; Pattichis, C.S. Virtual Reality Applications for Neurological Disease: A Review. Front. Robot. AI 2019, 6, 100. Available online: https://pubmed.ncbi.nlm.nih.gov/33501115/ (accessed on 26 September 2021). [CrossRef] [Green Version]
- Bezegová, E.; Ledgard, M.A.; Molemaker, R.-J.; Oberč, B.P.; Vigkos, A. Virtual Reality and Its Potential for Europe. Available online: https://ec.europa.eu/futurium/en/system/files/ged/vr_ecosystem_eu_report_0.pdf (accessed on 2 June 2021).
- Marín-Morales, J.; Llinares, C.; Guixeres, J.; Alcañiz, M. Emotion Recognition in Immersive Virtual Reality: From Statistics to Affective Computing. Sensors 2020, 20, 5163. Available online: www.mdpi.com/journal/sensors (accessed on 26 September 2021). [CrossRef]
- Van Duijnhoven, H.J.R.; Heeren, A.; Peters, M.A.M.; Veerbeek, J.M.; Kwakkel, G.; Geurts, A.C.H.; Weerdesteyn, V. Effects of Exercise Therapy on Balance Capacity in Chronic Stroke: Systematic Review and Meta-Analysis. Stroke 2016, 47, 2603–2610. [Google Scholar] [CrossRef] [Green Version]
- Vahlberg, B.; Cederholm, T.; Lindmark, B.; Zetterberg, L.; Hellström, K. Short-term and long-term effects of a progressive resistance and balance exercise program in individuals with chronic stroke: A randomized controlled trial. Disabil. Rehabil. 2017, 39, 1615–1622. [Google Scholar] [CrossRef]
- Ivey, F.M.; Prior, S.J.; Hafer-Macko, C.E.; Katzel, L.I.; Macko, R.F.; Ryan, A.S. Strength Training for Skeletal Muscle Endurance after Stroke. J. Stroke Cerebrovasc. Dis. 2017, 26, 787–794. [Google Scholar] [CrossRef]
- Rose, D.K.; Nadeau, S.E.; Wu, S.S.; Tilson, J.K.; Dobkin, B.H.; Pei, Q.; Duncan, P.W. Locomotor training and strength and balance exercises for walking recovery after stroke: Response to number of training sessions. Phys. Ther. 2017, 97, 1066–1074. [Google Scholar] [CrossRef]
- Berg, K.O.; Wood-Dauphinee, S.L.; Williams, J.I.; Maki, B. Measuring balance in the elderly: Validation of an instrument. Can. J. Public Health 1992, 83, S7–S11. [Google Scholar]
- Pickenbrock, H.M.; Diel, A.; Zapf, A. A comparison between the Static Balance Test and the Berg Balance Scale: Validity, reliability, and comparative resource use. Clin. Rehabil. 2016, 30, 288–293. [Google Scholar] [CrossRef]
- Alghadir, A.H.; Al-Eisa, E.S.; Anwer, S.; Sarkar, B. Reliability, validity, and responsiveness of three scales for measuring balance in patients with chronic stroke. BMC Neurol. 2018, 18, 141. Available online: https://pubmed.ncbi.nlm.nih.gov/30213258/ (accessed on 26 September 2021). [CrossRef] [Green Version]
- Kleim, J.A. Neural plasticity and neurorehabilitation: Teaching the new brain old tricks. J. Commun. Disord. 2011, 44, 521–528. [Google Scholar] [CrossRef]

| MEDLINE | PEDRO | COCHRANE | SCIELO | SCOPUS | |
|---|---|---|---|---|---|
| Boolean Osperator | AND/OR | AND/OR | AND/OR | AND/OR | AND/OR |
| Keywords | “Physiotherapy “or “Physical Therapy” “virtual Reality”, “Immersive Virtual Reality” or “Non Immersive Virtual Reality” “Stroke” “Balance” “static balance” “Dynamic balance” | “Physiotherapy “or “Physical Therapy” “virtual Reality”, “Immersive Virtual Reality” or “Non Immersive Virtual Reality” “Stroke” “Balance” “static balance” “Dynamic balance” | “Physiotherapy “or “Physical Therapy” “virtual Reality”, “Immersive Virtual Reality” or “Non Immersive Virtual Reality” “Stroke” “Balance” “static balance” “Dynamic balance” | “Physiotherapy “or “Physical Therapy” “virtual Reality”, “Immersive Virtual Reality” or “Non Immersive Virtual Reality” “Stroke” “Balance” “static balance” “Dynamic balance” | “Physiotherapy “or “Physical Therapy” “virtual Reality”, “Immersive Virtual Reality” or “Non Immersive Virtual Reality” “Stroke” “Balance” “static balance” “Dynamic balance” |
| Filters | “Article type” (RCT y clinical trial protocol) “Publication date” (last 10 years) species (humans) “languages” (English and Spanish) | “Subdisciplina”, Neurology “Method “, clinical trial “Published since”, 2010 | “Publication date”, 2010 “Type of article”, Trial | “Database”, was selected Article “Languages”. Spanish and English “Year of publication”, from 2010 to 2020 | “Time range of search”, from the year 2010 to the present. “Document type”, the article option was selected. “Selection fields”, the option was selected. Article title, Abstract y Keywords. |
| STUDY | POPULATION AND CHARACTERISTICS | INTERVENTION | TYPE OF VIRTUAL REALITY | COMPARISON | EVALUATION/FOLLOW-UP | MEASURING INSTRUMENTS |
|---|---|---|---|---|---|---|
| Karasu et al., 2018 [33] | 23 patients GE (n = 12): 62.3 ± 11.79 GC (n = 11): 64.1 ± 12.2 Ability to understand and follow simple commands | 20 sessions: conventional neurological rehabilitation + virtual reality (Wii) | Nonimmersive | Conventional neurological rehabilitation 20 sessions: 5 sessions of 2–3 h/week over 8 weeks | Static and dynamic balance assessment at baseline and 4 weeks. Follow-up at 8 weeks | Berg balance scale Functional reach test Postural assessment scale for stroke patients Timed up and go testS tatic balance index |
| Bergmann et al., 2018 [40] | 20 patients GE (n = 10): 62 ± 11 GC (n = 10): 65 ± 8 Inability to ambulate without help or assistance from another person (functional ambulation rating ≤2), Cognitive abilities to understand and follow simple verbal instructions. | Physiotherapy + virtual reality 8 sessions of physiotherapy + 12 sessions of virtual reality | Non-immersive | Physiotherapy + Lokomat 8 sessions of physiotherapy + 12 sessions of Lokomat | Assessment of dynamic balance at baseline and 4 weeks. Follow-up of dynamic balance at 8 weeks | Questionnaire IMI Functional ambulation classification 10 m walking test 6 min walking test Medical Research Council |
| Lee et al., 2017 [34] | 50 patients GE (n = 26): 59.35 ± 8.95 GC (n = 24): 55.76 ± 9.59 Ability to understand game instructions Ability to stand for 15 min | Conventional physiotherapy + virtual reality (kinetic sports), 12 sessions | Non-immersive | Conventional physiotherapy + balance exercise protocol 12 sessions | Static and dynamic balance assessment at baseline and 6 weeks Follow-up at 6 months | Berg balance scale Functional scope test Timed up and go test Barthel scale modified ABC specific test of balance stroke impact scale |
| Park et al., 2017 [35] | 20 patients GE (n = 10): 62.00 ± 17.14 GC (n = 10): 65.30 ± 10.51 Minimum score of 21 on mini-mental test Ability to walk 10 m with or without assistance | Conventional physiotherapy + virtual reality (Xbox), 12 sessions | Non-immersive | Conventional physiotherapy 12 sessions | Assessment of static and dynamic balance at baseline and 6 weeks No follow-up | Fugl–Meyer assessment Berg balance scale Timed up and go 10 m walking test |
| Lloréns, Noé, et al., 2015 [20] | 30 patients GE (n = 15): 55.47 ± 9.63 GC (n = 15): 55.60 ± 7.29 On the Brunel scale (Section 3), levels 7–12 More than 23 points in the mini-mental test | Virtual reality at home (Kinect) 20 sessions | Non-immersive | Virtual reality in the clinic 20 sessions | Static balance evaluation at baseline and 8 weeks Follow-up of static balance at 12 weeks | Berg balance scale Tinetti scale Brunel balance assessment Performance-oriented mobility assessment System usability scale Intrinsic motivation inventory |
| Lloréns et al., 2015 [24] | 20 patients GE (n = 10): 58.3 ± 11.6 GC (n = 10): 55 ± 11.6 More than 23 points in the mini-mental test Ability to remain in a standing position without assistance (Section 3, level 7 Brunel scale). | Conventional physiotherapy + virtual reality (virtual rehabilitation system), 20 sessions | Non-immersive | Conventional physiotherapy 20 sessions | Evaluation of static and dynamic balance at baseline and 4 weeks No follow-up | Berg balance scale Tinetti scale Brunel balance assessment 10 m walking test |
| Cho et al., 2014 [17] | 30 patients GE (n = 15): 63.53 ± 5.54 years GC (n = 15): 65.86 ± 5.73 years Ability to walk 10 m with or without assistance Ability to understand simple instructions (>24 on the mini mental test) | Conventional rehabilitation + immersive virtual reality (treadmill) 30 sessions | Immersive | Conventional physiotherapy 30 sessions | Static and dynamic balance assessment at baseline and 6 weeks No follow-up | Berg balance scale Timed up and go test Platform for postural and gait control |
| Cannell et al., 2018 [37] | 73 patients GE (n = 35): 72.8 ± 10.4 years GC (n = 38): 74.8 ± 11.9 years Ability to follow instructions and communicate with researchers | Conventional physiotherapy + virtual reality (Jintronix Rehabilitation SystemTM) 14 sessions | Non-immersive | Conventional physiotherapy + functional exercise protocol, strength, balance, and endurance (14 sessions) | Evaluation of static and dynamic balance at baseline and 8 weeks or at hospital discharge. No follow-up | Functional reach test Functional independence measure (FIM) Timed up and go test |
| Kim et al., 2016 [38] | 30 patients GVRCA (n = 10): 56.20 ± 7.56 years GCA (n = 10): 52.00 ± 7.27 years GC (n = 7): 48.71 ± 9.27 years Ability to walk 6 m without technical assistance More than 24 points in the mini mental test | GVRCA: conventional physiotherapy (8 sessions) + virtual reality on treadmill (12 sessions) | Immersive | 1-GCA: conventional physiotherapy (8 sessions) + walking in real environments (12 sessions) 2-GC: conventional physiotherapy: 8 sessions | Evaluation of dynamic balance at baseline and 4 weeks No follow-up | Timed up and go test ABC Scale 6 min walking test |
| Yom et al., 2015 [39] | 20 patients GE (n = 10): 64.60 years GC (n = 10): 78.10 years Score greater than 24 on the mini mental test | Conventional physiotherapy (previous) + virtual reality ankle exercises (30 sessions) | Non-immersive | Conventional physiotherapy (previous) + video observation of the same exercises (30 sessions) | Dynamic baseline evaluation at baseline and 6 weeks No follow-up | Timed up and go test Modified Ashworth Tardieu Scale GAITRite computerized evaluation system |
| Trial | Subjects Were Randomly Allocated to Groups (in a Crossover Study, Subjects Were Randomly Allocated an Order in Which Treatments Were Received). | Alloction Was Concealed. | The Groups Were Similar at Base-Line Regarding the Most Important PrognosticIndicators | There Was Blinding of All Subjects. | There Was Blinding of All Therapists Who Administered the Therapy. | There Was Blinding of All Assessors Who Measured at Least One Key Outcome. | Measures of at Least One Key Outcome Were Obtained from more than 85% of the Subjects Initially Allocated to Groups. | All Subjects for Whom Outcome Measures Were Available Received the Treatment or Control Condition as Allocated, or, Where This Was Not the Case, Data for at Least One Key Outcome Were Analyzed by “Intention to Treat”. | The Results of between-Group Statistical Comparisons Are Reported for at Least One Key Outcome. | The Study Provides Both Point Measures and Measures of Variability for at Least One Key Outcome. | Total Score PEDro Scale |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Karasu et al. [33] | Yes | Yes | Yes | No | No | Yes | Yes | No | Yes | Yes | 8 |
| Bergmann et al. [40] | Yes | Yes | Yes | No | No | Yes | No | No | Yes | Yes | 7 |
| Lee et al. [34] | Yes | No | Yes | No | No | Yes | Yes | Yes | Yes | Yes | 8 |
| Park et al. [35] | Yes | Yes | Yes | No | No | Yes | No | No | Yes | Yes | 7 |
| Lloréns et al. [24] | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes | 8 |
| Lloréns et al. [20] | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes | 8 |
| Cho et al. [17] | Yes | Yes | Yes | No | No | Yes | Yes | No | Yes | Yes | 7 |
| Cannell et al. [37] | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes | 8 |
| Kim et al. [38] | Yes | Yes | Yes | No | No | Yes | Yes | No | Yes | Yes | 7 |
| Yom et al. [39] | Yes | No | Yes | No | No | Yes | Yes | No | Yes | Yes | 6 |
| VARIABLES | ASSESSMENT INSTRUMENTS | STUDY | RESULTS | |
|---|---|---|---|---|
| NON-IMMERSIVE VIRTUAL REALITY | STATIC BALANCE | Berg scale | Karasu et al. [33] | No significant differences between groups in terms of primary and secondary outcome measures at admission (p > 0.05). |
| H. C. Lee et al. [34] | Significant improvements on Berg’s scale (p = 0.000). | |||
| Park et al. [35] | Significant improvements in the experimental group on the Berg scale (p < 0.05). | |||
| Lloréns, Gil-Gómez, et al. [24] | Significant improvement in both groups in terms of balance (p = 0.006). | |||
| Lloréns, Noé, et al. [20] | Significant improvements on the Berg scale (p < 0.05) in the experimental group. | |||
| Functional reach test | Karasu et al. [33] | No significant differences between groups in terms of primary and secondary outcome measures at admission (p > 0.05). | ||
| H. C. Lee et al. [34] | No significant changes were observed. | |||
| Cannell et al. [37] | No significant changes were found between the two groups. | |||
| DYNAMIC BALANCE | Timed up and go test | Karasu et al. [33] | No significant differences between groups on primary and secondary outcomes at entry (p > 0.05). | |
| H. C. Lee et al. [34] | Significant improvements in the TUG scale (p = 0.005.). | |||
| Park et al. [35] | Significant improvements in the experimental group for the timed up and go test (p < 0.05). | |||
| Yom et al. [39] | Significant improvements in dynamic balance (p < 0.05). | |||
| 10 m walking test | Bergmann et al. [40] | Significant improvements in both groups in terms of walking speed (p < 0.01). | ||
| Park et al. [35] | Significant improvements in the experimental group in the timed up and go and 10 m tests p < 0.05). | |||
| Lloréns, Gil-Gómez, et al. [24] | Significant improvement in both groups in terms of gait (p = 0.001). | |||
| IMMERSIVE VIRTUAL REALITY | STATIC BALANCE | Berg scale | K. H. Cho & Lee. [17] | There were significant improvements in terms of static balance (p < 0.01). |
| Functional reach test | ||||
| DYNAMIC BALANCE | Timed up and go test | K. H. Cho & Lee. [17] | Significant improvements in both groups in terms of dynamic balance and gait (p < 0.05). | |
| Kim et al. [38] | Significant changes in gait speed in each group (p < 0.01). | |||
| 10 m walking test |
| Certainly Assessment | № of Patients | Effect | Certainly | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| № of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | Virtual Reality | Other | Relative (95% CI) | Absolute (95% CI) | ||
| Static balance | ||||||||||||
| 7 | Randomized trials | Not serious | Not serious | Not serious | serious | None | 161 | 152 | - | SMD −0.18 (−0.81 to 0.45) | ⨁⨁⨁◯ MODERATE | NOT IMPORTANT |
| Dynamic balance | ||||||||||||
| 7 | Randomized trials | Not serious | Not serious | Not serious | Not serious | None | 123 | 114 | - | SMD −0.33 (−0.6 a −0.06) | ⨁⨁⨁⨁ HIGH | NOT IMPORTANT |
| Follow-up of Static balance | ||||||||||||
| 3 | Randomized trials | Not serious | Not serious | Not serious | serious | None | 53 | 47 | - | SMD −0.6 (−1.08 to −0.13) | ⨁⨁⨁◯ MODERATE | NOT IMPORTANT |
| Follow-up of dynamic balance | ||||||||||||
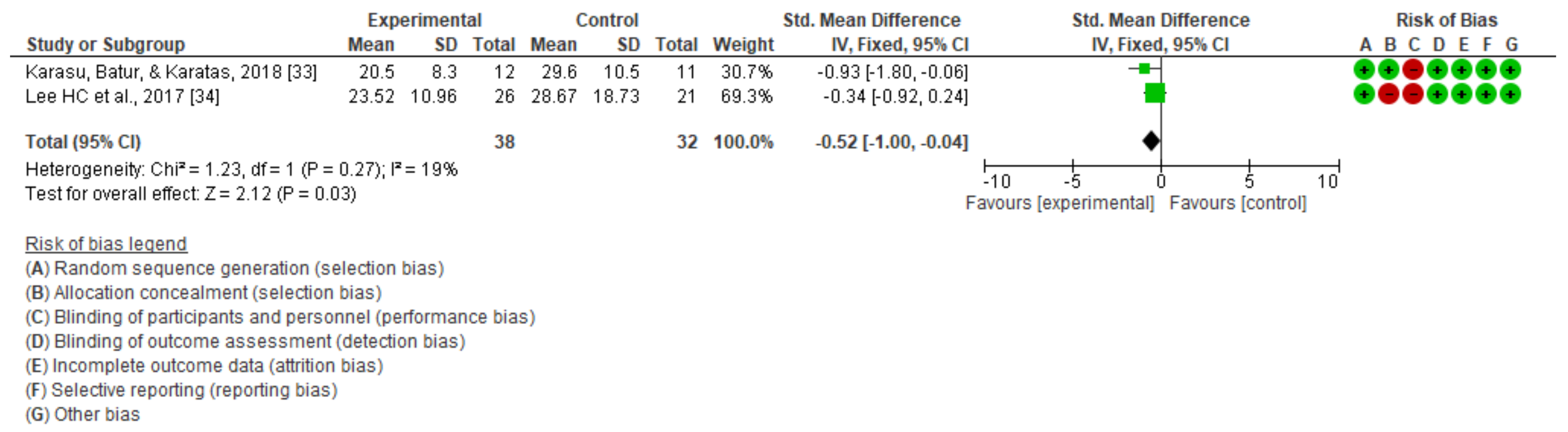
| 2 | Randomized trials | Not serious | Not serious | Not serious | serious | None | 38 | 33 | - | SMD −0.52 (−1 a −0.04) | ⨁⨁⨁◯ MODERATE | NOT IMPORTANT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garay-Sánchez, A.; Suarez-Serrano, C.; Ferrando-Margelí, M.; Jimenez-Rejano, J.J.; Marcén-Román, Y. Effects of Immersive and Non-Immersive Virtual Reality on the Static and Dynamic Balance of Stroke Patients: A Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 4473. https://doi.org/10.3390/jcm10194473
Garay-Sánchez A, Suarez-Serrano C, Ferrando-Margelí M, Jimenez-Rejano JJ, Marcén-Román Y. Effects of Immersive and Non-Immersive Virtual Reality on the Static and Dynamic Balance of Stroke Patients: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2021; 10(19):4473. https://doi.org/10.3390/jcm10194473
Chicago/Turabian StyleGaray-Sánchez, Aitor, Carmen Suarez-Serrano, Mercedes Ferrando-Margelí, Jose Jesus Jimenez-Rejano, and Yolanda Marcén-Román. 2021. "Effects of Immersive and Non-Immersive Virtual Reality on the Static and Dynamic Balance of Stroke Patients: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 10, no. 19: 4473. https://doi.org/10.3390/jcm10194473






