Comparison of the Effect of Pan-Retinal Photocoagulation and Intravitreal Conbercept Treatment on the Change of Retinal Vessel Density Monitored by Optical Coherence Tomography Angiography in Patients with Proliferative Diabetic Retinopathy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Treatment Protocol
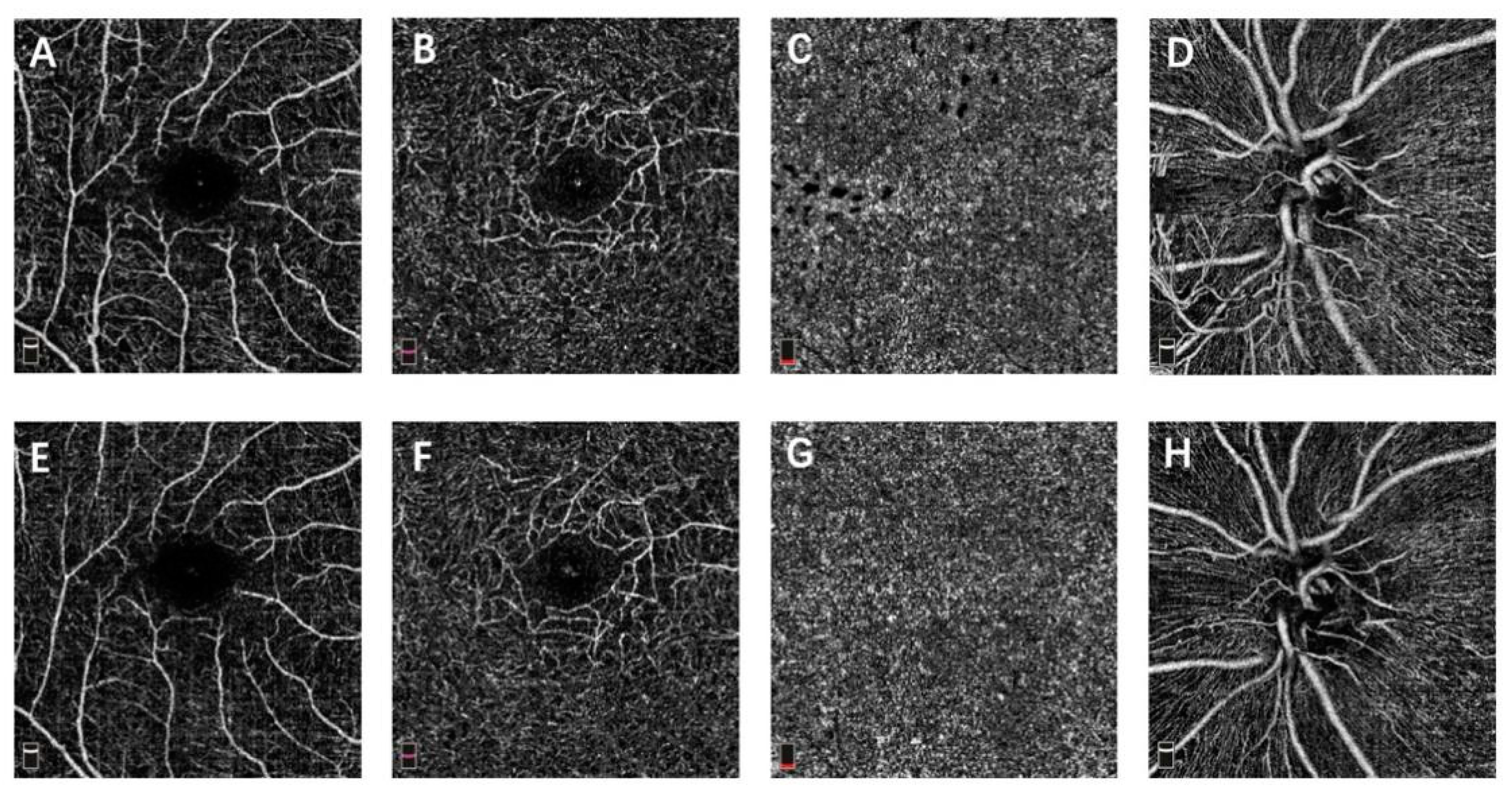
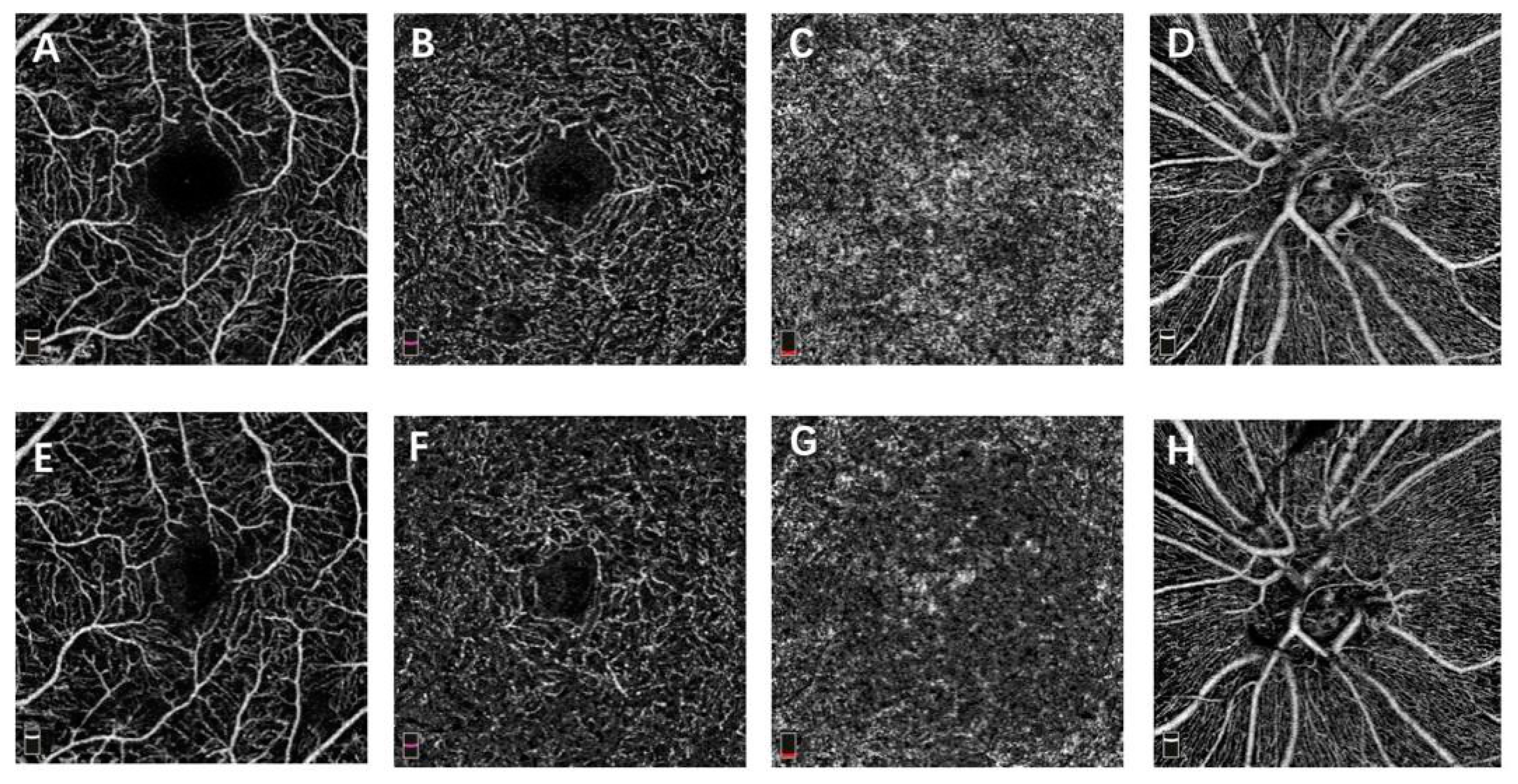
2.3. OCTA Image Acquisition
2.4. Statistical Analysis
3. Results
Vessel Density and FAZ on OCTA
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Early Treatment Diabetic Retinopathy Study Research Group. Early Photocoagulation for Diabetic Retinopathy: ETDRS Report Number 9. Ophthalmology 1991, 98, 766–785. [Google Scholar] [CrossRef]
- Royle, P.; Mistry, H.; Auguste, P.; Shyangdan, D.; Freeman, K.; Lois, N.; Waugh, N. Pan-retinal photocoagulation and other forms of laser treatment and drug therapies for non-proliferative diabetic retinopathy: Systematic review and economic evaluation. Health Technol. Assess. 2015, 19, 1–248. [Google Scholar] [CrossRef] [Green Version]
- Gross, J.G.; Glassman, A.R.; Jampol, L.M.; Inusah, S.; Aiello, L.P.; Antoszyk, A.N.; Baker, C.W.; Berger, B.B.; Bressler, N.M. Panretinal Photocoagulation vs Intravitreous Ranibizumab for Proliferative Diabetic Retinopathy: A randomized clinical trial. JAMA 2015, 314, 2137–2146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gross, J.G.; Glassman, A.R.; Liu, D.; Sun, J.K.; Antoszyk, A.N.; Baker, C.W.; Bressler, N.M.; Elman, M.J.; Ferris, F.L.; Gardner, T.W.; et al. Five-Year Outcomes of Panretinal Photocoagulation vs Intravitreous Ranibizumab for Proliferative Diabetic Retinopathy: A Randomized Clinical Trial. JAMA Ophthalmol. 2018, 136, 1138–1148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tey, K.Y.; Teo, K.; Tan, A.C.S.; Devarajan, K.; Tan, B.; Tan, J.; Schmetterer, L.; Ang, M. Optical coherence tomography angiography in diabetic retinopathy: A review of current applications. Eye Vis. 2019, 6, 1–10. [Google Scholar] [CrossRef]
- Durbin, M.K.; An, L.; Shemonski, N.D.; Soares, M.; Santos, T.; Lopes, M.; Neves, C.; Cunha-Vaz, J. Quantification of Retinal Microvascular Density in Optical Coherence Tomographic Angiography Images in Diabetic Retinopathy. JAMA Ophthalmol. 2017, 135, 370–376. [Google Scholar] [CrossRef]
- Zhu, Z.; Liang, Y.; Yan, B.; Meng, Z.; Long, K.; Zhang, Y.; Luo, J. Clinical effect of conbercept on improving diabetic macular ischemia by OCT angiography. BMC Ophthalmol. 2020, 20, 1–9. [Google Scholar] [CrossRef]
- Li, Z.J.; Xiao, J.H.; Zeng, P.; Zeng, R.; Gao, X.; Zhang, Y.C.; Lan, Y.Q. Optical coherence tomography angiography assessment of 577 nm laser effect on severe non-proliferative diabetic retinopathy with diabetic macular edema. Int. J. Ophthalmol. 2020, 45, 1257–1265. [Google Scholar] [CrossRef]
- Lorusso, M.; Milano, V.; Nikolopoulou, E.; Ferrari, L.M.; Cicinelli, M.V.; Querques, G.; Ferrari, T.M. Panretinal Photocoagulation Does Not Change Macular Perfusion in Eyes With Proliferative Diabetic Retinopathy. Ophthalmic Surg. Lasers Imaging Retin. 2019, 50, 174–178. [Google Scholar] [CrossRef]
- Alagorie, A.R.; Nittala, M.G.; Velaga, S.; Zhou, B.; Rusakevich, A.M.; Wykoff, C.C.; Sadda, S.R. Association of Intravitreal Aflibercept With Optical Coherence Tomography Angiography Vessel Density in Patients with Proliferative Diabetic Retinopathy: A Secondary Analysis of a Randomized Clinical Trial. JAMA Ophthalmol. 2020, 138, 851–857. [Google Scholar] [CrossRef]
- Camino, A.; Zhang, M.; Gao, S.S.; Hwang, T.; Sharma, U.; Wilson, D.J.; Huang, D.; Jia, Y. Evaluation of artifact reduction in optical coherence tomography angiography with real-time tracking and motion correction technology. Biomed. Opt. Express 2016, 7, 3905–3915. [Google Scholar] [CrossRef] [PubMed]
- Gawęcki, M. Micropulse Laser Treatment of Retinal Diseases. J. Clin. Med. 2019, 8, 242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomic, L.; Bjärnhall, G.; Mäepea, O.; Sperber, G.O.; Alm, A. Effects of oxygen and carbon dioxide on human retinal circulation: An investigation using blue field simulation and scanning laser ophthalmoscopy. Acta Ophthalmol. Scand. 2005, 83, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.A.; Stefansson, E.; Klombers, L.; Hubbard, L.D.; Kaufman, S.C.; Ferris, F.L. Optic Disk Neovascularization and Retinal Vessel Diameter in Diabetic Retinopathy. Am. J. Ophthalmol. 1988, 106, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Mendrinos, E.; Mangioris, G.; Papadopoulou, D.N.; Dosso, A.A.; Pournaras, C.J. Retinal Vessel Analyzer Measurements of the Effect of Panretinal Photocoagulation on the Retinal Arteriolar Diameter in Diabetic Retinopathy. Retina 2010, 30, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Bhanushali, D.; Anegondi, N.; Gadde, S.G.K.; Srinivasan, P.; Chidambara, L.; Yadav, N.K.; Roy, A.S. Linking Retinal Microvasculature Features with Severity of Diabetic Retinopathy Using Optical Coherence Tomography Angiography. Investig. Opthalmol. Vis. Sci. 2016, 57, 519–525. [Google Scholar] [CrossRef]
- Fayed, A.E.; AbdelBaki, A.M.; El Zawahry, O.M.; Fawzi, A.A. Optical coherence tomography angiography reveals progressive worsening of retinal vascular geometry in diabetic retinopathy and improved geometry after panretinal photocoagulation. PLoS ONE 2019, 14, e0226629. [Google Scholar] [CrossRef] [Green Version]
- Fawzi, A.A.; Fayed, A.E.; Linsenmeier, R.A.; Gao, J.; Yu, F. Improved Macular Capillary Flow on Optical Coherence Tomography Angiography After Panretinal Photocoagulation for Proliferative Diabetic Retinopathy. Am. J. Ophthalmol. 2019, 206, 217–227. [Google Scholar] [CrossRef]
- Wang, Q.; Li, T.; Wu, Z.; Wu, Q.; Ke, X.; Luo, D.; Wang, H. Novel VEGF Decoy Receptor Fusion Protein Conbercept Targeting Multiple VEGF Isoforms Provide Remarkable Anti-Angiogenesis Effect In Vivo. PLoS ONE 2013, 8, e70544. [Google Scholar] [CrossRef] [Green Version]
- He, F.; Yang, J.; Zhang, X.; Yu, W. Efficacy of conbercept combined with panretinal photocoagulation in the treatment of proliferative diabetic retinopathy. Sci. Rep. 2020, 10, 8778. [Google Scholar] [CrossRef]
- Xia, J.-P.; Liu, S.-Q.; Wang, S. Intravitreal conbercept improves outcome of proliferative diabetic retinopathy through inhibiting inflammation and oxidative stress. Life Sci. 2020, 265, 118795. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, D.N.; Mendrinos, E.; Mangioris, G.; Donati, G.; Pournaras, C.J. Intravitreal Ranibizumab May Induce Retinal Arteriolar Vasoconstriction in Patients with Neovascular Age-related Macular Degeneration. Ophthalmology 2009, 116, 1755–1761. [Google Scholar] [CrossRef]
- Manousaridis, K.; Talks, J. Macular ischaemia: A contraindication for anti-VEGF treatment in retinal vascular disease? Br. J. Ophthalmol. 2012, 96, 179–184. [Google Scholar] [CrossRef]
- Conti, F.F.; Song, W.; Rodrigues, E.B.; Singh, R.P. Changes in retinal and choriocapillaris density in diabetic patients receiving anti-vascular endothelial growth factor treatment using optical coherence tomography angiography. Int. J. Retin. Vitr. 2019, 5, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Early Treatment Diabetic Retinopathy Study Research Group. Classification of Diabetic Retinopathy from Fluorescein Angiograms. Ophthalmology 1991, 98, 807–822. [Google Scholar] [CrossRef]
- Salz, D.A.; De Carlo, T.E.; Adhi, M.; Moult, E.M.; Choi, W.; Baumal, C.R.; Witkin, A.J.; Duker, J.S.; Fujimoto, J.G.; Waheed, N.K. Select Features of Diabetic Retinopathy on Swept-Source Optical Coherence Tomographic Angiography Compared With Fluorescein Angiography and Normal Eyes. JAMA Ophthalmol. 2016, 134, 644–650. [Google Scholar] [CrossRef]
- Sivaprasad, S.; Hykin, P.; Prevost, A.T.; Vasconcelos, J.; Riddell, A.; Ramu, J.; Murphy, C.; Kelly, J.; Edwards, R.T.; Yeo, S.T.; et al. Intravitreal aflibercept compared with panretinal photocoagulation for proliferative diabetic retinopathy: The CLARITY non-inferiority RCT. Effic. Mech. Eval. 2018, 5, 1–112. [Google Scholar] [CrossRef] [PubMed]
| PRP (n = 29) | IVC (n = 26) | p | |
|---|---|---|---|
| Age (years) | 54.00 ± 10.46 | 55.12 ± 11.08 | 0.763 * |
| Gender (male/female) | 13/16 | 15/11 | 0.341 † |
| Glycated hemoglobin (%) | 8.02 ± 1.24 | 7.63 ± 1.75 | 0.342 * |
| Duration of diabetes (years) | 12.24 ± 6.73 | 10.56 ± 6.55 | 0.353 * |
| BCVA (letters) | 74.10 ± 9.66 | 75.57 ± 9.94 | 0.595 * |
| CFT (μm) | 285.8 ± 40.74 | 296.8 ± 42.38 | 0.343 * |
| Layers and Region | PRP (n = 29) | IVC (n = 26) | p |
|---|---|---|---|
| SCP—whole scan | 30.86 ± 4.11 | 31.40 ± 3.56 | 0.621 |
| SCP—foveal | 12.65 ± 3.60 | 11.60 ± 3.67 | 0.307 |
| SCP—parafoveal | 32.14 ± 4.59 | 33.45 ± 3.88 | 0.281 |
| DCP—whole scan | 42.22 ± 5.33 | 43.14 ± 5.48 | 0.542 |
| DCP—foveal | 28.04 ± 7.19 | 25.43 ± 6.94 | 0.194 |
| DCP—parafoveal | 43.99 ± 6.40 | 45.75 ± 5.92 | 0.313 |
| CC—whole scan | 52.75 ± 4.24 | 51.53 ± 3.74 | 0.286 |
| CC—foveal | 52.73 ± 6.19 | 49.37 ± 6.82 | 0.068 |
| CC—parafoveal | 52.77 ± 4.88 | 53.02 ± 3.28 | 0.838 |
| RPC—whole scan | 44.89 ± 3.19 | 43.66 ± 1.87 | 0.108 |
| RPC—inside disc | 40.69 ± 4.37 | 38.64 ± 5.97 | 0.159 |
| RPC—peripapilary | 47.31 ± 3.21 | 46.39 ± 2.23 | 0.244 |
| FAZ area (mm2) | 0.30 ± 0.09 | 0.33 ± 0.07 | 0.264 |
| Layers and Region | PRP (n = 29) | IVC (n = 26) | p |
|---|---|---|---|
| SCP—whole scan | 33.06 ± 5.48 | 33.04 ± 4.82 | 0.987 |
| SCP—foveal | 12.37 ± 2.34 | 11.23 ± 2.73 | 0.102 |
| SCP—parafoveal | 33.67 ± 6.48 | 34.88 ± 5.45 | 0.477 |
| DCP—whole scan | 42.72 ± 6.78 | 43.30 ± 5.39 | 0.743 |
| DCP—foveal | 26.91 ± 8.02 | 23.98 ± 5.09 | 0.134 |
| DCP—parafoveal | 45.48 ± 7.06 | 45.85 ± 5.71 | 0.840 |
| CC—whole scan | 53.19 ± 5.32 | 53.67 ± 6.45 | 0.772 |
| CC—foveal | 54.30 ± 6.95 | 51.05 ± 8.15 | 0.127 |
| CC—parafoveal | 53.12 ± 5.47 | 53.74 ± 6.56 | 0.709 |
| RPC—whole scan | 44.54 ± 2.56 | 44.36 ± 2.09 | 0.779 |
| RPC—inside disc | 40.62 ± 6.68 | 40.36 ± 9.21 | 0.908 |
| RPC—peripapilary | 47.20 ± 3.21 | 47.47 ± 2.43 | 0.765 |
| FAZ area (mm2) | 0.33 ± 0.05 | 0.35 ± 0.06 | 0.302 |
| Layers and Region | Significance of Difference between Baseline and Month 12 (p Value) | |
|---|---|---|
| PRP Group | IVC Group | |
| SCP—whole scan | 0.092 | 0.149 |
| SCP—foveal | 0.742 | 0.386 |
| SCP—parafoveal | 0.151 | 0.341 |
| DCP—whole scan | 0.713 | 0.913 |
| DCP—foveal | 0.522 | 0.303 |
| DCP—parafoveal | 0.395 | 0.942 |
| CC—whole scan | 0.670 | 0.228 |
| CC—foveal | 0.331 | 0.311 |
| CC—parafoveal | 0.798 | 0.571 |
| RPC—whole scan | 0.674 | 0.085 |
| RPC—inside disc | 0.964 | 0.744 |
| RPC—peripapilary | 0.879 | 0.096 |
| FAZ area | 0.602 | 0.464 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; Yu, M.; Zhou, L.; Li, C.; Lu, L.; Jin, C. Comparison of the Effect of Pan-Retinal Photocoagulation and Intravitreal Conbercept Treatment on the Change of Retinal Vessel Density Monitored by Optical Coherence Tomography Angiography in Patients with Proliferative Diabetic Retinopathy. J. Clin. Med. 2021, 10, 4484. https://doi.org/10.3390/jcm10194484
Zhao H, Yu M, Zhou L, Li C, Lu L, Jin C. Comparison of the Effect of Pan-Retinal Photocoagulation and Intravitreal Conbercept Treatment on the Change of Retinal Vessel Density Monitored by Optical Coherence Tomography Angiography in Patients with Proliferative Diabetic Retinopathy. Journal of Clinical Medicine. 2021; 10(19):4484. https://doi.org/10.3390/jcm10194484
Chicago/Turabian StyleZhao, Hongkun, Minzhong Yu, Lijun Zhou, Cong Li, Lin Lu, and Chenjin Jin. 2021. "Comparison of the Effect of Pan-Retinal Photocoagulation and Intravitreal Conbercept Treatment on the Change of Retinal Vessel Density Monitored by Optical Coherence Tomography Angiography in Patients with Proliferative Diabetic Retinopathy" Journal of Clinical Medicine 10, no. 19: 4484. https://doi.org/10.3390/jcm10194484






