Correlation between α1-Antitrypsin Deficiency and SARS-CoV-2 Infection: Epidemiological Data and Pathogenetic Hypotheses
Abstract
:1. Introduction
2. Genetics, Epidemiology, and Clinical Relevance of AATD
3. AATD and COVID-19: Geographical Overlap and Data from Clinical Registries
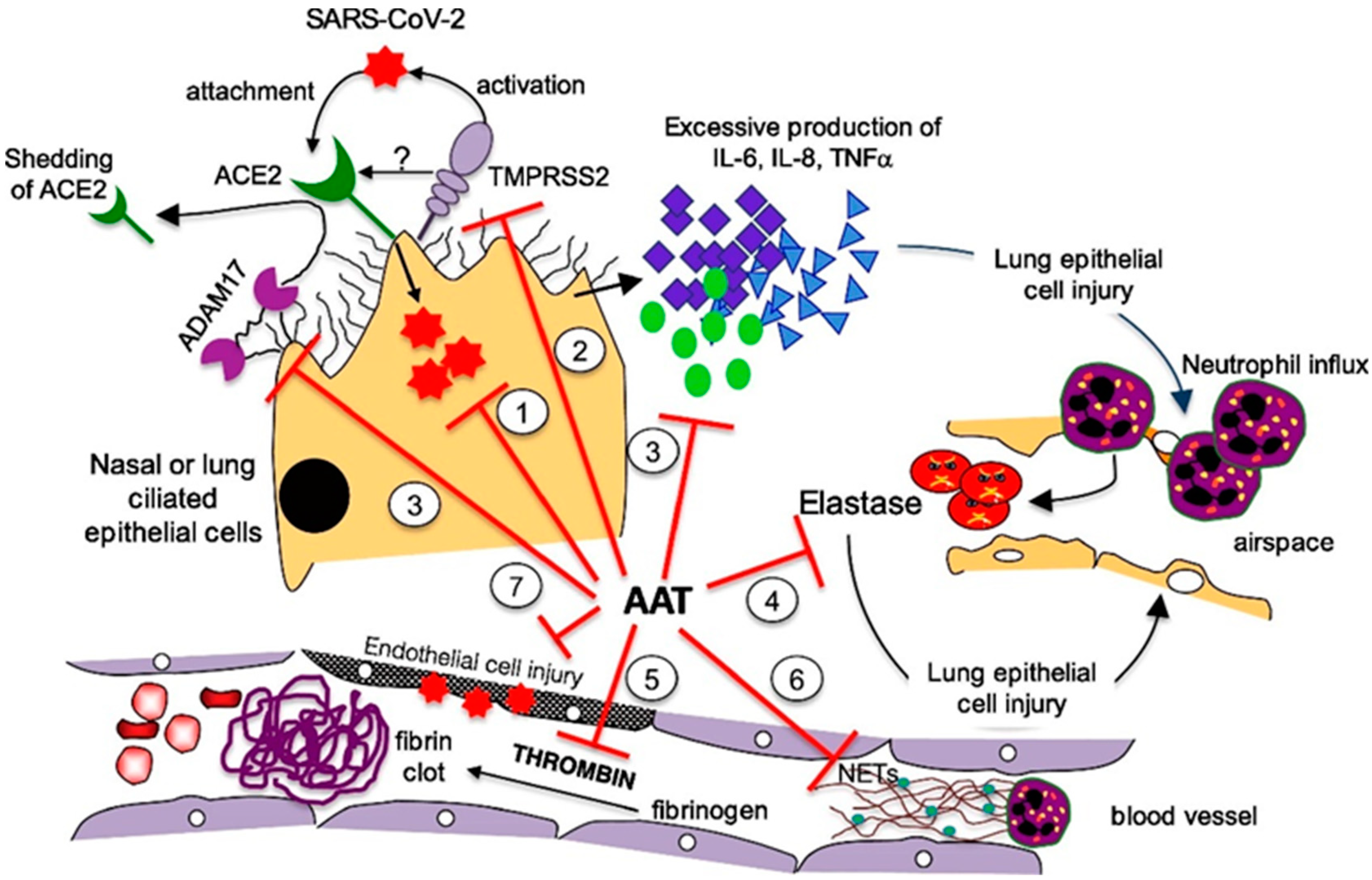
4. Shared Pathogenic Pathways
- Enhancement of host immunity: The antiviral effects of AAT have been documented for different RNA viruses, including the influenza virus [34] and human immunodeficiency virus (HIV) [35,36,37,38,39]. Indeed, AAT has been proven to block HIV entry into CD4+ lymphocytes and inhibit HIV replication. Moreover, AAT has been shown to enhance host immunity against Pseudomonas aeruginosa [40] and Mycobacterium intracellulare [41] by inducing autophagy, which is also implicated in the control of MERS-CoV infection [42].
- Inhibition of TMPRSS2: SARS-CoV-2 is an enveloped, single-stranded RNA virus. A pivotal role in cell entry is played by a viral surface “spike” protein, which is arranged in a trimeric form. The spike protein is composed of two subunits, S1 and S2. Two sequential cleavages are needed for the virus to enter the human cells. The first is performed by furin or furin-like proteases (which are ubiquitous in the human body) at the S1/S2 site. Subsequently, the spike protein binds to the host cell surface receptor angiotensin-converting enzyme 2 (ACE2) through its receptor-binding domain, undergoing conformational changes that make it possible for a second cleavage mediated by transmembrane serine protease 2 (TMPRSS2), commonly expressed in epithelial cells [43]. Following cleavage, SARS-CoV-2 may enter human cells through endosomal and/or non-endosomal pathways. In the first case, the virus enters the endosomes, whereas in the second case, the envelope directly fuses with the cell plasma membrane. In addition to furin/furin-like proteases and TMPRSS2, other proteases are involved in SARS-CoV-2 cell entry, which enhance the infectivity and transmissibility of the virus. In particular, several membrane-associated serine proteinases, including proprotein convertase 1 (PC1), trypsin, and matriptase-2, may synergize with or replace TMPRSS2 as the cellular activator of SARS-CoV-2 [44]. ACE2 is a component of the renin-angiotensin system that plays a role in the systemic regulation of the cardiovascular and renal systems, lungs, and liver by acting on blood pressure, electrolyte balance control mechanisms, and inflammation. ACE2 also plays a protective role against lung injury, diabetic cardiovascular complications, myocardial infarction, and disseminated intravascular coagulation. Interestingly, all of these conditions are associated with severe COVID-19 outcomes [45]. In vitro, TMPRSS2 inhibition has been demonstrated to prevent SARS-CoV-2 cell entry [46,47,48]. Based on the demonstration of the in vitro inhibition of SARS-CoV-2 cell entry by AAT [49,50], Wettstein et al. hypothesized that its effect could be related to the inhibition of TMPRSS2-mediated priming [50].
- Anti-inflammatory activity: AAT has strong anti-inflammatory properties, including the following:
- Inhibition of disintegrin/metalloproteinase 17 (ADAM17) [45]: ADAM17 is activated by the spike protein of coronaviruses and cleaves membrane-bound TNF-α to soluble TNF-α. Moreover, ADAM17 causes ACE2 shedding [58]. ACE2 shedding may increase inflammatory response by preventing the formation of the anti-inflammatory peptides, angiotensin-(1–7) and angiotensin-(1–9) [59].
- Protection against acute lung injury (ALI): AAT inhibits NE activity, which is known to mediate ALI at the sites of acute inflammation by inducing the release of IL-8 from neutrophil vesicles and facilitating the conversion of pro-IL-1β to IL-1β [60,61]. Moreover, AAT prevents ACE2 shedding by inhibiting ADAM17; increased ACE2 levels may inactivate bradykinin, which is essential for the leakage of exudate through the alveolar-capillary membrane in patients with non-cardiogenic pulmonary oedema [62].
- Inhibition of NETs adherence: NETs essentially consist of neutrophil-derived decondensed chromatin (cell-free DNA) combined with other proteins (i.e., elastase and cathepsin G), aimed at trapping and killing extracellular pathogens. In patients with COVID-19, the aberrant production of NETs plays a pathogenic role in immuno-thrombosis, mucous secretion, and cytokine production [64,65,66,67,68,69]. AAT may inhibit elastase, which is crucial for NET formation. Ex vivo studies have shown that AAT modifies the shape of NETs and reduces their adherence [70].
- Reduced levels of functional AAT would prompt the activation of TMPRSS2, thus promoting SARS-CoV-2 cell entry;
- The lack of thrombin and plasmin inhibition would increase the risk of coagulation disorders;
- Reduced anti-inflammatory, anti-cell death, anti-protease, and anti-coagulation activities would result in a greater probability of developing severe ALI [73].
5. Augmentation Therapy for Patients with COVID-19
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 21 September 2021).
- Yamamoto, N.; Yamamoto, R.; Ariumi, Y.; Mizokami, M.; Shimotohno, K.; Yoshikura, H. Does genetic predisposition contribute to the exacerbation of COVID-19 symptoms in individuals with comorbidities and explain the huge mortality disparity between the East and the West? Int. J. Mol. Sci. 2021, 22, 5000. [Google Scholar] [CrossRef] [PubMed]
- McElvaney, O.J.; McEvoy, N.L.; McElvaney, O.F.; Carroll, T.P.; Murphy, M.P.; Dunlea, D.M.; Ni Choileain, O.; Clarke, J.; O’Connor, E.; Hogan, G.; et al. Characterization of the Inflammatory Response to Severe COVID-19 Illness. Am. J. Respir. Crit. Care Med. 2020, 202, 812–821. [Google Scholar] [CrossRef] [PubMed]
- Van Der Made, C.I.; Simons, A.; Schuurs-Hoeijmakers, J.; Heuvel, G.V.D.; Mantere, T.; Kersten, S.; Van Deuren, R.C.; Steehouwer, M.; Van Reijmersdal, S.V.; Jaeger, M.; et al. Presence of Genetic Variants Among Young Men with Severe COVID-19. JAMA 2020, 324, 663. [Google Scholar] [CrossRef]
- McCoy, K.; Peterson, A.; Tian, Y.; Sang, Y. Immunogenetic Association Underlying Severe COVID-19. Vaccines 2020, 8, 700. [Google Scholar] [CrossRef] [PubMed]
- Souilmi, Y.; Lauterbur, M.E.; Tobler, R.; Huber, C.D.; Johar, A.S.; Moradi, S.V.; Johnston, W.A.; Krogan, N.J.; Alexandrov, K.; Enard, D. An ancient viral epidemic involving host coronavirus interacting genes more than 20,000 years ago in East Asia. Curr. Biol. 2021, 31, 3504–3514.e9. [Google Scholar] [CrossRef]
- Laurell, C.-B.; Eriksson, S. The Electrophoretic α1-Globulin Pattern of Serum in α1-Antitrypsin Deficiency. COPD J. Chronic Obstr. Pulm. Dis. 2013, 10, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Strnad, P.; McElvaney, N.G.; Lomas, D.A. Alpha1-Antitrypsin Deficiency. N. Engl. J. Med. 2020, 382, 1443–1455. [Google Scholar] [CrossRef]
- Wout, E.F.A.V.; Van Schadewijk, A.; Savage, N.D.L.; Stolk, J.; Hiemstra, P.S. α1-Antitrypsin Production by Proinflammatory and Antiinflammatory Macrophages and Dendritic Cells. Am. J. Respir. Cell Mol. Biol. 2012, 46, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Ferrarotti, I.; Ottaviani, S.; De Silvestri, A.; Corsico, A.G. Update on α1-antitrypsin deficiency. Breathe 2018, 14, e17–e24. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Keshavjee, S.; Liu, M. Alpha-1 Antitrypsin for COVID-19 Treatment: Dual Role in Antiviral Infection and Anti-Inflammation. Front. Pharmacol. 2020, 11, 615398. [Google Scholar] [CrossRef]
- Azouz, N.P.; Klingler, A.M.; Callahan, V.; Akhrymuk, I.V.; Elez, K.; Raich, L.; Henry, B.M.; Benoit, J.L.; Benoit, S.W.; Noé, F.; et al. Alpha 1 Antitrypsin Is an Inhibitor of the SARS-CoV-2–Priming Protease TMPRSS2. Pathog. Immun. 2021, 6, 55–74. [Google Scholar]
- Hatipoğlu, U.; Stoller, J.K. α1-Antitrypsin Deficiency. Clin. Chest Med. 2016, 37, 487–504. [Google Scholar] [CrossRef]
- Miravitlles, M.; Dirksen, A.; Ferrarotti, I.; Koblizek, V.; Lange, P.; Mahadeva, R.; McElvaney, N.G.; Parr, D.; Piitulainen, E.; Roche, N.; et al. European Respiratory Society statement: Diagnosis and treatment of pulmonary disease in α1-antitrypsin deficiency. Eur. Respir. J. 2017, 50, 1700610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vianello, A.; Caminati, M.; Senna, G.; Arcolaci, A.; Chieco-Bianchi, F.; Ferrarotti, I.; Guarnieri, G.; Molena, B.; Crisafulli, E. Effect of α1 antitrypsin deficiency on lung volume decline in severe asthmatic patients undergoing biologic therapy. J. Allergy Clin. Immunol. Pr. 2021, 9, 1414–1416. [Google Scholar] [CrossRef] [PubMed]
- Luisetti, M. 1-Antitrypsin deficiency 1: Epidemiology of 1-antitrypsin deficiency. Thorax 2004, 59, 164–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, C.E.; Vayalapra, S.; Hampson, J.A.; Mukherjee, D.; Stockley, R.A.; Turner, A. PiSZ alpha-1 antitrypsin deficiency (AATD): Pulmonary phenotype and prognosis relative to PiZZ AATD and PiMM COPD. Thorax 2015, 70, 939–945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanco, I.; Bueno, P.; Diego, I.; Pérez-Holanda, S.; Lara, B.; Casas, F.; Esquinas, C.; Miravitlles, M. Alpha-1 antitrypsin Pi*SZ genotype: Estimated prevalence and number of SZ subjects worldwide. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 1683–1694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Serres, F.; Blanco, I.; Fernández-Bustillo, E. Genetic epidemiology of alpha-1 antitrypsin deficiency in southern Europe: France, Italy, Portugal and Spain. Clin. Genet. 2003, 63, 490–509. [Google Scholar] [CrossRef]
- O’Brien, M.E.; Fee, L.; Browne, N.; Carroll, T.P.; Meleady, P.; Henry, M.; McQuillan, K.; Murphy, M.P.; Logan, M.; McCarthy, C.; et al. Activation of complement component 3 is associated with airways disease and pulmonary emphysema in alpha-1 antitrypsin deficiency. Thorax 2020, 75, 321–330. [Google Scholar] [CrossRef] [Green Version]
- Fischer, H.-P.; Ortiz-Pallardó, M.; Ko, Y.; Esch, C.; Zhou, H. Chronic liver disease in heterozygous α1-antitrypsin deficiency PiZ. J. Hepatol. 2000, 33, 883–892. [Google Scholar] [CrossRef]
- Hill, A.T.; Campbell, E.J.; Bayley, D.L.; Hill, S.L.; Stockley, R.A. Evidence for Excessive Bronchial Inflammation during an Acute Exacerbation of Chronic Obstructive Pulmonary Disease in Patients with α1-Antitrypsin Deficiency (PiZ). Am. J. Respir. Crit. Care Med. 1999, 160, 1968–1975. [Google Scholar] [CrossRef]
- Vogelmeier, C.F.; Criner, G.J.; Martinez, F.J.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Chen, R.; Decramer, M.; Fabbri, L.M.; et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report: GOLD Executive Summary. Eur. Respir. J. 2017, 49, 1700214. [Google Scholar] [CrossRef] [PubMed]
- Gulack, B.C.; Mulvihill, M.S.; Ganapathi, A.; Speicher, P.J.; Chery, G.; Snyder, L.; Davis, R.D.; Hartwig, M.G. Survival after lung transplantation in recipients with alpha-1-antitrypsin deficiency compared to other forms of chronic obstructive pulmonary disease: A national cohort study. Transpl. Int. 2017, 31, 45–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vianello, A.; Braccioni, F. Geographical Overlap Between Alpha-1 Antitrypsin Deficiency and COVID-19 Infection in Italy: Casual or Causal? Arch. Bronconeumol. 2020, 56, 609–610. [Google Scholar] [CrossRef]
- Italian Registry of Patients with Alpha-1 Antitrypsin Deficiency. Available online: http://alfa1antitripsina.it/it (accessed on 31 August 2021).
- Yoshikura, H. Epidemiological correlation between COVID-19 epidemic and prevalence of α-1 antitrypsin deficiency in the world. Glob. Health Med. 2021, 3, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Shapira, G.; Shomron, N.; Gurwitz, D. Ethnic differences in alpha-1 antitrypsin deficiency allele frequencies may partially explain national differences in COVID-19 fatality rates. FASEB J. 2020, 34, 14160–14165. [Google Scholar] [CrossRef]
- Bhattacharyya, C.; Das, C.; Ghosh, A.; Singh, A.K.; Mukherjee, S.; Majumder, P.P.; Basu, A.; Biswas, N.K. SARS-CoV-2 mutation 614G creates an elastase cleavage site enhancing its spread in high AAT-deficient regions. Infect. Genet. Evol. 2021, 90, 104760. [Google Scholar] [CrossRef]
- Ferrarotti, I.; Ottaviani, S.; Balderacchi, A.; Barzon, V.; De Silvestri, A.; Piloni, D.; Mariani, F.; Corsico, A. COVID-19 infection in severe Alpha 1-antitrypsin deficiency: Looking for a rationale. Respir. Med. 2021, 183, 106440. [Google Scholar] [CrossRef]
- Faria, N.; Costa, M.I.; Gomes, J.; Sucena, M. Alpha-1 antitrypsin deficiency severity and the risk of COVID-19: A Portuguese cohort. Respir. Med. 2021, 181, 106387. [Google Scholar] [CrossRef]
- Schneider, C.V.; Strnad, P. SARS-CoV-2 Infection in Alpha1-Antitrypsin Deficiency. Respir. Med. 2021, 184, 106466. [Google Scholar] [CrossRef]
- Bai, X.; Hippensteel, J.; Leavitt, A.; Maloney, J.P.; Beckham, D.; Garcia, C.; Li, Q.; Freed, B.M.; Ordway, D.; Sandhaus, R.A.; et al. Hypothesis: Alpha-1-antitrypsin is a promising treatment option for COVID-19. Med. Hypotheses 2020, 146, 110394. [Google Scholar] [CrossRef]
- Harbig, A.; Mernberger, M.; Bittel, L.; Pleschka, S.; Schughart, K.; Steinmetzer, T.; Stiewe, T.; Nist, A.; Böttcher-Friebertshäuser, E. Transcriptome profiling and protease inhibition experiments identify proteases that activate H3N2 influenza A and influenza B viruses in murine airways. J. Biol. Chem. 2020, 295, 11388–11407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, X.; Liu, Z.; Zhang, J.; Adelsberger, J.W.; Yang, J.; Burton, G.F. Alpha-1-antitrypsin interacts with gp41 to block HIV-1 entry into CD4+ T lymphocytes. BMC Microbiol. 2016, 16, 172. [Google Scholar] [CrossRef] [Green Version]
- Bryan, C.L.; Beard, K.S.; Pott, G.B.; Rahkola, J.; Gardner, E.M.; Janoff, E.N.; Shapiro, L. HIV infection is associated with reduced serum alpha-1-antitrypsin concentrations. Clin. Investig. Med. 2010, 33, E384–E389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, X.; Shapiro, L.; Fellingham, G.; Willardson, B.M.; Burton, G.F. HIV Replication in CD4+ T Lymphocytes in the Presence and Absence of Follicular Dendritic Cells: Inhibition of Replication Mediated by α-1-Antitrypsin through Altered IκBα Ubiquitination. J. Immunol. 2011, 186, 3148–3155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitney, J.B.; Asmal, M.; Geiben-Lynn, R. Serpin Induced Antiviral Activity of Prostaglandin Synthetase-2 against HIV-1 Replication. PLoS ONE 2011, 6, e18589. [Google Scholar] [CrossRef] [Green Version]
- Shapiro, L.; Pott, G.B.; Ralston, A.H. Alpha-1-antitrypsin inhibits human immunodeficiency virus type 1. FASEB J. 2000, 15, 115–122. [Google Scholar] [CrossRef]
- Pott, G.B.; Beard, K.S.; Bryan, C.L.; Merrick, D.T.; Shapiro, L. Alpha-1 Antitrypsin Reduces Severity of Pseudomonas Pneumonia in Mice and Inhibits Epithelial Barrier Disruption and Pseudomonas Invasion of Respiratory Epithelial Cells. Front. Public Health 2013, 1, 19. [Google Scholar] [CrossRef] [Green Version]
- Bai, X.; Bai, A.; Honda, J.R.; Eichstaedt, C.; Musheyev, A.; Feng, Z.; Huitt, G.; Harbeck, R.; Kosmider, B.; Sandhaus, R.A.; et al. Alpha-1-Antitrypsin Enhances Primary Human Macrophage Immunity Against Non-tuberculous Mycobacteria. Front. Immunol. 2019, 10, 1417. [Google Scholar] [CrossRef]
- Gassen, N.C.; Niemeyer, D.; Muth, D.; Corman, V.M.; Martinelli, S.; Gassen, A.; Hafner, K.; Papies, J.; Mösbauer, K.; Zellner, A.; et al. SKP2 attenuates autophagy through Beclin1-ubiquitination and its inhibition reduces MERS-Coronavirus infection. Nat. Commun. 2019, 10, 1–16. [Google Scholar] [CrossRef]
- Peng, R.; Wu, L.-A.; Wang, Q.; Qi, J.; Gao, G.F. Cell Entry by SARS-CoV-2. Trends Biochem. Sci. 2021, 46, 848–860. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Prior, P. Priming of SARS-CoV-2 S protein by several membrane-bound serine proteinases could explain enhanced viral infectivity and systemic COVID-19 infection. J. Biol. Chem. 2021, 296, 100135. [Google Scholar] [CrossRef] [PubMed]
- De Loyola, M.B.; dos Reis, T.T.A.; de Oliveira, G.X.L.M.; Palmeira, J.; Argañaraz, G.A.; Argañaraz, E.R. Alpha-1-antitrypsin: A possible host protective factor against Covid-19. Rev. Med. Virol. 2020, 31, e2157. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Kawase, M.; Shirato, K.; van der Hoek, L.; Taguchi, F.; Matsuyama, S. Simultaneous Treatment of Human Bronchial Epithelial Cells with Serine and Cysteine Protease Inhibitors Prevents Severe Acute Respiratory Syndrome Coronavirus Entry. J. Virol. 2012, 86, 6537–6545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuyama, S.; Nao, N.; Shirato, K.; Kawase, M.; Saito, S.; Takayama, I.; Nagata, N.; Sekizuka, T.; Katoh, H.; Kato, F.; et al. Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells. Proc. Natl. Acad. Sci. USA 2020, 117, 7001–7003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wettstein, L.; Conzelmann, C.; Müller, J.; Weil, T.; Groß, R.; Hirschenberger, M.; Seidel, A.; Klute, S.; Prelli Bozzo, C.; Zech, F.; et al. Alpha-1 Antitrypsin Inhibits SARS-CoV-2 Infection. bioRxiv 2020. [Google Scholar] [CrossRef]
- Oguntuyo, K.Y.; Stevens, C.S.; Siddiquey, M.N.; Schilke, R.M.; Woolard, M.D.; Zhang, H.; Acklin, J.A.; Ikegame, S.; Hung, C.-T.; Lim, J.K.; et al. In Plain Sight: The Role of Alpha-1-Antitrypsin in COVID-19 Pathogenesis and Therapeutics. bioRxiv 2020. [Google Scholar] [CrossRef]
- Chan, E.D.; Pott, G.B.; Silkoff, P.E.; Ralston, A.H.; Bryan, C.L.; Shapiro, L. Alpha-1-antitrypsin inhibits nitric oxide production. J. Leukoc. Biol. 2012, 92, 1251–1260. [Google Scholar] [CrossRef]
- Ehlers, M.R. Immune-modulating effects of alpha-1 antitrypsin. Biol. Chem. 2014, 395, 1187–1193. [Google Scholar] [CrossRef]
- Bergin, D.A.; Reeves, E.P.; Meleady, P.; Henry, M.; McElvaney, O.J.; Carroll, T.; Condron, C.; Chotirmall, S.H.; Clynes, M.; O’Neill, S.J.; et al. α-1 Antitrypsin regulates human neutrophil chemotaxis induced by soluble immune complexes and IL-8. J. Clin. Investig. 2010, 120, 4236–4250. [Google Scholar] [CrossRef] [Green Version]
- Bai, D.; Ueno, L.; Vogt, P.K. Akt-mediated regulation of NFκB and the essentialness of NFκB for the oncogenicity of PI3K and Akt. Int. J. Cancer 2009, 125, 2863–2870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.; Sun, K.; Guo, S.; Wang, J.; Li, A.; Rong, X.; Wang, T.; Shang, Y.; Chang, W.; Wang, S. Early Warning Indicators of Severe COVID-19: A Single-Center Study of Cases from Shanghai, China. Front. Med. 2020, 7, 432. [Google Scholar] [CrossRef] [PubMed]
- Bucurenci, N.; Blake, D.R.; Chidwick, K.; Winyard, P. Inhibition of neutrophil superoxide production by human plasma α1-antitrypsin. FEBS Lett. 1992, 300, 21–24. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.-Z.; Zhang, R.-Y.; Bai, J. An anti-oxidative therapy for ameliorating cardiac injuries of critically ill COVID-19-infected patients. Int. J. Cardiol. 2020, 312, 137–138. [Google Scholar] [CrossRef]
- Heurich, A.; Hofmann-Winkler, H.; Gierer, S.; Liepold, T.; Jahn, O.; Pöhlmann, S. TMPRSS2 and ADAM17 Cleave ACE2 Differentially and Only Proteolysis by TMPRSS2 Augments Entry Driven by the Severe Acute Respiratory Syndrome Coronavirus Spike Protein. J. Virol. 2013, 88, 1293–1307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwai, M.; Horiuchi, M. Devil and angel in the renin–angiotensin system: ACE–angiotensin II–AT1 receptor axis vs. ACE2–angiotensin-(1–7)–Mas receptor axis. Hypertens. Res. 2009, 32, 533–536. [Google Scholar] [CrossRef] [Green Version]
- Belaaouaj, A.; McCarthy, R.; Baumann, M.; Gao, Z.; Ley, T.J.; Abraham, S.N.; Shapiro, S.D. Mice lacking neutrophil elastase reveal impaired host defense against gram negative bacterial sepsis. Nat. Med. 1998, 4, 615–618. [Google Scholar] [CrossRef]
- Ishii, T.; Doi, K.; Okamoto, K.; Imamura, M.; Dohi, M.; Yamamoto, K.; Fujita, T.; Noiri, E. Neutrophil Elastase Contributes to Acute Lung Injury Induced by Bilateral Nephrectomy. Am. J. Pathol. 2010, 177, 1665–1673. [Google Scholar] [CrossRef]
- Ackermann, M.; Verleden, S.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef]
- Levi, M.; Hunt, B.J. Thrombosis and coagulopathy in COVID-19: An illustrated review. Res. Pr. Thromb. Haemost. 2020, 4, 744–751. [Google Scholar] [CrossRef]
- Middleton, E.A.; He, X.-Y.; Denorme, F.; Campbell, R.A.; Ng, D.; Salvatore, S.P.; Mostyka, M.; Baxter-Stoltzfus, A.; Borczuk, A.C.; Loda, M.; et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood 2020, 136, 1169–1179. [Google Scholar] [CrossRef]
- Rao, A.N.; Kazzaz, N.M.; Knight, J.S. Do neutrophil extracellular traps contribute to the heightened risk of thrombosis in inflammatory diseases? World J. Cardiol. 2015, 7, 829–842. [Google Scholar] [CrossRef]
- Zuo, Y.; Yalavarthi, S.; Shi, H.; Gockman, K.; Zuo, M.; Madison, J.A.; Blair, C.N.; Weber, A.; Barnes, B.J.; Egeblad, M.; et al. Neutrophil extracellular traps in COVID-19. JCI Insight 2020, 5, e138999. [Google Scholar] [CrossRef] [Green Version]
- Zuo, Y.; Yalavarthi, S.; Shi, H.; Gockman, K.; Zuo, M.; Madison, J.A.; Blair, C.; Weber, A.; Barnes, B.J.; Egeblad, M.; et al. Neutrophil Extracellular Traps (NETs) as Markers of Disease Severity in COVID-19. medRxiv 2020, 2020, 2009. [Google Scholar] [CrossRef] [Green Version]
- Zuo, Y.; Zuo, M.; Yalavarthi, S.; Gockman, K.; Madison, J.A.; Shi, H.; Woodard, W.; Lezak, S.P.; Lugogo, N.L.; Knight, J.S.; et al. Neutrophil extracellular traps and thrombosis in COVID-19. J. Thromb. Thrombolysis 2020, 51, 446–453. [Google Scholar] [CrossRef]
- Barnes, B.J.; Adrover, J.M.; Baxter-Stoltzfus, A.; Borczuk, A.; Cools-Lartigue, J.; Crawford, J.M.; Daßler-Plenker, J.; Guerci, P.; Huynh, C.; Knight, J.S.; et al. Targeting potential drivers of COVID-19: Neutrophil extracellular traps. J. Exp. Med. 2020, 217, e20200652. [Google Scholar] [CrossRef] [PubMed]
- Frenzel, E.; Korenbaum, E.; Hegermann, J.; Ochs, M.; Koepke, J.; Koczulla, A.R.; Welte, T.; Köhnlein, T.; Janciauskiene, S. Does Augmentation with Alpha1-Antitrypsin Affect Neutrophil Extracellular Traps Formation? Int. J. Biol. Sci. 2012, 8, 1023–1025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrache, I.; Fijalkowska, I.; Medler, T.R.; Skirball, J.; Cruz, P.; Zhen, L.; Petrache, H.I.; Flotte, T.R.; Tuder, R.M. α-1 Antitrypsin Inhibits Caspase-3 Activity, Preventing Lung Endothelial Cell Apoptosis. Am. J. Pathol. 2006, 169, 1155–1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrache, I.; Fijalkowska, I.; Zhen, L.; Medler, T.R.; Brown, E.; Cruz, P.; Choe, K.-H.; Taraseviciene-Stewart, L.; Scerbavicius, R.; Shapiro, L.; et al. A Novel Antiapoptotic Role for α1-Antitrypsin in the Prevention of Pulmonary Emphysema. Am. J. Respir. Crit. Care Med. 2006, 173, 1222–1228. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Chapman, K.R.; Wong, A.; Liu, M. α1-Antitrypsin deficiency and the risk of COVID-19: An urgent call to action. Lancet Respir. Med. 2021, 9, 337–339. [Google Scholar] [CrossRef]
- Shimi, G.; Zand, H. Association of alpha-1-antitrypsin deficiency with vitamin D status: Who is most at risk of getting severe COVID-19? Inflamm. Res. 2021, 70, 375–377. [Google Scholar] [CrossRef] [PubMed]
- Study to Evaluate the Safety and Efficacy of Liquid Alpha1-Proteinase Inhibitor (Human) in Hospitalized Participants with Coronavirus Disease (COVID-19)—Tabular View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/record/NCT04547140 (accessed on 27 November 2020).
- Trial of Alpha One Antitrypsin Inhalation in Treating Patient with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)—Tabular View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/record/NCT04385836 (accessed on 27 November 2020).
- McEvoy, N.L.; Clarke, J.L.; Mc Elvaney, O.J.; Mc Elvaney, O.F.; Boland, F.; Hyland, D.; Geoghegan, P.; Donnelly, K.; Friel, O.; Cullen, A.; et al. A randomised, double-blind, placebo-controlled, pilot trial of intravenous plasma purified alpha-1 antitrypsin for SARS-CoV-2-induced Acute Respiratory Distress Syndrome: A structured summary of a study protocol for a randomised, controlled trial. Trials 2021, 22, 1–3. [Google Scholar] [CrossRef] [PubMed]
- McElvaney, O.J.; O’Connor, E.; McEvoy, N.L.; Fraughan, D.D.; Clarke, J.; McElvaney, O.F.; Gunaratnam, C.; O’Rourke, J.; Curley, G.F.; McElvaney, N.G. Alpha-1 antitrypsin for cystic fibrosis complicated by severe cytokinemic COVID-19. J. Cyst. Fibros. 2020, 20, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Ritzmann, F.; Chitirala, P.; Krüger, N.; Hoffmann, M.; Zuo, W.; Lammert, F.; Smola, S.; Tov, N.; Alagem, N.; Lepper, P.M.; et al. Therapeutic Application of alpha-1-antitrypsin in COVID-19. Am. J. Respir. Crit. Care Med. 2021, 204, 2. [Google Scholar] [CrossRef]
- Martini, F.; De Mattei, M.; Contini, C.; Tognon, M.G. Potential Use of Alpha-1 Anti-trypsin in the Covid-19 Treatment. Front. Cell Dev. Biol. 2020, 8, 577528. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Nicolau, D.V.; Langford, B.; Mahdi, M.; Jeffers, H.; Mwasuku, C.; Krassowska, K.; Fox, R.; Binnian, I.; Glover, V.; et al. Inhaled budesonide in the treatment of early COVID-19 (STOIC): A phase 2, open-label, randomised controlled trial. Lancet Respir. Med. 2021, 9, 763–772. [Google Scholar] [CrossRef]
| Authors | Geographical Area | Population Size | Main Findings |
|---|---|---|---|
| Vianello et al. [25] | Italy | 60,461,826 | Overlap between variants of the SERPINA1 gene and severe cases of COVID-19 |
| Yoshikura [28] | Global scale | 7.9 billion | Prevalence of AATD correlates with distribution of COVID-19 |
| Shapira et al. [29] | Global scale | NA | Correlation between AATD prevalence and COVID-19 mortality rates between Middle East/Far East and South Europe |
| Bhattacharyya et al. [30] | Europe, North America | NA | Increased risk of SARS-CoV-2 subtype 614G infection explained by higher AATD prevalence |
| Ferrarotti et al. [31] | Italy | 209 | Higher frequency of SARS-CoV-2 infection in AATD cohort compared to national data |
| Faria et al. [32] | Portugal | 77 | PiZZ genotype associated with greater COVID-19 incidence |
| Schneider et al. [33] | United Kingdom | 500,000 | Mild AATD genotypes not associated with increased SARS-CoV-2 infection or fatality rates |
| Protective Effect | Underlying Mechanism |
|---|---|
| Antiviral | Enhancement of host immunity |
| Inhibition of TMPRSS2 | |
| Anti-inflammatory | Reduced IL-8 release; IL-8 binding |
| Inhibition of NFκB and ADAM17 | |
| Prevention of acute lung injury | Inhibition of NE and ADAM17 |
| Prevention of thromboembolism | Thrombin antagonization |
| Inhibition of NET adherence | |
| Prevention of endothelial cell injury | Inhibition of caspase-3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vianello, A.; Guarnieri, G.; Braccioni, F.; Molena, B.; Lococo, S.; Achille, A.; Lionello, F.; Salviati, L.; Caminati, M.; Senna, G. Correlation between α1-Antitrypsin Deficiency and SARS-CoV-2 Infection: Epidemiological Data and Pathogenetic Hypotheses. J. Clin. Med. 2021, 10, 4493. https://doi.org/10.3390/jcm10194493
Vianello A, Guarnieri G, Braccioni F, Molena B, Lococo S, Achille A, Lionello F, Salviati L, Caminati M, Senna G. Correlation between α1-Antitrypsin Deficiency and SARS-CoV-2 Infection: Epidemiological Data and Pathogenetic Hypotheses. Journal of Clinical Medicine. 2021; 10(19):4493. https://doi.org/10.3390/jcm10194493
Chicago/Turabian StyleVianello, Andrea, Gabriella Guarnieri, Fausto Braccioni, Beatrice Molena, Sara Lococo, Alessia Achille, Federico Lionello, Leonardo Salviati, Marco Caminati, and Gianenrico Senna. 2021. "Correlation between α1-Antitrypsin Deficiency and SARS-CoV-2 Infection: Epidemiological Data and Pathogenetic Hypotheses" Journal of Clinical Medicine 10, no. 19: 4493. https://doi.org/10.3390/jcm10194493
APA StyleVianello, A., Guarnieri, G., Braccioni, F., Molena, B., Lococo, S., Achille, A., Lionello, F., Salviati, L., Caminati, M., & Senna, G. (2021). Correlation between α1-Antitrypsin Deficiency and SARS-CoV-2 Infection: Epidemiological Data and Pathogenetic Hypotheses. Journal of Clinical Medicine, 10(19), 4493. https://doi.org/10.3390/jcm10194493









