High-Frequency Ultrasound to Assess Activity in Connective Tissue Panniculitis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design
2.2. Patients
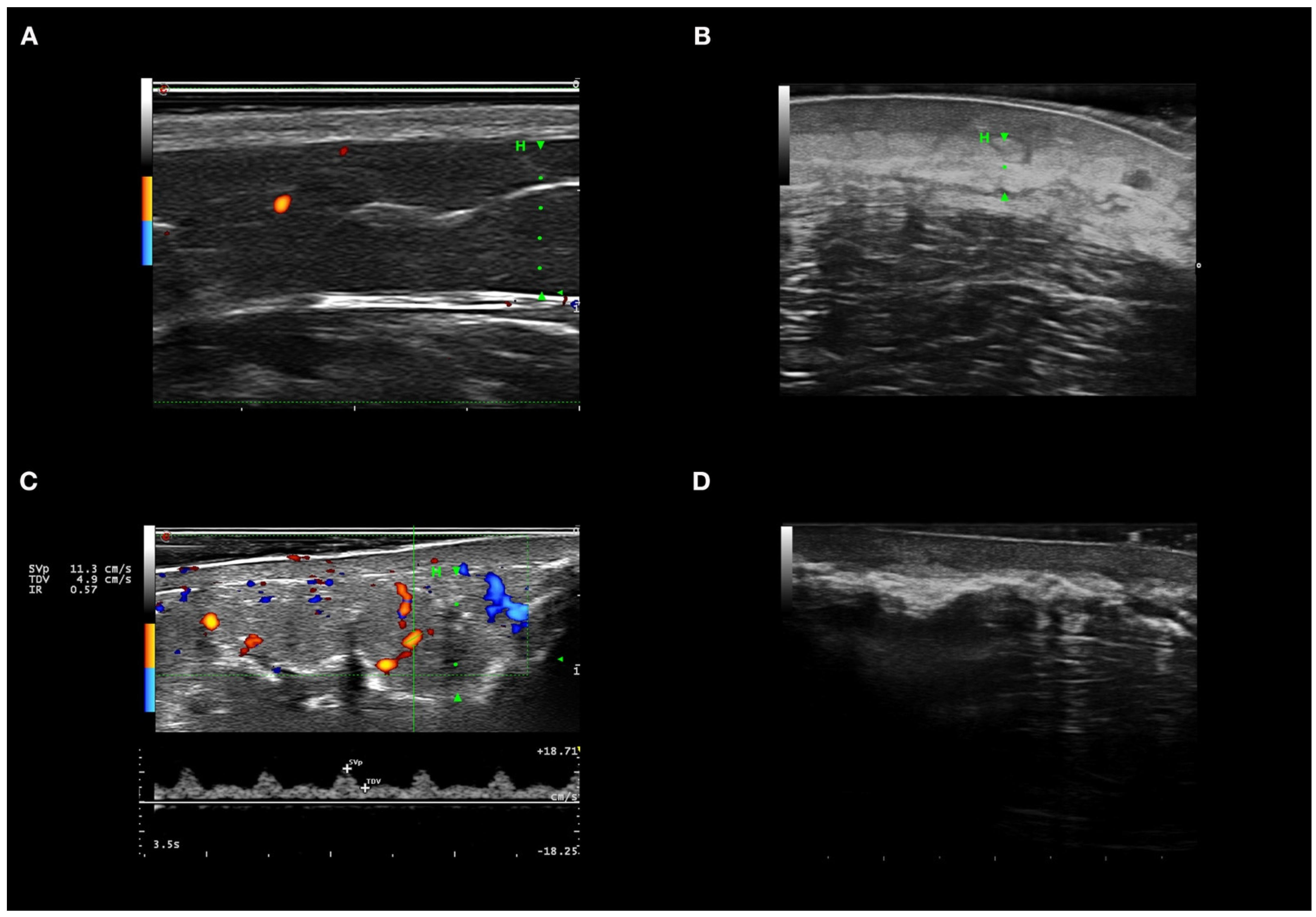
2.3. Skin Ultrasound
2.4. Statistical Analyses
3. Results
3.1. Baseline and Clinical Characteristics
3.2. Patient Assessment Three Months after Diagnosis
3.3. Clinical Ultrasound Follow-Up during the Complete Study Period
3.4. The Usefulness of Ultrasound in the Presence of Nonspecific Clinical Findings
3.5. Ultrasound Features of Inflammation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CT | computed tomography |
| CTP | connective tissue panniculitis |
| DM | dermatomyositis |
| HFUS | high-frequency ultrasound |
| LE | lupus erythematosus |
| MRI | magnetic resonance imaging |
References
- González-Cruz, C.; Aparicio Español, G.; Ferrer Fàbrega, B.; Cabezas Calderón, V.; Giner Pichel, M.; García-Patos Briones, V. Lupus Panniculitis: Clinicopathological Features of a Series of 12 Patients. Med. Clin. (Barc.) 2018, 151, 444–449. [Google Scholar] [CrossRef]
- Sharma, A.; Blank, A.; Komforti, M.K. Rare Initial Manifestation of Lupus as Lobular Panniculitis of the Breast-A Case Report and Review of the Literature. Am. J. Dermatopathol. 2020, 43, 381–385. [Google Scholar] [CrossRef]
- Santos-Briz, A.; Calle, A.; Linos, K.; Semans, B.; Carlson, A.; Sangüeza, O.P.; Metze, D.; Cerroni, L.; Díaz-Recuero, J.L.; Alegría-Landa, V.; et al. Dermatomyositis Panniculitis: A Clinicopathological and Immunohistochemical Study of 18 Cases. J. Eur. Acad. Dermatol. Venereol. JEADV 2018, 32, 1352–1359. [Google Scholar] [CrossRef]
- Arai, S.; Katsuoka, K. Clinical Entity of Lupus Erythematosus Panniculitis/Lupus Erythematosus Profundus. Autoimmun. Rev. 2009, 8, 449–452. [Google Scholar] [CrossRef] [PubMed]
- Requena, L.; Sánchez Yus, E. Panniculitis. Part II. Mostly Lobular Panniculitis. J. Am. Acad. Dermatol. 2001, 45, 325–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, P.P.-L.; Tan, S.H.; Tan, T. Lupus Erythematosus Panniculitis: A Clinicopathologic Study. Int. J. Dermatol. 2002, 41, 488–490. [Google Scholar] [CrossRef]
- Ud-Din, S.; Bayat, A. Classification of Distinct Endotypes in Human Skin Scarring: SCAR—A Novel Perspective on Dermal Fibrosis. Adv. Wound Care 2021. [Google Scholar] [CrossRef] [PubMed]
- Rangel, L.K.; Villa-Ruiz, C.; Lo, K.; Cobos, G.; Lo Sicco, K.; Vleugels, R.A.; Femia, A.N. Clinical Characteristics of Lupus Erythematosus Panniculitis/Profundus: A Retrospective Review of 61 Patients. JAMA Dermatol. 2020, 156, 1264–1266. [Google Scholar] [CrossRef]
- Catalano, O.; Wortsman, X. Dermatology Ultrasound. Imaging Technique, Tips and Tricks, High-Resolution Anatomy. Ultrasound Q. 2020, 36, 321–327. [Google Scholar] [CrossRef]
- Wortsman, X.; Wortsman, J. Clinical Usefulness of Variable-Frequency Ultrasound in Localized Lesions of the Skin. J. Am. Acad. Dermatol. 2010, 62, 247–256. [Google Scholar] [CrossRef]
- Giavedoni, P.; Pousa-Martínez, M.; Estany-Destal, A.; Ginarte, M.; Vázquez-Veiga, H.; Tamez, L.; Mascaró, J.M. Usefulness of High-Frequency Doppler Ultrasound Skin Thickness Measurement for Disease Staging and Assessing Treatment Response in Patients with Scleredema: A Case-Control Study. J. Am. Acad. Dermatol. 2021. [Google Scholar] [CrossRef]
- Giavedoni, P.; Martinez, C.; Podlipnik, S.; Suárez-Lleidó, M.; Martí-Martí, I.; Morgado-Carrasco, D.; Rovira, M.; Fernández-Avilés, F.; Gutiérrez, G.; Rosiñol, L.; et al. Assessment of Sclerodermoid Chronic Graft-versus-Host Disease with Colour Doppler Ultrasound. Acta Derm. Venereol. 2021, 101. [Google Scholar] [CrossRef]
- Wortsman, X.; Wortsman, J.; Sazunic, I.; Carreño, L. Activity Assessment in Morphea Using Color Doppler Ultrasound. J. Am. Acad. Dermatol. 2011, 65, 942–948. [Google Scholar] [CrossRef]
- Romaní, J.; Giavedoni, P.; Roé, E.; Vidal, D.; Luelmo, J.; Wortsman, X. Inter- and Intra-Rater Agreement of Dermatologic Ultrasound for the Diagnosis of Lobular and Septal Panniculitis. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2020, 39, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Sabaté, J.M.; Gómez, A.; Torrubia, S.; Salinas, T.; Clotet, M.; Lerma, E. Lupus Panniculitis Involving the Breast. Eur. Radiol. 2006, 16, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Kimball, H.; Kimball, D.; Siroy, A.; Tuna, I.S.; Boyce, B.J.; Albayram, M.S. Novel Diagnostic Imaging Features of Facial Lupus Panniculitis: Ultrasound, CT, and MR Imaging with Histopathology Correlate. Clin. Imaging 2019, 58, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Aringer, M.; Costenbader, K.; Daikh, D.; Brinks, R.; Mosca, M.; Ramsey-Goldman, R.; Smolen, J.S.; Wofsy, D.; Boumpas, D.T.; Kamen, D.L.; et al. 2019 European League Against Rheumatism/American College of Rheumatology Classification Criteria for Systemic Lupus Erythematosus. Ann. Rheum. Dis. 2019, 78, 1151–1159. [Google Scholar] [CrossRef] [Green Version]
- Hoogendijk, J.E.; Amato, A.A.; Lecky, B.R.; Choy, E.H.; Lundberg, I.E.; Rose, M.R.; Vencovsky, J.; de Visser, M.; Hughes, R.A. 119th ENMC International Workshop: Trial Design in Adult Idiopathic Inflammatory Myopathies, with the Exception of Inclusion Body Myositis, 10–12 October 2003, Naarden, The Netherlands. Neuromuscul. Disord. 2004, 14, 337–345. [Google Scholar] [CrossRef]
- Wortsman, X.; Alfageme, F.; Roustan, G.; Arias-Santiago, S.; Martorell, A.; Catalano, O.; Scotto di Santolo, M.; Zarchi, K.; Bouer, M.; Gonzalez, C.; et al. Guidelines for Performing Dermatologic Ultrasound Examinations by the DERMUS Group. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2016, 35, 577–580. [Google Scholar] [CrossRef] [Green Version]
- Alfageme Roldán, F.; Mollet Sánchez, J.; Cerezo López, E. Principios físicos y generalidades. Actas Dermo-Sifiliográficas 2015, 106, 3–9. [Google Scholar] [CrossRef]
- Wortsman, X. Dermatologic Ultrasound with Clinical and Histologic Correlations; Springer Science & Business Media: Berlin, Germany, 2013. [Google Scholar]
- Mlosek, R.K.; Migda, B.; Migda, M. High-Frequency Ultrasound in the 21st Century. J. Ultrason. 2021, 20, e233–e241. [Google Scholar] [CrossRef] [PubMed]
- Dopytalska, K.; Sobolewski, P.; Mikucka-Wituszyńska, A.; Gnatowski, M.; Szymańska, E.; Walecka, I. Noninvasive Skin Imaging in Esthetic Medicine-Why Do We Need Useful Tools for Evaluation of the Esthetic Procedures. J. Cosmet. Dermatol. 2021, 20, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Vidal, D.; Ruiz-Villaverde, R.; Alfageme, F.; Roustan, G.; Mollet, J.; Ruiz-Carrascosa, J.C.; Haychein, S.; Gavin, J.; Arias-Santiago, S.; Martorell, A.; et al. Use of High Frequency Ultrasonography in Dermatology Departments in Spain. Dermatol. Online J. 2016, 22. [Google Scholar] [CrossRef]
- Giavedoni, P.; del Prado Sanz, M.E.; Vila, J.B.; Crespo, E.R.; Ortigosa, J.S.; Vidal, D. Uso de La Ecografía Doppler En Servicios de Dermatología En Cataluña y Aragón, EspañaUse of Doppler Ultrasound in Dermatology Departments in Catalonia and Aragon, Spain. Piel 2019, 34, 69–73. [Google Scholar] [CrossRef]
- Varela-Ponte, R.; Martínez-Lago, N.; Vieito-Villar, M.; Carreira-Villamor, J.M. Impact of Risk Factors on the Efficacy and Complications of Ultrasound-Guided Percutaneous Liver Biopsy of Space-Occupying Lesions. Radiologia 2020. [Google Scholar] [CrossRef]
- He, L.-J.; Xie, C.; Li, Y.; Luo, L.-N.; Pan, K.; Gao, X.-Y.; Liu, L.-Z.; Gao, J.-M.; Luo, G.-Y.; Shan, H.-B.; et al. Ultrasound-Guided Fine Needle Aspiration of Retropharyngeal Lymph Nodes after Radiotherapy for Nasopharyngeal Carcinoma: A Novel Technique for Accurate Diagnosis. Cancer Commun. Lond. Engl. 2018, 38, 20. [Google Scholar] [CrossRef] [Green Version]
- Echeverría-García, B.; Borbujo, J.; Alfageme, F. Incorporación de la ecografía en Dermatología. Actas Dermo-Sifiliográficas 2014, 105, 887–890. [Google Scholar] [CrossRef]
- Marti-Marti, I.; Morgado-Carrasco, D.; Podlipnik, S.; Rizo-Potau, D.; Bosch-Amate, X.; Lledó, G.M.; Suárez-Lledó, M.; Espinosa, G.; Martínez, C.; Mascaró, J.M.; et al. Usefulness of High-Frequency Ultrasound in the Evaluation and Monitoring of Sclerosing Dermatoses. A Cohort Study. Clin. Exp. Dermatol. 2021. [Google Scholar] [CrossRef]
- Dermatologic Ultrasound in the Management of Childhood Linear Morphea-PubMed. Available online: https://pubmed-ncbi-nlm-nih-gov.sire.ub.edu/34391329/ (accessed on 17 September 2021).
- Flower, V.A.; Barratt, S.L.; Hart, D.J.; Mackenzie, A.B.; Shipley, J.A.; Ward, S.G.; Pauling, J.D. High-Frequency Ultrasound Assessment of Systemic Sclerosis Skin Involvement: Intraobserver Repeatability and Relationship With Clinician Assessment and Dermal Collagen Content. J. Rheumatol. 2021, 48, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Worstman, X. Atlas of Dermatologic Ultrasound; Springer Science & Business Media: Berlin, Germany, 2018. [Google Scholar]
- Naredo, E.; Pascau, J.; Damjanov, N.; Lepri, G.; Gordaliza, P.M.; Janta, I.; Ovalles-Bonilla, J.G.; López-Longo, F.J.; Matucci-Cerinic, M. Performance of Ultra-High-Frequency Ultrasound in the Evaluation of Skin Involvement in Systemic Sclerosis: A Preliminary Report. Rheumatol. Oxf. Engl. 2020, 59, 1671–1678. [Google Scholar] [CrossRef]
- Urdiales-Gálvez, F.; Barres-Caballer, J.; Carrasco-Sánchez, S. Ultrasound Assessment of Tissue Integration of the Crosslinked Hyaluronic Acid Filler VYC-25L in Facial Lower-Third Aesthetic Treatment: A Prospective Multicenter Study. J. Cosmet. Dermatol. 2020, 20, 1439–1449. [Google Scholar] [CrossRef] [PubMed]
- López-Sundh, A.E.; Quintana-Sancho, A.; Durán-Vian, C.; Reguero-DelCura, L.; Corrales-Martínez, A.F.; Gómez-Fernández, C.; González-López, M.A. Clinical and Ultrasound Response to Intralesional Sodium Thiosulfate for the Treatment of Calcinosis Cutis in the Setting of Systemic Sclerosis. A Case-Based Review. Clin. Rheumatol. 2020, 40, 2985–2989. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Tiwari, S.; Yadav, T.; Khera, P.S.; Garg, P.; Sureka, B.; Budania, A.; Singh, S. Parry Romberg Syndrome: Imaging Features in 4 Consecutive Cases and Review of Literature. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas. 2020, 76, 249–253. [Google Scholar] [CrossRef] [PubMed]

| Overall (N = 23) | |
|---|---|
| Age (years) | |
| Median | 44 (18–78) |
| Sex | |
| Female | 22 (95.7%) |
| Male | 1 (4.3%) |
| Autoimmune disease | |
| Lupus erythematosus * | 18 (78.3) |
| Isolated lupus panniculitis | 9 (50%) |
| Systemic lupus erythematosus | 6 (33.3%) |
| Chronic discoid lupus | 5 (27.8%) |
| Subacute cutaneous lupus | 1 (5.5%) |
| Dermatomyositis | 5 (21.7%) |
| Classic dermatomyositis | 3 (60%) |
| Amyopathic dermatomyositis | 2 (40%) |
| Other cutaneous manifestations associated with lupus | |
| Cicatricial alopecia | 3 (16.7%) |
| Perniosis | 2 (11.2%) |
| Oral ulcers | 1 (5.6%) |
| Other systemic manifestations associated with lupus | |
| Arthritis | 4 (22.2%) |
| Arthralgia | 2 (11.2%) |
| Nephritis | 2 (11.2%) |
| Serositis | 1 (5.6%) |
| Cytopenia | 1 (5.6%) |
| Myositis | 1 (5.6%) |
| Other cutaneous manifestations associated with dermatomyositis | |
| Calcinosis | 4 (80%) |
| Gottron papules | 4 (80%) |
| Heliotrope erythema | 3 (60%) |
| Periungual telangiectasias | 3 (60%) |
| Cutaneous ulcers | 2 (40%) |
| V-shaped erythema at the chest | 2 (40%) |
| Erythema on proximal thighs | 2 (40%) |
| Facial erythema | 1 (20%) |
| Other systemic manifestations associated with dermatomyositis | |
| Myositis | 3 (60%) |
| Interstitial lung disease | 1 (20%) |
| Panniculitis location | |
| Arms | 15 (65.2%) |
| Scalp | 8 (34.8%) |
| Thighs | 6 (26.1%) |
| Buttocks | 6 (26.1%) |
| Face | 3 (13%) |
| Breasts | 3 (13%) |
| Trunk | 3 (13%) |
| Forearms | 3 (13%) |
| Legs | 2 (8.7%) |
| Number of systemic treatments, mean (range) | 3 (1 -17) |
| Number of ultrasounds performed per patient | |
| Median (range) | 3 (2–13) |
| Clinical Active (N = 14) | Clinical Inactive (N = 40) | Total (N = 54) | p Value | Sensitivity | Specificity | |
|---|---|---|---|---|---|---|
| Ultrasound activity | <0.001 | 100% | 97.50% | |||
| Active | 14 (100.0%) | 1 (2.5%) | 15 (27.8%) | |||
| Inactive | 0 (0.0%) | 39 (97.5%) | 39 (72.2%) | |||
| Hypoechoic dermis | <0.001 | 78.57% | 92.50% | |||
| Yes | 11 (78.6%) | 3 (7.5%) | 14 (25.9%) | |||
| No | 3 (21.4%) | 37 (92.5%) | 40 (74.1%) | |||
| Dermo-hypodermic limit | <0.001 | 78.57% | 75% | |||
| Undefined | 11 (78.6%) | 10 (25.0%) | 21 (38.9%) | |||
| Defined | 3 (21.4%) | 30 (75.0%) | 33 (61.1%) | |||
| Hypoechoic septa | <0.001 | 42.86% | 97.50% | |||
| Yes | 6 (42.9%) | 1 (2.5%) | 7 (13.0%) | |||
| No | 8 (57.1%) | 39 (97.5%) | 47 (87.0%) | |||
| Thickened septa | 0.003 | 21.43% | 100% | |||
| Yes | 3 (21.4%) | 0 (0.0%) | 3 (5.6%) | |||
| No | 11 (78.6%) | 40 (100.0%) | 51 (94.4%) | |||
| Hyperechoic lobules | <0.001 | 92.86% | 72.50% | |||
| Yes | 13 (92.9%) | 11 (27.5%) | 24 (44.4%) | |||
| No | 1 (7.1%) | 29 (72.5%) | 30 (55.6%) | |||
| Vessel diameter >1 mm | <0.001 | 85.71% | 100% | |||
| Yes | 12 (85.7%) | 0 (0.0%) | 12 (22.2%) | |||
| No | 2 (14.3%) | 40 (100.0%) | 42 (77.8%) | |||
| Peak systolic flow >10 cm/s | <0.001 | 100% | 97.50% | |||
| Yes | 14 (100.0%) | 1 (2.5%) | 15 (27.8%) | |||
| No | 0 (0.0%) | 39 (97.5%) | 39 (72.2%) | |||
| Resistive index >0.7 | <0.001 | 64.29% | 97.50% | |||
| Yes | 9 (64.3%) | 1 (2.5%) | 10 (18.5%) | |||
| No | 5 (35.7%) | 39 (97.5%) | 44 (81.5%) | |||
| Calcinosis cutis | 0.347 | 21.43% | 65% | |||
| Yes | 3 (21.4%) | 14 (35.0%) | 17 (31.5%) | |||
| No | 11 (78.6%) | 26 (65.0%) | 37 (68.5%) | |||
| Subcutaneous tissue atrophy | 0.348 | 92.86% | 17.50% | |||
| Yes | 13 (92.9%) | 33 (82.5%) | 46 (85.2%) | |||
| No | 1 (7.1%) | 7 (17.5%) | 8 (14.8%) |
| Undetermined Clinical Activity | 80 (100%) |
|---|---|
| Therapeutic changes based on HFUS | 57 (71.2%) |
| Decrease/Stop treatment | 29 (36.2%) |
| stop prednisone | 1 (3.4%) |
| stop hydroxychloroquine | 1 (3.4%) |
| decrease prednisone | 17 (58.6%) |
| decrease hydroxychloroquine | 5 (17.2%) |
| decrease ruxolitinib | 2 (6.9%) |
| decrease mepacrine | 1 (3.4%) |
| decrease methotrexate | 1 (3.4%) |
| Increase treatment | 28 (35%) |
| start hydroxychloroquine | 8 (28.6%) |
| start prednisone | 2 (7.1) |
| start methotrexate | 1 (3.6%) |
| start mepacrine | 1 (3.6%) |
| start mycophenolate | 1 (3.6%) |
| start hydroxychloroquine | 1 (3.6%) |
| start belimumab | 1 (3.6%) |
| start tofacitinib | 1 (3.6%) |
| increase prednisone | 4 (14.3%) |
| increase hydroxychloroquine | 3 (10.7%) |
| increase methotrexate | 3 (10.7%) |
| increase mycophenolate | 1 (3.6%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giavedoni, P.; Podlipnik, S.; Fuertes de Vega, I.; Iranzo, P.; Mascaró, J.M., Jr. High-Frequency Ultrasound to Assess Activity in Connective Tissue Panniculitis. J. Clin. Med. 2021, 10, 4516. https://doi.org/10.3390/jcm10194516
Giavedoni P, Podlipnik S, Fuertes de Vega I, Iranzo P, Mascaró JM Jr. High-Frequency Ultrasound to Assess Activity in Connective Tissue Panniculitis. Journal of Clinical Medicine. 2021; 10(19):4516. https://doi.org/10.3390/jcm10194516
Chicago/Turabian StyleGiavedoni, Priscila, Sebastian Podlipnik, Irene Fuertes de Vega, Pilar Iranzo, and José Manuel Mascaró, Jr. 2021. "High-Frequency Ultrasound to Assess Activity in Connective Tissue Panniculitis" Journal of Clinical Medicine 10, no. 19: 4516. https://doi.org/10.3390/jcm10194516
APA StyleGiavedoni, P., Podlipnik, S., Fuertes de Vega, I., Iranzo, P., & Mascaró, J. M., Jr. (2021). High-Frequency Ultrasound to Assess Activity in Connective Tissue Panniculitis. Journal of Clinical Medicine, 10(19), 4516. https://doi.org/10.3390/jcm10194516








