Personalization of Treatment for Patients with Childhood-Abuse-Related Posttraumatic Stress Disorder
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedures
2.3. Treatment
3. Measures
3.1. Outcome Measures
3.2. Predictor Variables
3.2.1. Patient Expectancies
3.2.2. Demographics
3.2.3. Social Support
3.2.4. Trauma Background
3.2.5. General Health Status
3.2.6. Self-Reported Psychiatric Symptoms
3.2.7. Clinician-Assessed Psychiatric Symptoms and Disorders
4. Statistical Analysis
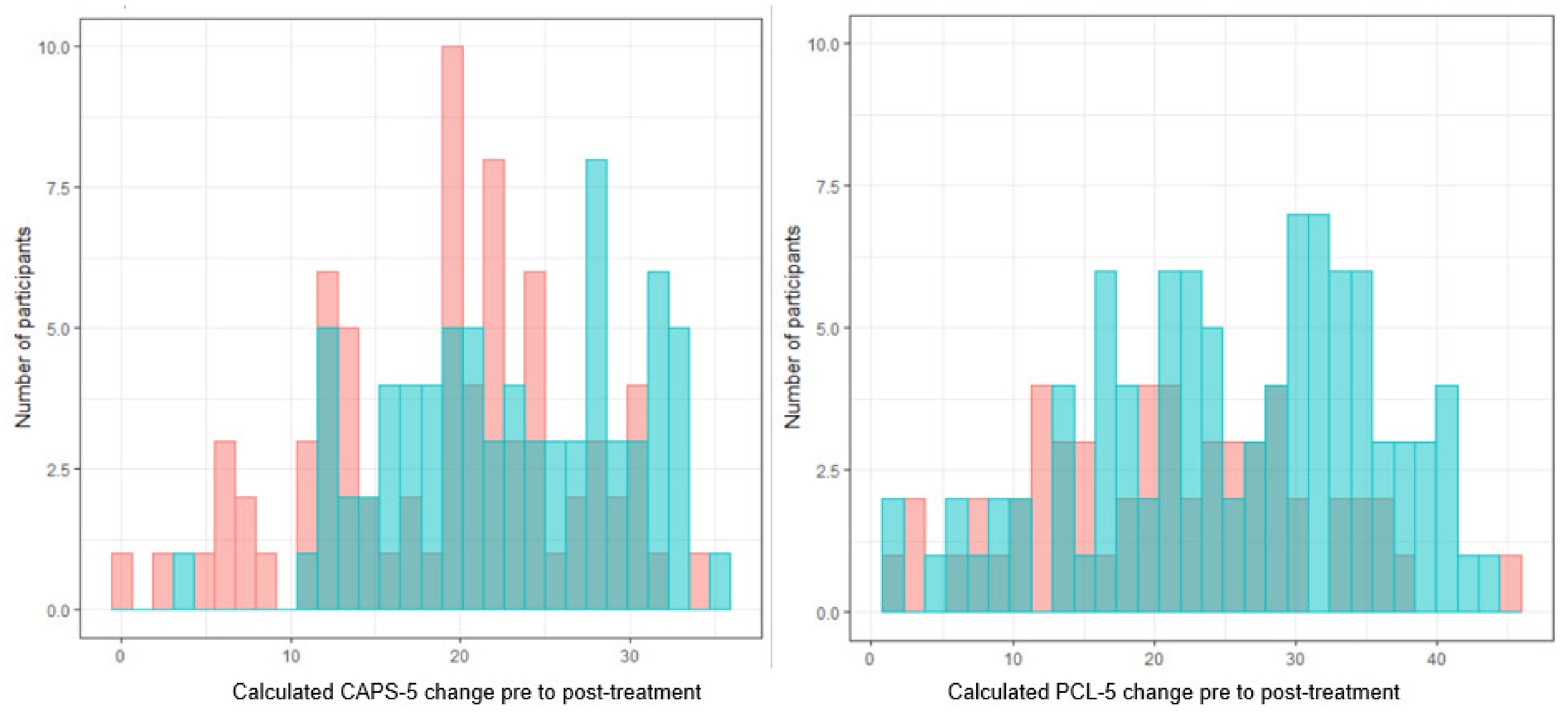
4.1. Outcome
4.2. Initial Predictor Selection with Boruta
4.3. Further Predictor Selection Using Bootstrap Procedure
4.4. Personalized Advantage Index
4.5. Results
4.6. Variable Selection for Exposure Therapies and STAIR+PE
4.7. Personalized Advantage Index
5. Discussion
Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| CAPS | PCL | |||||
|---|---|---|---|---|---|---|
| Predictors | Boruta | Bootstrap | Boruta | Bootstrap | ||
| Number of Hits (%) | Important | Important | Number of Hits (%) | Important | Important | |
| Patient expectancies | ||||||
| Expected burden | 0.03 | No | 0 | No | ||
| Credibility | 0.58 | Yes | No | 0.53 | Tentative | |
| Demographics | ||||||
| Age, y | <0.01 | No | 0.14 | No | ||
| Gender, female | 0 | No | 0 | No | ||
| Cultural background, western 1 | 0 | No | 0 | No | ||
| Education, high 2 | 0 | No | 0 | No | ||
| Employment, yes | 0 | No | 0 | No | ||
| Social support | ||||||
| MOS total score | 1 | Yes | Yes | 0.93 | Yes | Yes |
| Trauma background | ||||||
| CTQ childhood emotional abuse | 0 | No | 0 | No | ||
| CTQ childhood emotional neglect | 0 | No | 0 | No | ||
| CTQ childhood physical abuse | 0 | No | 0 | No | ||
| CTQ childhood sexual abuse | 0.67 | Yes | Yes | 0 | No | |
| General health status | ||||||
| EQ-5D-5L general health status | 0 | No | 0 | No | ||
| Self-reported psychiatric symptoms | ||||||
| BDI total score | 1 | Yes | Yes | 0.97 | Yes | Yes |
| PTCI total score | 1 | Yes | No | 0.94 | Yes | No |
| IIP total score | <0.01 | No | <0.01 | No | ||
| RSES total score | 0 | No | 0 | No | ||
| DERS total score | 0.59 | Yes | No | 0.64 | Yes | No |
| SDQ-5 total score | 0 | No | 0.01 | No | ||
| Psychotropic medication | 0 | No | 0 | No | ||
| Clinician-assessed psychiatric symptoms and disorders | ||||||
| Any SCID-2 personality disorder | 0.04 | No | <0.01 | No | ||
| DSP-I total score | 0 | No | 0 | No | ||
| Axis-1 MINI diagnoses, excluding PTSD | 0.71 | Yes | Yes | 0 | No | |
| CAPS-5 baseline total score | 1 | Yes | No | 0.01 | No | |
Appendix B
| CAPS | PCL | |||||
|---|---|---|---|---|---|---|
| Predictors | Boruta | Bootstrap | Boruta | Bootstrap | ||
| Number of Hits (%) | Important | Important | Number of Hits (%) | Important | Important | |
| Patient expectancies | ||||||
| Expected burden | 0 | No | 0 | No | ||
| Credibility | 0 | No | 0 | No | ||
| Demographics | ||||||
| Age, y | 0 | No | 0.54 | Tentative | ||
| Gender, female | 0 | No | 0 | No | ||
| Cultural background, western 1 | 0 | No | 0 | No | ||
| Education, high 2 | 0 | No | 0 | No | ||
| Employment, yes | <0.01 | No | 0 | No | ||
| Social support | ||||||
| MOS total score | 0 | No | 0 | No | ||
| Trauma background | ||||||
| CTQ childhood emotional abuse | <0.01 | No | 0 | No | ||
| CTQ childhood emotional neglect | 0 | No | 0 | No | ||
| CTQ childhood physical abuse | 0 | No | 0 | No | ||
| CTQ childhood sexual abuse | 0 | No | 0 | No | ||
| General health status | ||||||
| EQ-5D-5L general health status | 0.84 | Yes | Yes | 0.89 | Yes | Yes |
| Self-reported psychiatric symptoms | ||||||
| BDI total score | 0.89 | Yes | No | 0.82 | Yes | No |
| PTCI total score | 0.94 | Yes | No | 0.90 | Yes | No |
| IIP total score | 0.82 | Yes | No | 0 | No | |
| RSES total score | 0 | No | 0 | No | ||
| DERS total score | 1 | Yes | Yes | 0.86 | Yes | Yes |
| SDQ-5 total score | 0 | No | 0 | No | ||
| Psychotropic medication | 0 | No | 0 | No | ||
| Clinician-assessed psychiatric symptoms and disorders | ||||||
| Any SCID-2 personality disorder | 0 | No | 0 | No | ||
| DSP-I total score | 0 | No | 0 | No | ||
| Axis-1 MINI diagnoses, excluding PTSD | 0.33 | No | 0 | No | ||
| CAPS-5 baseline total score | 1 | Yes | Yes | 0 | No | |
References
- De Vries, G.J.; Olff, M. The Lifetime Prevalence of Traumatic Events and Posttraumatic Stress Disorder in the Netherlands. J. Trauma. Stress 2009, 22, 259–267. [Google Scholar] [CrossRef]
- APA. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Kessler, R.C.; Green, J.G.; Gruber, M.J.; Sampson, N.A.; Bromet, E.; Cuitan, M.; Furukawa, T.A.; Gureje, O.; Hinkov, H.; Hu, C.Y.; et al. Screening for serious mental illness in the general population with the K6 screening scale: Results from the WHO World Mental Health (WMH) survey initiative. Int. J. Meth. Psychiatr. Res. 2010, 19, 4–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pagotto, L.F.; Mendlowicz, M.V.; Coutinho, E.S.F.; Figueira, I.; Luz, M.P.; Araujo, A.X.; Berger, W. The impact of posttraumatic symptoms and comorbid mental disorders on the health-related quality of life in treatment-seeking PTSD patients. Compr. Psychiatry 2015, 58, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Mavranezouli, I.; Megnin-Viggars, O.; Daly, C.; Dias, S.; Welton, N.J.; Stockton, S.; Bhutani, G.; Grey, N.; Leach, J.; Greenberg, N.; et al. Psychological treatments for post-traumatic stress disorder in adults: A network meta-analysis. Psychol. Med. 2020, 50, 542–555. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C.; Roberts, N.P.; Gibson, S.; Bisson, J.I. Dropout from psychological therapies for post-traumatic stress disorder (PTSD) in adults: Systematic review and meta-analysis. Eur. J. Psychotraumatol. 2020, 11, 1709709, Retraction in 2020, 11, 1729633. [Google Scholar] [CrossRef] [PubMed]
- Mavranezouli, I.; Megnin-Viggars, O.; Grey, N.; Bhutani, G.; Leach, J.; Daly, C.; Dias, S.; Welton, N.J.; Katona, C.; El-Leithy, S.; et al. Cost-effectiveness of psychological treatments for post-traumatic stress disorder in adults. PLoS ONE 2020, 15, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Bradley, R. A multidimensional meta-analysis of psychotherapy for PTSD. Am. J. Psychiatry 2005, 162, 214–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehring, T.; Welboren, R.; Morina, N.; Wicherts, J.M.; Freitag, J.; Emmelkamp, P.M. Meta-analysis of psychological treatments for posttraumatic stress disorder in adult survivors of childhood abuse. Clin. Psychol. Rev. 2014, 34, 645–657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watkins, L.E.; Sprang, K.R.; Rothbaum, B.O. Treating PTSD: A Review of Evidence-Based Psychotherapy Interventions. Front. Behav. Neurosci. 2018, 12, 258. [Google Scholar] [CrossRef]
- Wilson, S.A.; Becker, L.A.; Tinker, R.H. Eye-Movement Desensitization and Reprocessing (Emdr) Treatment for Psychologically Traumatized Individuals. J. Consult. Clin. Psychol. 1995, 63, 928–937. [Google Scholar] [CrossRef]
- Cloitre, M.; Koenen, K.C.; Cohen, L.R.; Han, H. Skills training in affective and interpersonal regulation followed by exposure: A phase-based treatment for PTSD related to childhood abuse. J. Consult. Clin. Psychol. 2002, 70, 1067–1074. [Google Scholar] [CrossRef]
- Hendriks, L.; de Kleine, R.A.; Broekman, T.G.; Hendriks, G.J.; van Minnen, A. Intensive prolonged exposure therapy for chronic PTSD patients following multiple trauma and multiple treatment attempts. Eur. J. Psychotraumatol. 2018, 9, 1425574. [Google Scholar] [CrossRef] [Green Version]
- Ehlers, A.; Hackmann, A.; Grey, N.; Wild, J.; Liness, S.; Albert, I.; Deale, A.; Stott, R.; Clark, D.M. A Randomized Controlled Trial of 7-Day Intensive and Standard Weekly Cognitive Therapy for PTSD and Emotion-Focused Supportive Therapy. Am. J. Psychiatry 2014, 171, 294–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.G.; Sun, L.N.; Wang, Y.; Wu, L.L.; Sun, Z.E.; Zhang, F.; Liu, W.Z. Developments of prolonged exposure in treatment effect of post-traumatic stress disorder and controlling dropout rate: A meta-analytic review. Clin. Psychol. Psychol. 2020, 27, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Oprel, D.A.C.; Hoeboer, C.M.; Schoorl, M.; de Kleine, R.A.; Cloitre, M.; Wigard, I.G.; van Minnen, A.; van der Does, W. Effect of Prolonged Exposure, intensified Prolonged Exposure and STAIR+Prolonged Exposure in patients with PTSD related to childhood abuse: A randomized controlled trial. Eur. J. Psychotraumatol. 2021, 12, 1851511. [Google Scholar] [CrossRef]
- Seidler, G.H.; Wagner, F.E. Comparing the efficacy of EMDR and trauma-focused cognitive-behavioral therapy in the treatment of PTSD: A meta-analytic study. Psychol. Med. 2006, 36, 1515–1522. [Google Scholar] [CrossRef]
- Becker, C.B.; Zayfert, C.; Anderson, E. A survey of psychologists’ attitudes towards and utilization of exposure therapy for PTSD. Behav. Res. Ther. 2004, 42, 277–292. [Google Scholar] [CrossRef] [Green Version]
- De Haan, K.B.L.; Lee, C.S.W.; Correia, H.; Menninga, S.; Fassbinder, E.; Koehne, S.; Arntz, A. Patient and Therapist Perspectives on Treatment for Adults with PTSD from Childhood Trauma. J. Clin. Med. 2021, 10, 954. [Google Scholar] [CrossRef]
- Van Minnen, A.; Hendriks, L.; Olff, M. When do trauma experts choose exposure therapy for PTSD patients? A controlled study of therapist and patient factors. Behav. Res. Ther. 2010, 48, 312–320. [Google Scholar] [CrossRef]
- Perlis, R.H. Abandoning personalization to get to precision in the pharmacotherapy of depression. World Psychiatry 2016, 15, 228–235. [Google Scholar] [CrossRef] [Green Version]
- Waller, G. Evidence-based treatment and therapist drift. Behav. Res. Ther. 2009, 47, 119–127. [Google Scholar] [CrossRef]
- Deisenhofer, A.K.; Delgadillo, J.; Rubel, J.A.; Bohnke, J.R.; Zimmermann, D.; Schwartz, B.; Lutz, W. Individual treatment selection for patients with posttraumatic stress disorder. Depress. Anxiety 2018, 35, 541–550. [Google Scholar] [CrossRef]
- Keefe, J.R.; Stirman, S.W.; Cohen, Z.D.; DeRubeis, R.J.; Smith, B.N.; Resick, P.A. In rape trauma PTSD, patient characteristics indicate which trauma-focused treatment they are most likely to complete. Depress. Anxiety 2018, 35, 330–338. [Google Scholar] [CrossRef]
- Petkova, E.; Park, H.; Ciarleglio, A.; Ogden, R.T.; Tarpey, T. Optimising treatment decision rules through generated effect modifiers: A precision medicine tutorial. Bjpsych Open 2020, 6. [Google Scholar] [CrossRef]
- Cloitre, M.; Petkova, E.; Su, Z.; Weiss, B. Patient characteristics as a moderator of post-traumatic stress disorder treatment outcome: Combining symptom burden and strengths. BJPsych Open 2016, 2, 101–106. [Google Scholar] [CrossRef]
- Oprel, D.A.C.; Hoeboer, C.M.; Schoorl, M.; De Kleine, R.A.; Wigard, I.G.; Cloitre, M.; Van Minnen, A.; Van der Does, W. Improving treatment for patients with childhood abuse related posttraumatic stress disorder (IMPACT study): Protocol for a multicenter randomized trial comparing prolonged exposure with intensified prolonged exposure and phase-based treatment. BMC Psychiatry 2018, 18, 385. [Google Scholar] [CrossRef] [Green Version]
- Boeschoten, M.A.; Van der Aa, N.; Bakker, A.; Ter Heide, F.J.J.; Hoofwijk, M.C.; Jongedijk, R.A.; Van Minnen, A.; Elzinga, B.M.; Olff, M. Development and Evaluation of the Dutch Clinician-Administered PTSD Scale for DSM-5 (CAPS-5). Eur. J. Psychotraumatol. 2018, 9, 1546085. [Google Scholar] [CrossRef] [PubMed]
- Foa, E.B.; Hembree, E.A.; Rothbaum, B.O. Prolonged Exposure Therapy for PTSD: Emotional Processing of Traumatic Experiences: Therapist Guide; Oxford University Press: Oxford, UK; New York, NY, USA, 2007; p. viii. 146p. [Google Scholar]
- Weathers, F.W.; Blake, D.D.; Schnurr, P.P.; Kaloupek, D.G.; Marx, B.P.; Keane, T.M. The Clinician-Administered PTSD Scale for DSM-5 (CAPS-5). Available online: www.ptsd.va.gov (accessed on 26 February 2021).
- Blevins, C.A.; Weathers, F.W.; Davis, M.T.; Witte, T.K.; Domino, J.L. The Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5): Development and Initial Psychometric Evaluation. J. Trauma. Stress 2015, 28, 489–498. [Google Scholar] [CrossRef] [PubMed]
- De Bont, P.A.; van den Berg, D.P.; van der Vleugel, B.M.; de Roos, C.; Mulder, C.L.; Becker, E.S.; de Jongh, A.; van der Gaag, M.; van Minnen, A. A multi-site single blind clinical study to compare the effects of prolonged exposure, eye movement desensitization and reprocessing and waiting list on patients with a current diagnosis of psychosis and co morbid post traumatic stress disorder: Study protocol for the randomized controlled trial Treating Trauma in Psychosis. Trials 2013, 14, 151. [Google Scholar] [CrossRef] [Green Version]
- Kempen, G.I. Assessment of health status of the elderly. Application of a Dutch version of the MOS scale. Tijdschr. Gerontol. Geriatr. 1992, 23, 132–140. [Google Scholar]
- Bernstein, D.P.; Fink, L. CTQ Childhood Trauma Questionnaire. A Retrospective Self-Report. Manual; The Psychological Corporation: San Antonio, TX, USA, 1998. [Google Scholar]
- Brooks, R. EuroQol: The current state of play. Health Policy 1996, 37, 53–72. [Google Scholar] [CrossRef]
- Le, Q.A.; Doctor, J.N.; Zoellner, L.A.; Feeny, N.C. Minimal clinically important differences for the EQ-5D and QWB-SA in Post-traumatic Stress Disorder (PTSD): Results from a Doubly Randomized Preference Trial (DRPT). Health Qual. Life Outcomes 2013, 11, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beck, A.T.; Steer, R.A.; Brown, G.K. BDI-II, Beck Depression Inventory: Manual, 2nd ed.; Psychological Corp.: Boston, MA, USA, 1996; Volume p. vi, 38p. [Google Scholar]
- Foa, E.B.; Ehlers, A.; Clark, D.M.; Tolin, D.F.; Orsillo, S.M. The Posttraumatic Cognitions Inventory (PTCI): Development and validation. Psychol. Assess. 1999, 11, 303–314. [Google Scholar] [CrossRef]
- Barkham, M.; Hardy, G.E.; Startup, M. The IIP-32: A short version of the inventory of interpersonal problems. Br. J. Clin. Psychol. 1996, 35, 21–35. [Google Scholar] [CrossRef]
- Schmitt, D.P.; Allik, J. Simultaneous administration of the Rosenberg Self-Esteem Scale in 53 nations: Exploring the universal and culture-specific features of global self-esteem. J. Pers. Soc. Psychol. 2005, 89, 623–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.J.; Witte, T.K.; Bardeen, J.R.; Davis, M.T.; Weathers, F.W. A Factor Analytic Evaluation of the Difficulties in Emotion Regulation Scale. J. Clin. Psychol. 2016, 72, 933–946. [Google Scholar] [CrossRef] [PubMed]
- Nijenhuis, E.R.S.; Spinhoven, P.; VanDyck, R.; VanderHart, O.; Vanderlinden, J. The development and psychometric characteristics of the somatoform dissociation questionnaire (SDQ-20). J. Nerv. Ment. Dis. 1996, 184, 688–694. [Google Scholar] [CrossRef]
- Weertman, A.; Arntz, A.; Dreessen, L.; van Velzen, C.; Vertommen, S. Short-interval test-retest interrater reliability of the Dutch version of the structured clinical interview for DSM-IV personality disorders (SCID-II). J. Pers. Disord. 2003, 17, 562–567. [Google Scholar] [CrossRef]
- Sheehan, D.V.; Lecrubier, Y.; Sheehan, K.H.; Amorim, P.; Janavs, J.; Weiller, E.; Hergueta, T.; Baker, R.; Dunbar, G.C. The Mini-International Neuropsychiatric Interview (MINI): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 1998, 59, 22–33. [Google Scholar]
- Eidhof, M.B.; ter Heide, F.J.J.; van der Aa, N.; Schreckenbach, M.; Schmidt, U.; Brand, B.L.; Lanius, R.A.; Loewenstein, R.J.; Spiegel, D.; Vermetten, E. The Dissociative Subtype of PTSD Interview (DSP-I): Development and Psychometric Properties. J. Trauma Dissociation 2019, 20, 564–581. [Google Scholar] [CrossRef]
- Bates, D.; Machler, M.; Bolker, B.M.; Walker, S.C. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Raudenbush, S.W.; Bryk, A.S. Hierarchical Linear Models: Applications and Data Analysis Methods, 2nd ed.; SAGA: Newbury Park, CA, USA, 2002. [Google Scholar]
- Kursa, M.B.; Rudnicki, W.R. Feature Selection with the Boruta Package. J. Stat. Softw. 2010, 36, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Rizopoulos, D. BootStepAIC: Bootstrap StepAIC. R Package Version 1.2-0. Available online: https://CRAN.R-project.org/package=bootStepAIC (accessed on 26 February 2021).
- Dewar, M.; Paradis, A.; Fortin, C.A. Identifying Trajectories and Predictors of Response to Psychotherapy for Post-Traumatic Stress Disorder in Adults: A Systematic Review of Literature. Can. J. Psychiatry 2020, 65, 71–86. [Google Scholar] [CrossRef]
- Barawi, K.S.; Lewis, C.; Simon, N.; Bisson, J.I. A systematic review of factors associated with outcome of psychological treatments for post-traumatic stress disorder. Eur. J. Psychotraumatol. 2020, 11, 1774240. [Google Scholar] [CrossRef]
- Hoskins, M.; Pearce, J.; Bethell, A.; Dankova, L.; Barbui, C.; Tol, W.A.; van Ommeren, M.; de Jong, J.; Seedat, S.; Chen, H.H.; et al. Pharmacotherapy for post-traumatic stress disorder: Systematic review and meta-analysis. Br. J. Psychiatry 2015, 206, 93–100. [Google Scholar] [CrossRef]
- James, G.; Witten, D.; Hastie, T.; Tibshirani, R. An Introduction to Statistical Learning: With Applications in R; Springer: New York, NY, USA, 2013; Volume p. xvi, 426p. [Google Scholar]
- Delgadillo, J.; Duhne, P.G.S. Targeted Prescription of Cognitive-Behavioral Therapy Versus Person-Centered Counseling for Depression Using a Machine Learning Approach. J. Consult. Clin. Psychol. 2020, 88, 14–24. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, B.; Cohen, Z.D.; Rubel, J.A.; Zimmermann, D.; Wittmann, W.W.; Lutz, W. Personalized treatment selection in routine care: Integrating machine learning and statistical algorithms to recommend cognitive behavioral or psychodynamic therapy. Psychother. Res. 2020, 31, 33–51. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.K.; Gilthorpe, M.S. Revisiting the relation between change and initial value: A review and evaluation—Author’s reply. Stat. Med. 2007, 26, 3206–3208. [Google Scholar] [CrossRef]
- Delgadillo, J.; Lutz, W. A Development Pathway Towards Precision Mental Health Care. JAMA Psychiatry 2020, 77, 889–890. [Google Scholar] [CrossRef] [PubMed]
- Lutz, W.; Deisenhofer, A.K.; Justus, J.R.; Bennemann, B.; Giesemann, J.; Poster, K.; Schwartz, B. Prospective evaluation of a clinical decision support system in psychological therapy. J. Consult. Clin. Psychol. 2021. [Google Scholar] [CrossRef]
- Lutz, W.; Rubel, J.A.; Schwartz, B.; Schilling, V.; Deisenhofer, A.K. Towards integrating personalized feedback research into clinical practice: Development of the Trier Treatment Navigator (TTN). Behav. Res. Ther. 2019, 120, 103438. [Google Scholar] [CrossRef]

| Predictors 1 | Possible Range of Predictor Scores Min-Max | Exposure Therapies (n = 99) Mean (SD) or % | STAIR+PE (n = 50) Mean (SD) or % |
|---|---|---|---|
| Patient expectancies | |||
| Expected burden | 0–10 | 5.98 (2.56) | 6.73 (2.37) |
| Credibility | 0–10 | 6.75 (1.89) | 6.72 (1.74) |
| Demographics | |||
| Age, y | 36.76 (11.47) | 37.07 (12.39) | |
| Gender, female | 75.76 | 78.00 | |
| Cultural background, western | 39.39 | 52.00 | |
| Education, high | 21.21 | 18.00 | |
| Employment, yes | 40.40 | 34.00 | |
| Social support | |||
| MOS total score | 1–5 | 3.41 (1.10) | 3.32 (1.04) |
| Trauma background | |||
| CTQ childhood emotional abuse | 5–25 | 17.06 (6.04) | 17.54 (6.21) |
| CTQ childhood emotional neglect | 5–25 | 17.74 (5.08) | 19.84 (5.38) |
| CTQ childhood physical abuse | 5–25 | 13.09 (6.97) | 14.42 (6.36) |
| CTQ childhood sexual abuse | 5–25 | 15.48 (7.12) | 15.62 (7.68) |
| General health status | |||
| EQ-5D-5L general health status | 0–100 | 55.56 (26.31) | 58.18 (20.03) |
| Self-reported psychiatric symptoms | |||
| BDI total score | 0–63 | 33.63 (10.06) | 34.88 (11.15) |
| PTCI total score | 33–231 | 133.26 (36.40) | 149.64 (31.64) |
| IIP total score | 0–4 | 1.65 (0.62) | 1.70 (0.50) |
| RSES total score | 0–30 | 12.52 (5.84) | 11.32 (6.14) |
| DERS total score | 36–180 | 115.63 (21.27) | 117.46 (20.46) |
| SDQ-5 total score | 5–25 | 6.78 (2.93) | 7.64 (3.11) |
| Psychotropic medication | 49.49 | 44.00 | |
| Clinician-assessed psychiatric symptoms and disorders | |||
| Any SCID-2 personality disorder | 59.60 | 62.00 | |
| DSP-I total score | 0–36 | 1.78 (3.20) | 3.22 (5.65) |
| Axis-1 MINI diagnoses, excluding PTSD | 2.99 | 3.38 | |
| CAPS-5 baseline total score | 0–80 | 40.28 (8.73) | 43.56 (10.46) |
| Exposure Therapies | Estimate | Std. Error | t-Value | p |
|---|---|---|---|---|
| BDI | −0.24 | 0.07 | −3.40 | <0.001 |
| MOS | 2.23 | 0.62 | 3.63 | <0.001 |
| Axis-1 MINI diagnoses | −0.89 | 0.37 | −2.42 | 0.02 |
| CTQ sexual abuse | −0.18 | 0.09 | −2.04 | 0.04 |
| STAIR+PE | ||||
| EQ-5D-5L | 0.07 | 0.04 | 1.97 | 0.05 |
| DERS | −0.10 | 0.04 | −2.54 | 0.01 |
| CAPS-5 baseline | −0.26 | 0.08 | −3.19 | 0.003 |
| Exposure Therapies | Estimate | Std. Error | t-Value | p |
|---|---|---|---|---|
| BDI | −0.26 | 0.10 | −2.65 | 0.01 |
| MOS | 2.59 | 0.89 | 2.90 | 0.005 |
| STAIR+PE | ||||
| EQ-5D-5L | 0.11 | 0.06 | 1.78 | 0.08 |
| DERS | −0.16 | 0.06 | −2.75 | 0.009 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoeboer, C.M.; Oprel, D.A.C.; De Kleine, R.A.; Schwartz, B.; Deisenhofer, A.-K.; Schoorl, M.; Van Der Does, W.A.J.; van Minnen, A.; Lutz, W. Personalization of Treatment for Patients with Childhood-Abuse-Related Posttraumatic Stress Disorder. J. Clin. Med. 2021, 10, 4522. https://doi.org/10.3390/jcm10194522
Hoeboer CM, Oprel DAC, De Kleine RA, Schwartz B, Deisenhofer A-K, Schoorl M, Van Der Does WAJ, van Minnen A, Lutz W. Personalization of Treatment for Patients with Childhood-Abuse-Related Posttraumatic Stress Disorder. Journal of Clinical Medicine. 2021; 10(19):4522. https://doi.org/10.3390/jcm10194522
Chicago/Turabian StyleHoeboer, Chris M., Danielle A. C. Oprel, Rianne A. De Kleine, Brian Schwartz, Anne-Katharina Deisenhofer, Maartje Schoorl, Willem A. J. Van Der Does, Agnes van Minnen, and Wolfgang Lutz. 2021. "Personalization of Treatment for Patients with Childhood-Abuse-Related Posttraumatic Stress Disorder" Journal of Clinical Medicine 10, no. 19: 4522. https://doi.org/10.3390/jcm10194522
APA StyleHoeboer, C. M., Oprel, D. A. C., De Kleine, R. A., Schwartz, B., Deisenhofer, A.-K., Schoorl, M., Van Der Does, W. A. J., van Minnen, A., & Lutz, W. (2021). Personalization of Treatment for Patients with Childhood-Abuse-Related Posttraumatic Stress Disorder. Journal of Clinical Medicine, 10(19), 4522. https://doi.org/10.3390/jcm10194522






