Prognosis of Laboratory-Confirmed Influenza and Respiratory Syncytial Virus in Acute Heart Failure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Nucleic Acid Analysis Using Multiplex Real-Time PCR
2.3. Statistical Analysis
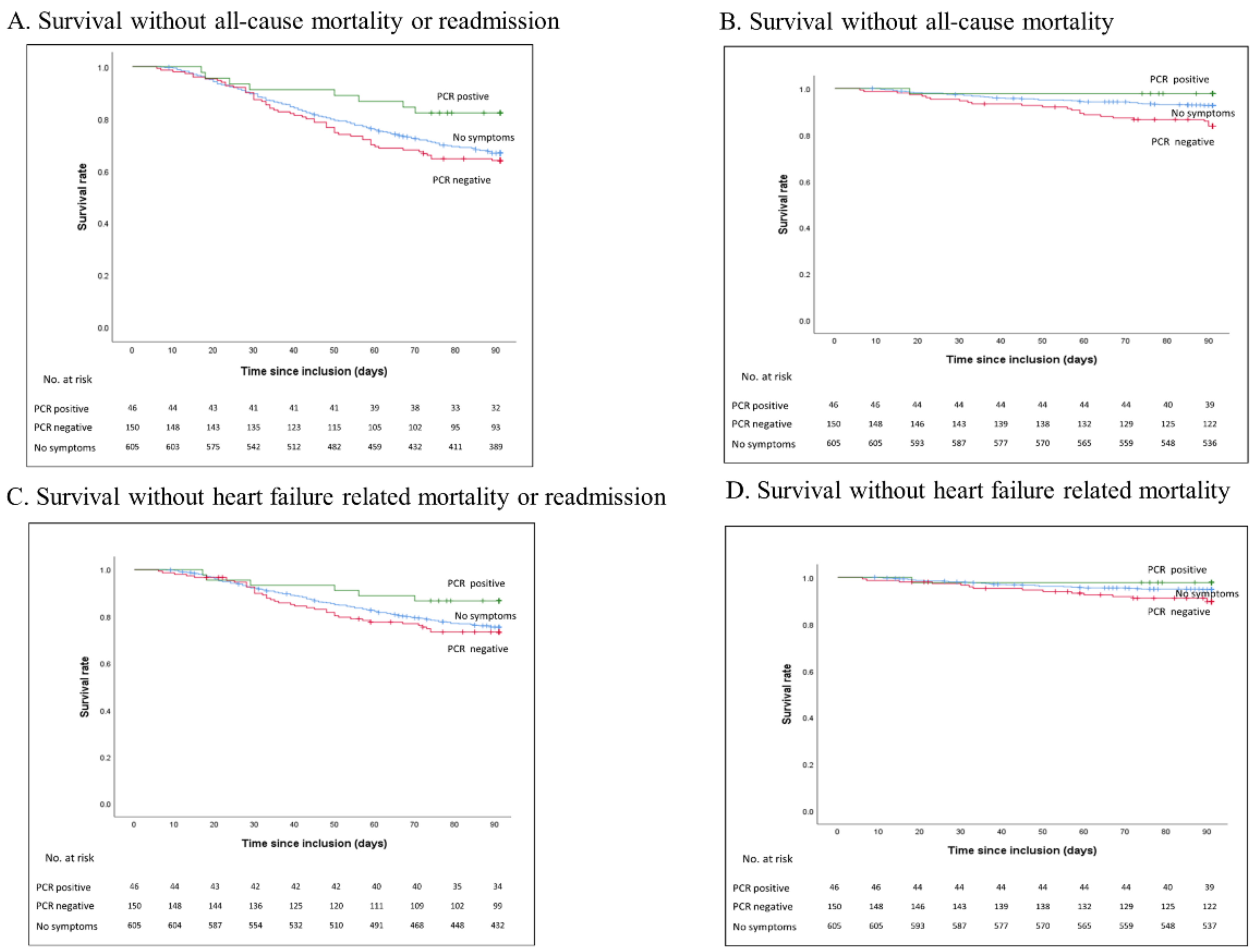
3. Results
Survival Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jhund, P.S.; Macintyre, K.; Simpson, C.R.; Lewsey, J.D.; Stewart, S.; Redpath, A.; Chalmers, J.W.; Capewell, S.; McMurray, J.J. Long-term trends in first hospitalization for heart failure and subsequent survival between 1986 and 2003: A population study of 5.1 million people. Circulation 2009, 119, 515–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levy, D.; Kenchaiah, S.; Larson, M.G.; Benjamin, E.J.; Kupka, M.J.; Ho, K.K.; Murabito, J.M.; Vasan, R.S. Long-term trends in the incidence of and survival with heart failure. N. Engl. J. Med. 2002, 347, 1397–1402. [Google Scholar] [CrossRef] [PubMed]
- Roger, V.L.; Go, A.S.; Lloyd-Jones, D.M.; Benjamin, E.J.; Berry, J.D.; Borden, W.B.; Bravata, D.M.; Dai, S.; Ford, E.S.; Fox, C.S.; et al. Executive summary: Heart disease and stroke statistics—2012 update: A report from the American Heart Association. Circulation 2012, 125, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, L.; Clarke, M.J.; Williamson, G.D.; Stroup, D.F.; Arden, N.H.; Schonberger, L.B. The impact of influenza epidemics on mortality: Introducing a severity index. Am. J. Public Health 1997, 87, 1944–1950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simonsen, L.; Fukuda, K.; Schonberger, L.B.; Cox, N.J. The impact of influenza epidemics on hospitalizations. J. Infect. Dis. 2000, 181, 831–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berra, G.; Garin, N.; Stirnemann, J.; Jannot, A.S.; Martin, P.Y.; Perrier, A.; Carballo, S. Outcome in acute heart failure: Prognostic value of acute kidney injury and worsening renal function. J. Card. Fail. 2015, 21, 382–390. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; Gonzalez-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef]
- Rahimi, K.; Bennett, D.; Conrad, N.; Williams, T.M.; Basu, J.; Dwight, J.; Woodward, M.; Patel, A.; McMurray, J.; MacMahon, S. Risk prediction in patients with heart failure: A systematic review and analysis. JACC Heart Fail. 2014, 2, 440–446. [Google Scholar] [CrossRef]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Drazner, M.H.; Fonarow, G.C.; Geraci, S.A.; Horwich, T.; Januzzi, J.L.; et al. 2013 ACCF/AHA guideline for the management of heart failure: Executive summary: A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013, 128, 1810–1852. [Google Scholar] [CrossRef]
- Flattet, Y.; Garin, N.; Serratrice, J.; Perrier, A.; Stirnemann, J.; Carballo, S. Determining prognosis in acute exacerbation of COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2017, 12, 467–475. [Google Scholar] [CrossRef] [Green Version]
- Modin, D.; Jorgensen, M.E.; Gislason, G.; Jensen, J.S.; Kober, L.; Claggett, B.; Hegde, S.M.; Solomon, S.D.; Torp-Pedersen, C.; Biering-Sorensen, T. Influenza Vaccine in Heart Failure. Circulation 2019, 139, 575–586. [Google Scholar] [CrossRef]
- Panhwar, M.S.; Kalra, A.; Gupta, T.; Kolte, D.; Khera, S.; Bhatt, D.; Ginwalla, M. Relation of Concomitant Heart Failure to Outcomes in Patients Hospitalized With Influenza. Am. J. Cardiol. 2019, 123, 1478–1480. [Google Scholar] [CrossRef]
- Sandoval, C.; Walter, S.D.; Krueger, P.; Loeb, M.B. Comparing estimates of influenza-associated hospitalization and death among adults with congestive heart failure based on how influenza season is defined. BMC Public Health 2008, 8, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandoval, C.; Walter, S.D.; Krueger, P.; Smieja, M.; Smith, A.; Yusuf, S.; Loeb, M.B. Risk of hospitalization during influenza season among a cohort of patients with congestive heart failure. Epidemiol. Infect. 2007, 135, 574–582. [Google Scholar] [CrossRef]
- Visseaux, B.; Burdet, C.; Voiriot, G.; Lescure, F.X.; Chougar, T.; Brugiere, O.; Crestani, B.; Casalino, E.; Charpentier, C.; Descamps, D.; et al. Prevalence of respiratory viruses among adults, by season, age, respiratory tract region and type of medical unit in Paris, France, from 2011 to 2016. PLoS ONE 2017, 12, e0180888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivey, K.S.; Edwards, K.M.; Talbot, H.K. Respiratory Syncytial Virus and Associations With Cardiovascular Disease in Adults. J. Am. Coll. Cardiol. 2018, 71, 1574–1583. [Google Scholar] [CrossRef]
- Harling, R.; Hayward, A.; Watson, J.M. Implications of the incidence of influenza-like illness in nursing homes for influenza chemoprophylaxis: Descriptive study. BMJ 2004, 329, 663–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pieske, B.; Tschope, C.; de Boer, R.A.; Fraser, A.G.; Anker, S.D.; Donal, E.; Edelmann, F.; Fu, M.; Guazzi, M.; Lam, C.S.P.; et al. How to diagnose heart failure with preserved ejection fraction: The HFA-PEFF diagnostic algorithm: A consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur. Heart J. 2019, 40, 3297–3317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Federal Office of Public Health. Seasonal Flu (Influenza). Available online: https://www.bag.admin.ch/bag/en/home/begriffe-a-z/saisonale-grippe (accessed on 24 April 2020).
- Carballo, S.; Musso, P.; Garin, N.; Muller, H.; Serratrice, J.; Mach, F.; Carballo, D.; Stirnemann, J. Prognostic Value of the Echocardiographic Probability of Pulmonary Hypertension in Patients with Acute Decompensated Heart Failure. J. Clin. Med. 2019, 8, 1684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glezen, W.P.; Greenberg, S.B.; Atmar, R.L.; Piedra, P.A.; Couch, R.B. Impact of respiratory virus infections on persons with chronic underlying conditions. JAMA 2000, 283, 499–505. [Google Scholar] [CrossRef]
- Thompson, W.W.; Shay, D.K.; Weintraub, E.; Brammer, L.; Cox, N.; Anderson, L.J.; Fukuda, K. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003, 289, 179–186. [Google Scholar] [CrossRef]
- Jain, S.; Self, W.H.; Wunderink, R.G.; Fakhran, S.; Balk, R.; Bramley, A.M.; Reed, C.; Grijalva, C.G.; Anderson, E.J.; Courtney, D.M.; et al. Community-Acquired Pneumonia Requiring Hospitalization among U.S. Adults. N. Engl. J. Med. 2015, 373, 415–427. [Google Scholar] [CrossRef] [Green Version]
- Musher, D.M.; Roig, I.L.; Cazares, G.; Stager, C.E.; Logan, N.; Safar, H. Can an etiologic agent be identified in adults who are hospitalized for community-acquired pneumonia: Results of a one-year study. J. Infect. 2013, 67, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Musher, D.M.; Rueda, A.M.; Kaka, A.S.; Mapara, S.M. The association between pneumococcal pneumonia and acute cardiac events. Clin. Infect. Dis. 2007, 45, 158–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwong, J.C.; Schwartz, K.L.; Campitelli, M.A.; Chung, H.; Crowcroft, N.S.; Karnauchow, T.; Katz, K.; Ko, D.T.; McGeer, A.J.; McNally, D.; et al. Acute Myocardial Infarction after Laboratory-Confirmed Influenza Infection. N. Engl. J. Med. 2018, 378, 345–353. [Google Scholar] [CrossRef]
- Panhwar, M.S.; Kalra, A.; Gupta, T.; Kolte, D.; Khera, S.; Bhatt, D.L.; Ginwalla, M. Effect of Influenza on Outcomes in Patients with Heart Failure. JACC Heart Fail. 2019, 7, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Falsey, A.R.; Hennessey, P.A.; Formica, M.A.; Cox, C.; Walsh, E.E. Respiratory syncytial virus infection in elderly and high-risk adults. N. Engl. J. Med. 2005, 352, 1749–1759. [Google Scholar] [CrossRef]
- Lee, N.; Lui, G.C.; Wong, K.T.; Li, T.C.; Tse, E.C.; Chan, J.Y.; Yu, J.; Wong, S.S.; Choi, K.W.; Wong, R.Y.; et al. High morbidity and mortality in adults hospitalized for respiratory syncytial virus infections. Clin. Infect. Dis. 2013, 57, 1069–1077. [Google Scholar] [CrossRef]
- Iversen, K.K.; Kjaergaard, J.; Akkan, D.; Kober, L.; Torp-Pedersen, C.; Hassager, C.; Vestbo, J.; Kjoller, E.; Group, E.C.-L.F.S. Chronic obstructive pulmonary disease in patients admitted with heart failure. J. Intern. Med. 2008, 264, 361–369. [Google Scholar] [CrossRef]
- Rusinaru, D.; Saaidi, I.; Godard, S.; Mahjoub, H.; Battle, C.; Tribouilloy, C. Impact of chronic obstructive pulmonary disease on long-term outcome of patients hospitalized for heart failure. Am. J. Cardiol. 2008, 101, 353–358. [Google Scholar] [CrossRef]
- Hawkins, N.M.; Virani, S.; Ceconi, C. Heart failure and chronic obstructive pulmonary disease: The challenges facing physicians and health services. Eur. Heart J. 2013, 34, 2795–2803. [Google Scholar] [CrossRef] [Green Version]
- Aronson, D.; Darawsha, W.; Atamna, A.; Kaplan, M.; Makhoul, B.F.; Mutlak, D.; Lessick, J.; Carasso, S.; Reisner, S.; Agmon, Y.; et al. Pulmonary hypertension, right ventricular function, and clinical outcome in acute decompensated heart failure. J. Card. Fail. 2013, 19, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Tisdale, R.L.; Haddad, F.; Kohsaka, S.; Heidenreich, P.A. Trends in Left Ventricular Ejection Fraction for Patients With a New Diagnosis of Heart Failure. Circ. Heart Fail. 2020, 13, e006743. [Google Scholar] [CrossRef] [PubMed]
- Zurcher, K.; Zwahlen, M.; Berlin, C.; Egger, M.; Fenner, L. Trends in influenza vaccination uptake in Switzerland: Swiss Health Survey 2007 and 2012. Swiss Med. Wkly. 2019, 149, w14705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salez, N.; Vabret, A.; Leruez-Ville, M.; Andreoletti, L.; Carrat, F.; Renois, F.; de Lamballerie, X. Evaluation of Four Commercial Multiplex Molecular Tests for the Diagnosis of Acute Respiratory Infections. PLoS ONE 2015, 10, e0130378. [Google Scholar] [CrossRef] [Green Version]
| Symptomatic Patients (N = 196) | Patients without Suggestive Symptoms N (%) (N = 607) | |||
|---|---|---|---|---|
| PCR positive N (%) (N = 45) | PCR negative N (%) (N = 151) | p | ||
| Age (mean (SD)) | 77.9 (±10.3) | 77.1 (±10.2) | 77.2 (±11.2) | 0.93 |
| Sex | 0.49 | |||
| Male | 23 (51) | 84 (56) | 354 (58) | |
| Female | 22 (48) | 67 (44) | 253 (42) | |
| BMI (Kg/m2) | 27.8 (6.0) | 27.7 (7.2) | 26.5 (6.3) | 0.09 |
| Left ventricular ejection fraction | 0.71 | |||
| Preserved (>50%) | 23 (55) | 81 (57) | 322 (54) | |
| Mid-range (40–49%) | 8 (19) | 18 (13) | 77 (13) | |
| Reduced (<40%) | 11 (26) | 43 (30) | 198 (33) | |
| Comorbidities | ||||
| Hypertension | 34 (76) | 121 (80) | 482 (19) | 0.03 |
| Diabetes | 17 (39) | 44 (29) | 197 (33) | 0.43 |
| Chronic kidney disease | 19 (48) | 58 (39) | 205 (36) | 0.29 |
| COPD | 9 (21) | 35 (24) | 70 (12) | <0.001 |
| Atrial fibrillation | 21 (47) | 70 (47) | 276 (47) | 0.99 |
| Anaemia | 21 (47) | 62 (41) | 245 (40) | 0.06 |
| Etiology of heart failure | ||||
| Ischaemic | 15 (33.3) | 57 (37.7) | 192 (31.6) | 0.05 |
| Hypertensive | 11 (24.4) | 40 (26.5) | 123 (20.3) | 0.03 |
| Valvular | 13 (28.9) | 29 (19.2) | 128 (21.1) | 0.05 |
| Arythmic | 16 (34.8) | 50 (33.3) | 180 (29.7) | 0.07 |
| Active smoking | 10 (22) | 23 (15) | 102 (17) | 0.55 |
| NYHA class | 0.01 | |||
| I | 0 | 1 (1) | 6 (1) | |
| II | 3 (7) | 9 (6) | 35 (6) | |
| III | 6 (13) | 45 (31) | 219 (39) | |
| IV | 36 (80) | 90 (62) | 296 (53) | |
| De novo heart failure | 9 (20) | 43 (29) | 204 (34) | 0.21 |
| Parameters (mean (SD)) | ||||
| Systolic blood pressure | 143 (±28) | 137 (±25) | 142 (±27) | 0.12 |
| Diastolic blood pressure | 77 (±18) | 77 (±16) | 83 (±20) | 0.003 |
| Heart rate | 92 (±21) | 95 (±59) | 90 (±25) | 0.19 |
| Temperature (°C) | 37.3 (±0.9) | 36.9 (±0.8) | 36.8 (±0.6) | <0.001 |
| Fever (>38 °C) N(%) | 10 (22.2) | 14 (9.3) | 22 (3.6) | <0.001 |
| BNP (n = 123) | 1549 (±1520) | 1264 (±1563) | 1150 (±1021) | 0.53 |
| NT-proBNP (n = 678) | 7752 (±12060) | 8979 (±12302) | 8187 (±11091) | 0.76 |
| Hb (g/L) | 125 (±26) | 123 (±23) | 124 (±23) | 0.75 |
| eGFR (mL/min) | 51 (±23) | 53 (±22) | 53 (±24) | 0.77 |
| CRP (n = 786) | 48 (±64) | 55 (±74) | 24 (±43) | <0.001 |
| Leucocytes | 8.7 (±3.6) | 12.6 (±26.7) | 10.6 (±19.6) | 0.43 |
| Medication at admission | ||||
| ACEi | 11 (24) | 42 (28) | 166 (27) | 0.52 |
| ARB | 19 (42) | 40 (27) | 177 (29) | 0.19 |
| Loop diuretics | 24 (51) | 88 (58) | 268(44) | 0.07 |
| Betablockers | 24 (53) | 80 (53) | 342 (56) | 0.52 |
| MRA | 5 (11) | 20 (13) | 79 (13) | 0.99 |
| Medication at discharge | ||||
| ACEi | 16 (36) | 63 (42) | 231 (38) | 0.96 |
| ARB | 9 (20) | 29 (19) | 138 (23) | 0.86 |
| Loop diuretics | 35 (78) | 114 (76) | 445 (73) | 0.19 |
| Betablockers | 30 (67) | 97 (64) | 417 (69) | 0.82 |
| MRA | 6 (13) | 21 (14) | 110 (18) | 0.34 |
| All-Cause Mortality or Readmission (HR (95% CI) | p Value | |
|---|---|---|
| Sex (male) | 1.10 (0.86–1.40) | 0.46 |
| Age, year | 1.00 (0.99–1.01) | 0.70 |
| BMI, Kg/m2 | 0.99 (0.97–1.01) | 0.42 |
| Hypertension | 1.14 (0.84–1.56) | 0.40 |
| COPD | 1.72 (1.27–2.33) | <0.001 |
| Diabetes | 1.13 (0.87–1.46) | 0.36 |
| Chronic kidney disease | 1.25 (0.97–1.61) | 0.08 |
| Chronic anemia | 1.25 (0.98–1.60) | 0.08 |
| LVEF (<40%) a | 0.94 (0.73–1.22) | 0.66 |
| Symptomatic Patients | ||||
|---|---|---|---|---|
| PCR Positive Patients HR * (95% CI) p Value | PCR Negative Patients HR * (95% CI) p Value | |||
| Unadjusted | Adjusted | Unadjusted | Adjusted | |
| All-cause mortality or readmission | 0.44 (0.21–0.93) 0.03 | 0.40 (0.18–0.91) 0.03 | 1.14 (0.85–1.54) 0.38 | 1.11 (0.80–1.53) 0.66 |
| All-cause mortality | 0.31 (0.42–2.23) 0.24 | 0.30 (0.04–2.20) 0.24 | 2.27 (1.28–3.73) 0.001 | 2.41 (1.40–4.15) <0.01 |
| Heart failure related mortality or readmission | 0.44 (0.18–1.07) 0.07 | 0.36 (0.13–0.99) 0.05 | 1.13 (0.70–1.60) 0.51 | 1.16 (0.80–1.68) 0.44 |
| Heart failure related mortality | 0.44 (0.06–3.19) 0.41 | 0.43 (0.06–3.20) 0.41 | 2.02 (1.09–3.74) 0.03 | 2.00 (1.01–3.96) 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carballo, D.; Garin, N.; Stirnemann, J.; Mamin, A.; Prendki, V.; Meyer, P.; Marti, C.; Mach, F.; Reny, J.-L.; Serratrice, J.; et al. Prognosis of Laboratory-Confirmed Influenza and Respiratory Syncytial Virus in Acute Heart Failure. J. Clin. Med. 2021, 10, 4546. https://doi.org/10.3390/jcm10194546
Carballo D, Garin N, Stirnemann J, Mamin A, Prendki V, Meyer P, Marti C, Mach F, Reny J-L, Serratrice J, et al. Prognosis of Laboratory-Confirmed Influenza and Respiratory Syncytial Virus in Acute Heart Failure. Journal of Clinical Medicine. 2021; 10(19):4546. https://doi.org/10.3390/jcm10194546
Chicago/Turabian StyleCarballo, David, Nicolas Garin, Jérôme Stirnemann, Aline Mamin, Virginie Prendki, Philippe Meyer, Christophe Marti, Francois Mach, Jean-Luc Reny, Jacques Serratrice, and et al. 2021. "Prognosis of Laboratory-Confirmed Influenza and Respiratory Syncytial Virus in Acute Heart Failure" Journal of Clinical Medicine 10, no. 19: 4546. https://doi.org/10.3390/jcm10194546






