Impact of Infliximab Biosimilar CT-P13 Dose and Infusion Interval on Real-World Drug Survival and Effectiveness in Patients with Ankylosing Spondylitis
Abstract
1. Introduction
2. Materials and Methods
2.1. RAAS Study Design and Patients
2.2. Dose and Infusion Interval Analysis
2.3. Assessments
2.4. Statistical Analyses
3. Results
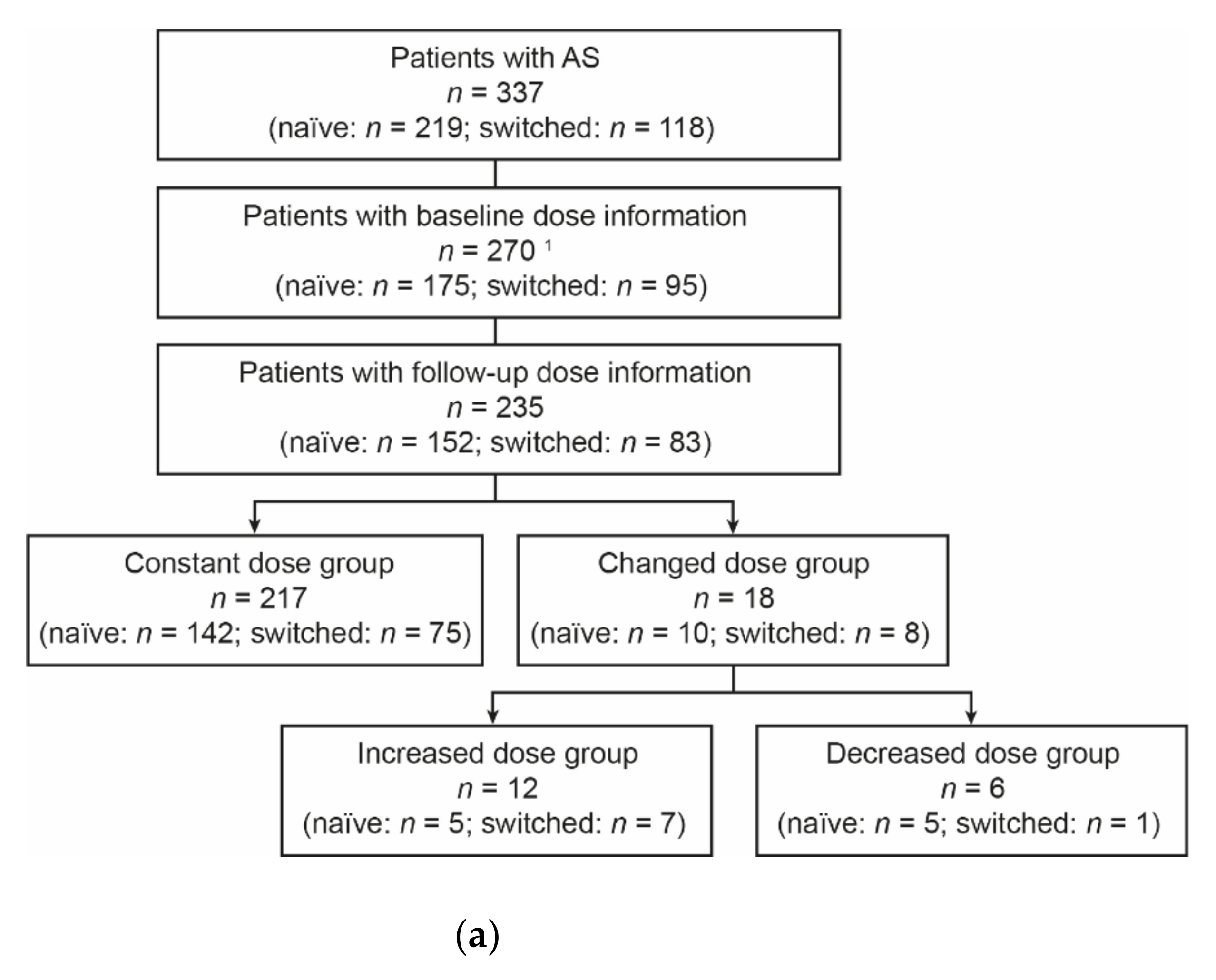
3.1. Patients
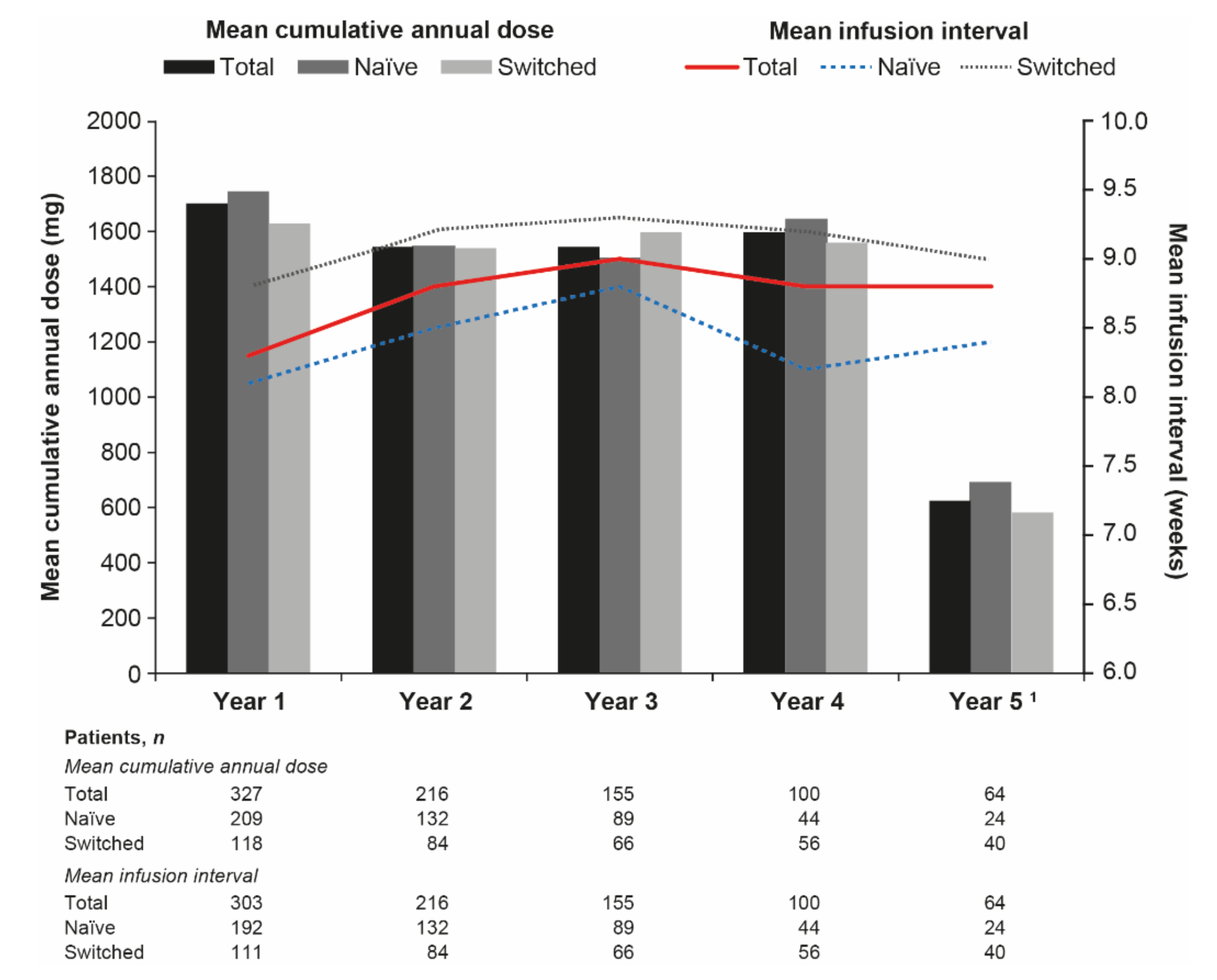
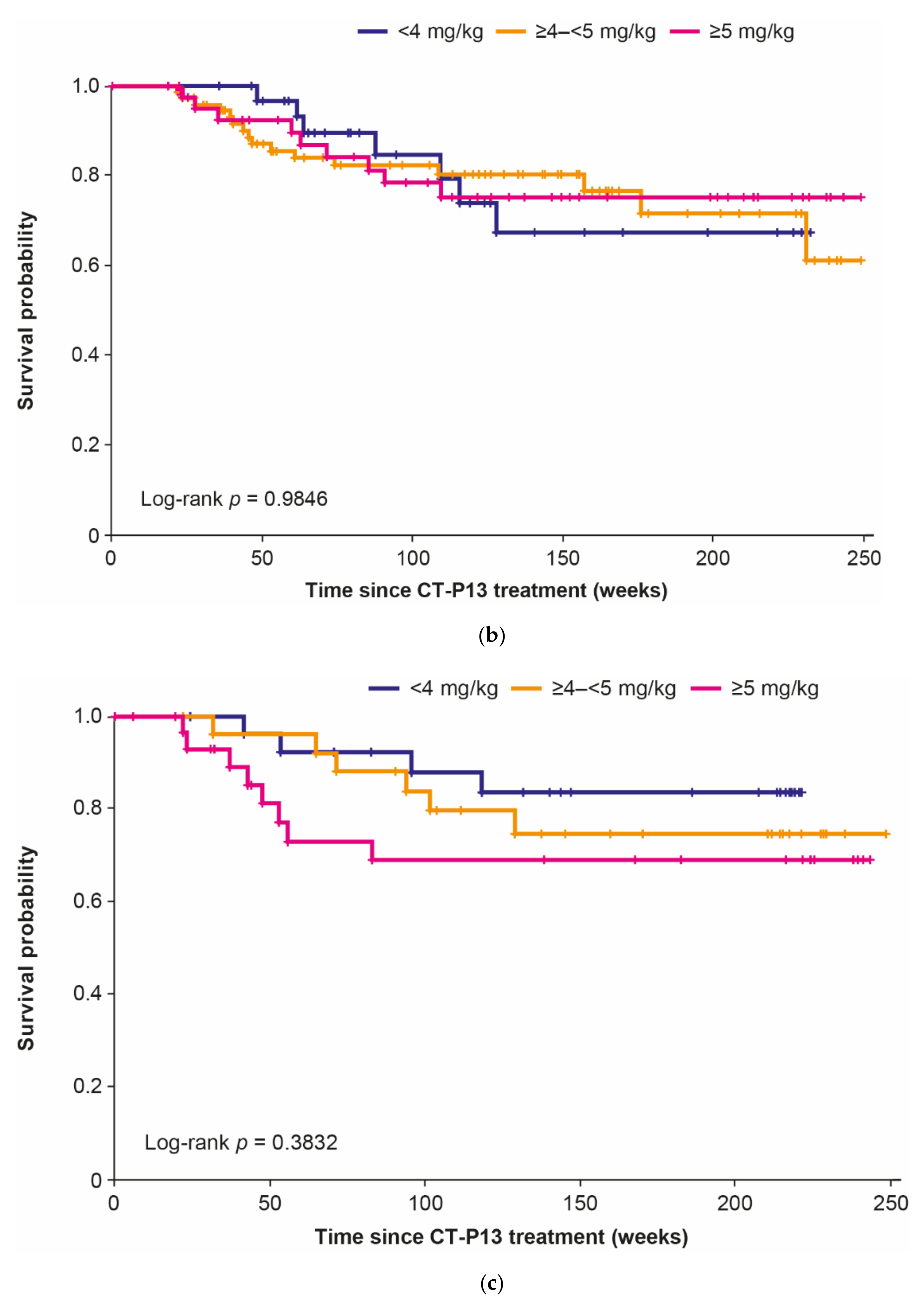
3.2. Dose Analyses
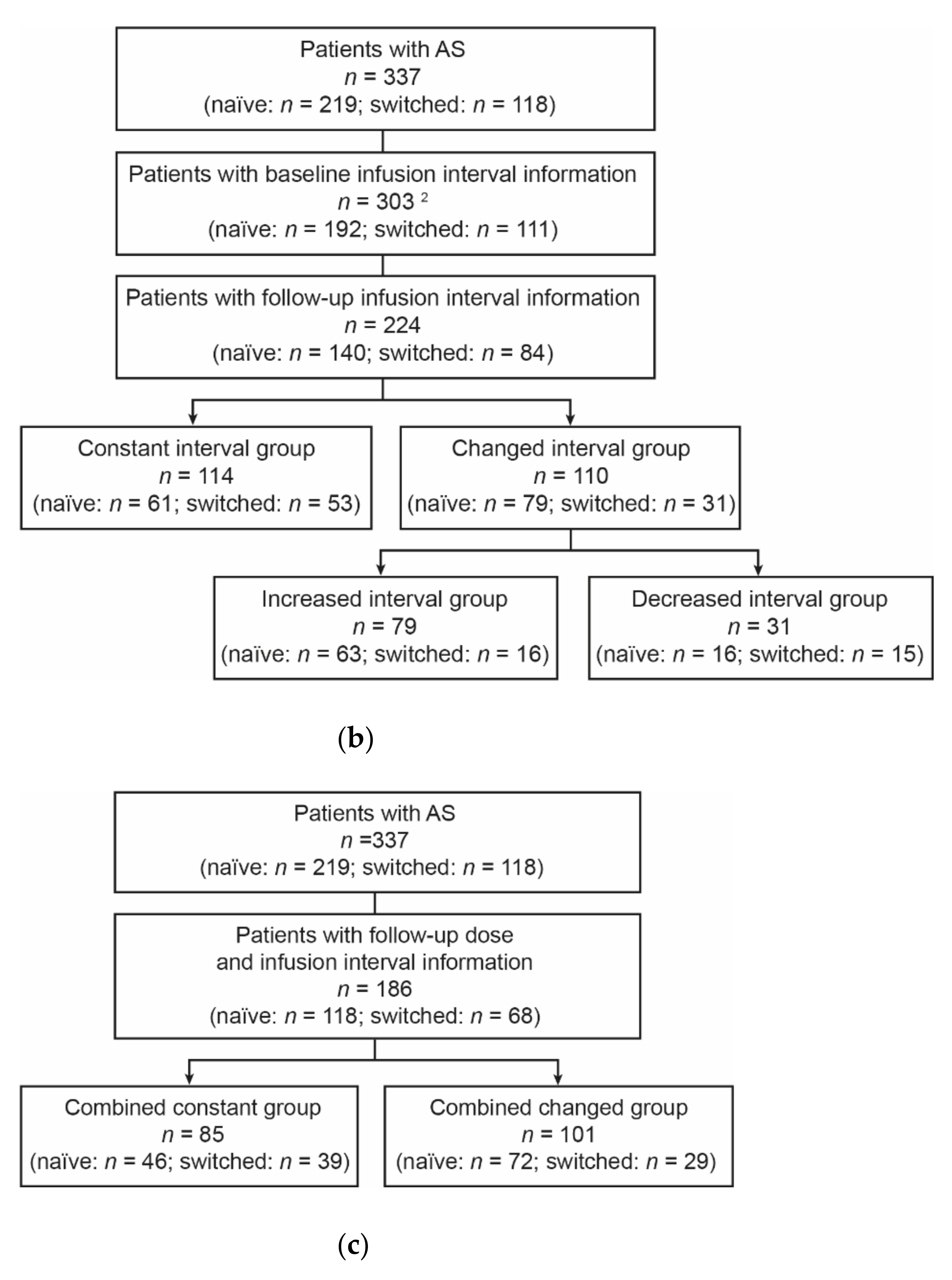
3.3. Infusion Interval Analyses
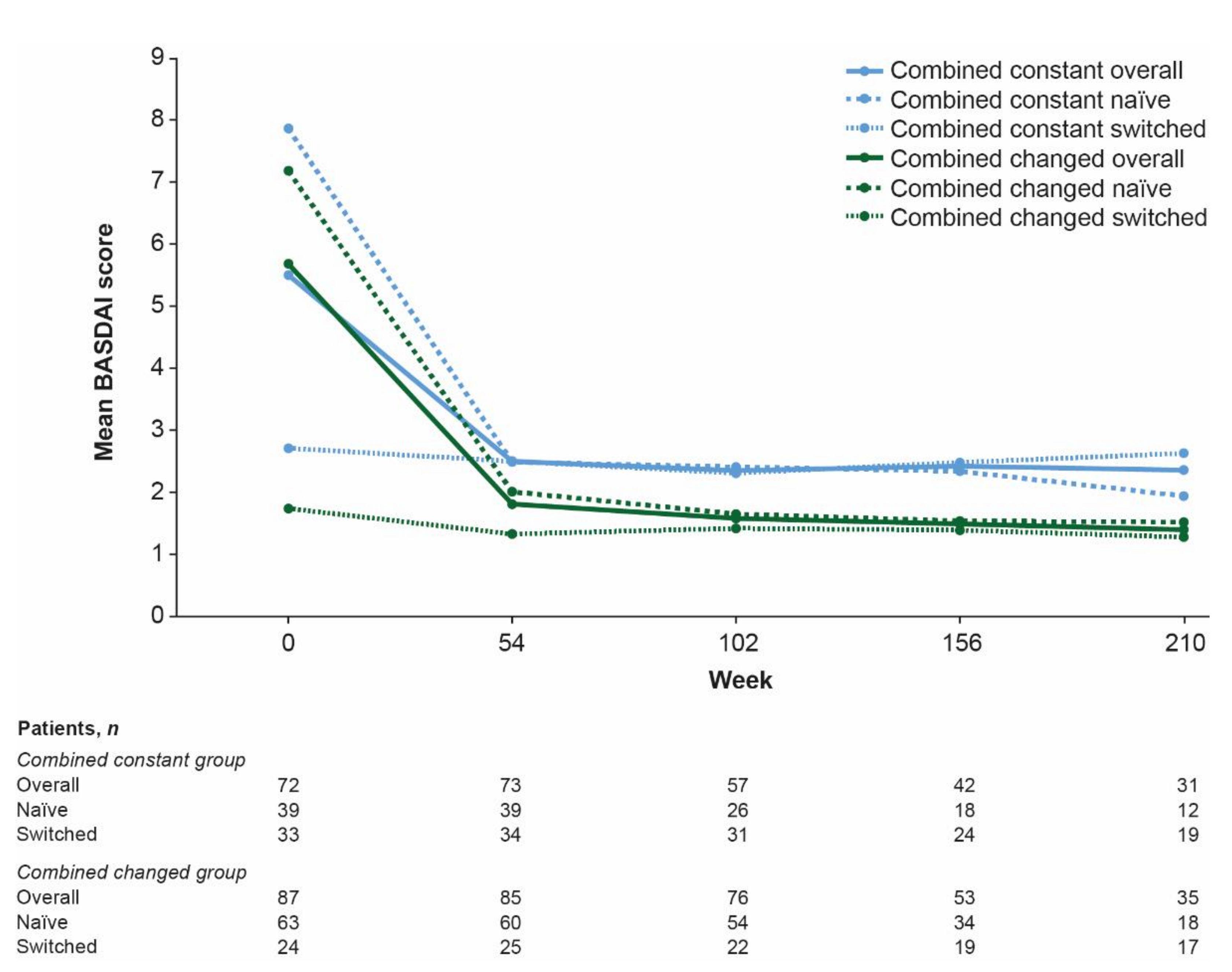
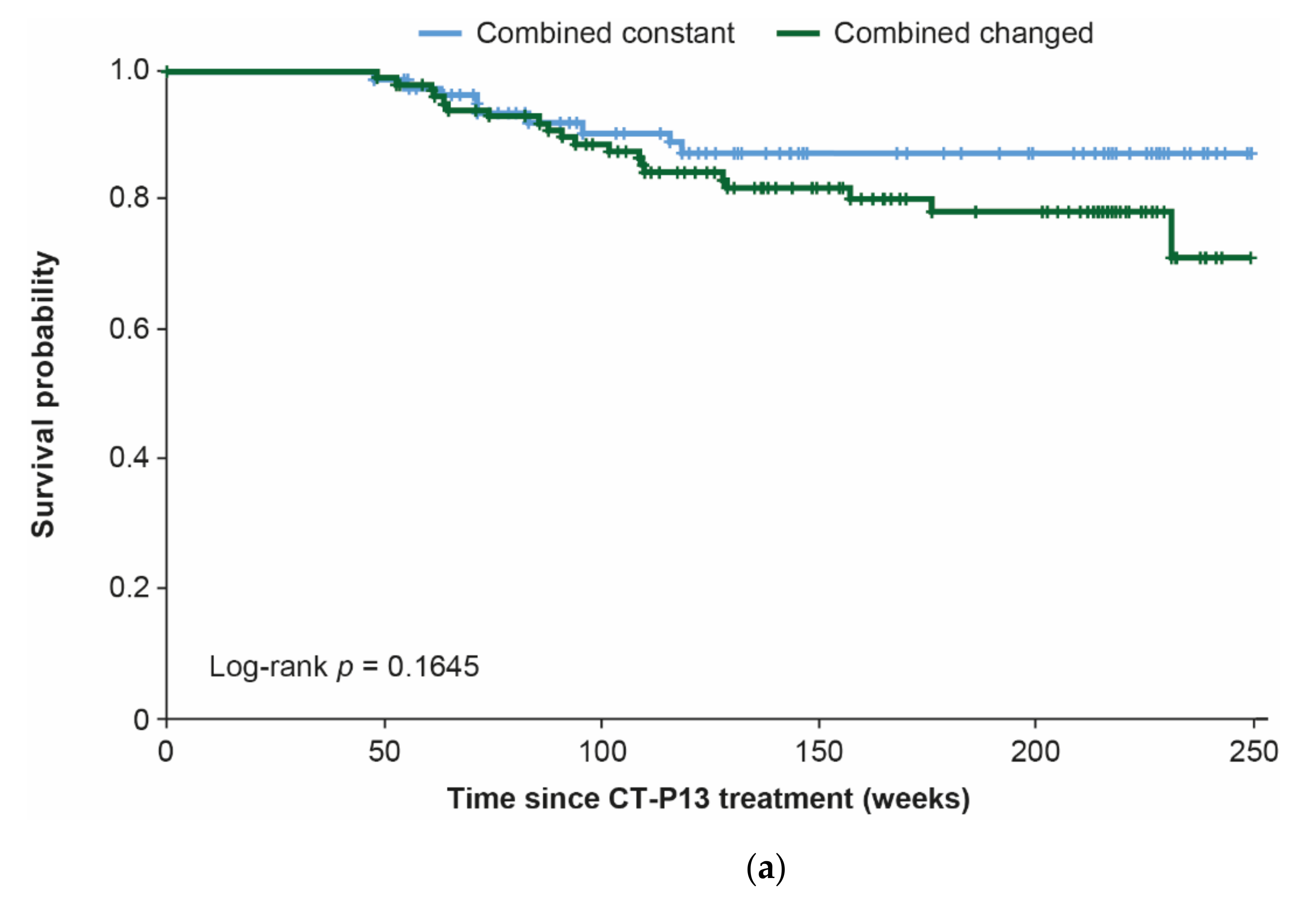
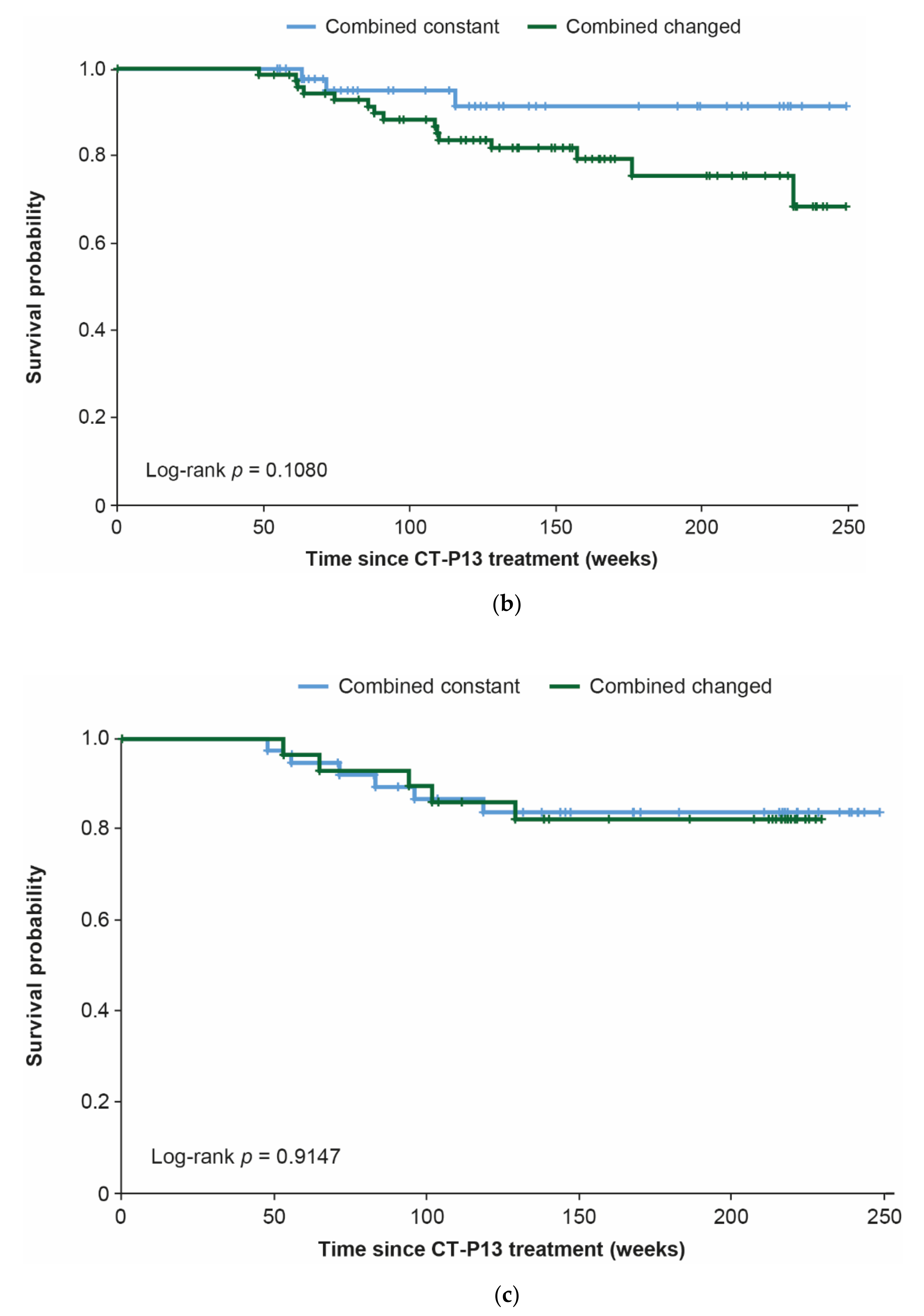
3.4. Combination Analyses
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, W.; Hrycaj, P.; Jeka, S.; Kovalenko, V.; Lysenko, G.; Miranda, P.; Mikazane, H.; Gutierrez-Ureña, S.; Lim, M.; Lee, Y.A.; et al. A randomised, double-blind, multicentre, parallel-group, prospective study comparing the pharmacokinetics, safety, and efficacy of CT-P13 and innovator infliximab in patients with ankylosing spondylitis: The PLANETAS study. Ann. Rheum. Dis. 2013, 72, 1605–1612. [Google Scholar] [CrossRef]
- Park, W.; Yoo, D.H.; Miranda, P.; Brzosko, M.; Wiland, P.; Gutierrez-Ureña, S.; Mikazane, H.; Lee, Y.A.; Smiyan, S.; Lim, M.J.; et al. Efficacy and safety of switching from reference infliximab to CT-P13 compared with maintenance of CT-P13 in ankylosing spondylitis: 102-week data from the PLANETAS extension study. Ann. Rheum. Dis. 2017, 76, 346–354. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Inflectra Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/125544s009lbl.pdf (accessed on 16 February 2021).
- European Medicines Agency. Remsima Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/remsima-epar-product-information_en.pdf (accessed on 16 February 2021).
- US Food and Drug Administration. Remicade Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/103772s5389s5391s5394lbl.pdf (accessed on 16 February 2021).
- European Medicines Agency. Inflectra Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/inflectra-epar-product-information_en.pdf (accessed on 16 February 2021).
- European Medicines Agency. Remicade Prescribing Information. Available online: https://www.ema.europa.eu/en/documents/product-information/remicade-epar-product-information_en.pdf (accessed on 16 February 2021).
- Ministry of Food and Drug Safety. Biological products (recombinant DNA products): Celltrion, Inc. Available online: https://www.mfds.go.kr/eng/brd/m_30/view.do?seq=69797 (accessed on 23 February 2021).
- Maksymowych, W.P.; Jhangri, G.S.; Lambert, R.G.; Mallon, C.; Buenviaje, H.; Pedrycz, E.; Luongo, R.; Russell, A.S. Infliximab in ankylosing spondylitis: A prospective observational inception cohort analysis of efficacy and safety. J. Rheumatol. 2002, 29, 959–965. [Google Scholar]
- Maksymowych, W.P.; Salonen, D.; Inman, R.D.; Rahman, P.; Lambert, R.G. Low-dose infliximab (3 mg/kg) significantly reduces spinal inflammation on magnetic resonance imaging in patients with ankylosing spondylitis: A randomized placebo-controlled study. J. Rheumatol. 2010, 37, 1728–1734. [Google Scholar] [CrossRef]
- Cherouvim, E.P.; Zintzaras, E.; Boki, K.A.; Moutsopoulos, H.M.; Manoussakis, M.N. Infliximab therapy for patients with active and refractory spondyloarthropathies at the dose of 3 mg/kg: A 20-month open treatment. J. Clin. Rheumatol. 2004, 10, 162–168. [Google Scholar] [CrossRef]
- Tenga, G.; Goëb, V.; Lequerré, T.; Bacquet-Deschryver, H.; Daragon, A.; Pouplin, S.; Lanfant-Weybel, K.; Le Loët, X.; Dieu, B.; Vittecoq, O. A 3 mg/kg starting dose of infliximab in active spondyloarthritis resistant to conventional treatments is efficient, safe and lowers costs. Jt. Bone Spine 2011, 78, 50–55. [Google Scholar] [CrossRef]
- Inman, R.D.; Maksymowych, W.P. A double-blind, placebo-controlled trial of low dose infliximab in ankylosing spondylitis. J. Rheumatol. 2010, 37, 1203–1210. [Google Scholar] [CrossRef]
- Keeling, S.; Oswald, A.; Russell, A.S.; Maksymowych, W.P. Prospective observational analysis of the efficacy and safety of low-dose (3 mg/kg) infliximab in ankylosing spondylitis: 4-year followup. J. Rheumatol. 2006, 33, 558–561. [Google Scholar]
- Jois, R.N.; Leeder, J.; Gibb, A.; Gaffney, K.; Macgregor, A.; Somerville, M.; Scott, D.G. Low-dose infliximab treatment for ankylosing spondylitis—clinically- and cost-effective. Rheumatology (Oxford) 2006, 45, 1566–1569. [Google Scholar] [CrossRef]
- Glintborg, B.; Gudbjornsson, B.; Steen Krogh, N.; Omerovic, E.; Manilo, N.; Holland-Fischer, M.; Lindegaard, H.M.; Gitte Loft, A.; Nordin, H.; Johnsen, L.; et al. Impact of different infliximab dose regimens on treatment response and drug survival in 462 patients with psoriatic arthritis: Results from the nationwide registries DANBIO and ICEBIO. Rheumatology 2014, 53, 2100–2109. [Google Scholar] [CrossRef]
- Gudbjornsson, B.; Geirsson, A.J.; Krogh, N.S. Low starting dosage of infliximab with possible escalating dosage in psoriatic arthritis gives the same treatment results as standard dosage of adalimumab or etanercept: Results from the nationwide Icelandic ICEBIO registry. Psoriasis (Auckl) 2018, 8, 13–19. [Google Scholar] [CrossRef]
- Nozaki, Y.; Nagare, Y.; Ashida, C.; Tomita, D.; Okada, A.; Inoue, A.; Kinoshita, K.; Funauchi, M.; Matsumura, I. Infliximab dose adjustment can improve the clinical and radiographic outcomes of rheumatoid arthritis patients: REVIVE study results. Biologics 2018, 12, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.B.; Kremer, J.M.; Dandreo, K.J.; Reed, G.W.; Magner, R.; Shan, Y.; Kafka, S.; DeHoratius, R.J.; Ellis, L.; Parenti, D. Outcomes of infliximab dose escalation in patients with rheumatoid arthritis. Clin. Rheumatol. 2019, 38, 2501–2508. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-H.; Lee, S.-S.; Park, W.; Song, Y.W.; Suh, C.-H.; Kim, S.; Lee, Y.N.; Yoo, D.H. A 5-year retrospective analysis of drug survival, safety, and effectiveness of the infliximab biosimilar CT-P13 in patients with rheumatoid arthritis and ankylosing spondylitis. Clin. Drug Investig. 2020, 40, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Kim, T.H.; Park, W.; Song, Y.W.; Suh, C.H.; Kim, S.; Yoo, D. POS0911 Similar clinical responses achieved with lower versus standard doses of infliximab biosimilar CT-P13 in patients with ankylosing spondylitis: Real-world results from the RAAS study. Ann. Rheum. Dis. 2021, 80, 716. [Google Scholar] [CrossRef]
- Walsh, J.A.; Adejoro, O.; Chastek, B.; Park, Y. Treatment patterns of biologics in US patients with ankylosing spondylitis: Descriptive analyses from a claims database. J. Comp. Eff. Res. 2018, 7, 369–380. [Google Scholar] [CrossRef]
- Kaltsonoudis, E.; Pelechas, E.; Voulgari, P.V.; Drosos, A.A. Maintained clinical remission in ankylosing spondylitis patients switched from reference infliximab to its biosimilar: An 18-month comparative open-label study. J. Clin. Med. 2019, 8, 956. [Google Scholar] [CrossRef]
- Benucci, M.; Gobbi, F.L.; Bandinelli, F.; Damiani, A.; Infantino, M.; Grossi, V.; Manfredi, M.; Parisi, S.; Fusaro, E.; Batticciotto, A.; et al. Safety, efficacy and immunogenicity of switching from innovator to biosimilar infliximab in patients with spondyloarthritis: A 6-month real-life observational study. Immunol. Res. 2017, 65, 419–422. [Google Scholar] [CrossRef]
- Valido, A.; Silva-Dinis, J.; Saavedra, M.J.; Iria, I.; Gonçalves, J.; Lopes, J.P.; Fonseca, J.E. Efficacy, immunogenicity and cost analysis of a systematic switch from originator infliximab to biosimilar CT-P13 of all patients with inflammatory arthritis from a single center. Acta Reumatol. Port. 2019, 44, 303–311. [Google Scholar]
- Glintborg, B.; Sørensen, I.J.; Loft, A.G.; Lindegaard, H.; Linauskas, A.; Hendricks, O.; Hansen, I.M.J.; Jensen, D.V.; Manilo, N.; Espesen, J.; et al. A nationwide non-medical switch from originator infliximab to biosimilar CT-P13 in 802 patients with inflammatory arthritis: 1-year clinical outcomes from the DANBIO registry. Ann. Rheum. Dis. 2017, 76, 1426–1431. [Google Scholar] [CrossRef]
- Jørgensen, K.K.; Olsen, I.C.; Goll, G.L.; Lorentzen, M.; Bolstad, N.; Haavardsholm, E.A.; Lundin, K.E.A.; Mørk, C.; Jahnsen, J.; Kvien, T.K. Switching from originator infliximab to biosimilar CT-P13 compared with maintained treatment with originator infliximab (NOR-SWITCH): A 52-week, randomised, double-blind, non-inferiority trial. Lancet 2017, 389, 2304–2316. [Google Scholar] [CrossRef]
- Lie, E.; Kristensen, L.E.; Forsblad-d’Elia, H.; Zverkova-Sandström, T.; Askling, J.; Jacobsson, L.T. The effect of comedication with conventional synthetic disease modifying antirheumatic drugs on TNF inhibitor drug survival in patients with ankylosing spondylitis and undifferentiated spondyloarthritis: Results from a nationwide prospective study. Ann. Rheum. Dis. 2015, 74, 970–978. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.-H.; Park, D.-J.; Lee, J.-W.; Lee, K.-E.; Wen, L.; Kim, T.-J.; Park, Y.-W.; Lee, S.-S. Drug survival rates of tumor necrosis factor inhibitors in patients with rheumatoid arthritis and ankylosing spondylitis. J. Korean Med. Sci. 2014, 29, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Saougou, I.; Markatseli, T.E.; Voulgari, P.V.; Drosos, A.A. Maintained clinical response of infliximab treatment in ankylosing spondylitis: A 6-year long-term study. Jt. Bone Spine 2010, 77, 325–329. [Google Scholar] [CrossRef]
- Jeong, H.; Eun, Y.H.; Kim, I.Y.; Kim, H.; Ahn, J.K.; Lee, J.; Koh, E.-M.; Cha, H.-S. Drug survival of tumor necrosis factor α inhibitors in patients with ankylosing spondylitis in Korea. Korean J. Intern. Med. 2018, 33, 407–416. [Google Scholar] [CrossRef]
- Lorenzin, M.; Ortolan, A.; Frallonardo, P.; Oliviero, F.; Punzi, L.; Ramonda, R. Predictors of response and drug survival in ankylosing spondylitis patients treated with infliximab. BMC Musculoskelet. Disord. 2015, 16, 166. [Google Scholar] [CrossRef][Green Version]
- Venetsanopoulou, A.I.; Voulgari, P.V.; Alamanos, Y.; Papadopoulos, C.G.; Markatseli, T.E.; Drosos, A.A. Persistent clinical response of infliximab treatment, over a 4-year period in ankylosing spondylitis. Rheumatol. Int. 2007, 27, 935–939. [Google Scholar] [CrossRef]
- Kim, H.-A.; Lee, E.; Lee, S.-K.; Park, Y.-B.; Shin, K. Retention rate and efficacy of the biosimilar CT-P13 versus reference infliximab in patients with ankylosing spondylitis: A propensity score-matched analysis from the Korean College of Rheumatology Biologics registry. BioDrugs 2020, 34, 529–539. [Google Scholar] [CrossRef]
- Lee, S.J.; Baek, K.; Lee, S.; Lee, Y.J.; Park, J.E.; Lee, S.G. Post-marketing pooled safety analysis for CT-P13 treatment of patients with immune-mediated inflammatory diseases in observational cohort studies. BioDrugs 2020, 34, 513–528. [Google Scholar] [CrossRef]
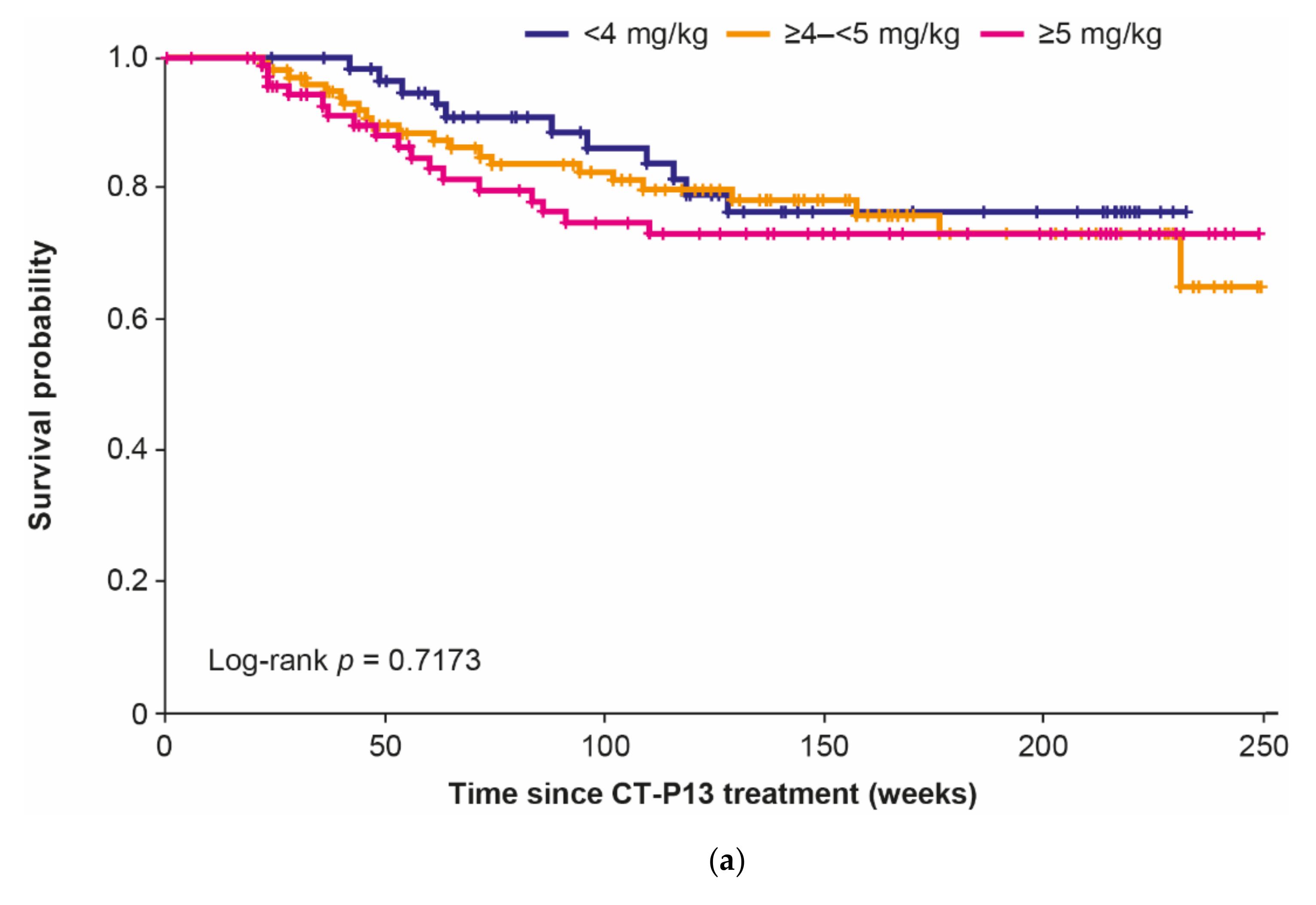
| Overall (n = 270 1) | Baseline Dose | p-Value 2 | |||
|---|---|---|---|---|---|
| <4 mg/kg (n = 71) | ≥4–<5 mg/kg (n = 117) | ≥5 mg/kg (n = 82) | |||
| Sex, n (%) | |||||
| Female | 68 (25.2) | 20 (28.2) | 24 (20.5) | 24 (29.3) | 0.2987 3 |
| Male | 202 (74.8) | 51 (71.8) | 93 (79.5) | 58 (70.7) | |
| Median (IQR) treatment duration, months | 24.2 (7.50–45.1) | 24.3 (9.97–47.8) | 23.8 (7.47–36.6) | 24.2 (7.30–48.1) | 0.6747 |
| Median (IQR) disease duration, years | 3 (1–6) | 3 (1–6) | 2 (1–7) | 3 (1–6) | 0.5956 |
| Median (IQR) age, years | 38 (30–51) | 43 (31–54) | 33 (27–44) | 40 (32–51) | 0.0013 |
| Body weight, kg | |||||
| n | 264 | 68 | 116 | 80 | |
| Median (IQR) | 67.0 (59.0–74.4) | 66.1 (58.1–79.0) | 68.0 (62.0–73.0) | 60.0 (57.0–74.4) | 0.0529 |
| BMI, kg/m2 | |||||
| n | 223 | 63 | 85 | 75 | |
| Median (IQR) | 23.38 (21.10–25.71) | 23.80 (22.44–26.93) | 22.96 (21.10–24.68) | 23.06 (20.69–25.90) | 0.0633 |
| BASDAI score | |||||
| n | 212 | 55 | 88 | 69 | |
| Median (IQR) | 6.66 (3.86–7.90) | 5.47 (0.58–7.20) | 7.25 (4.80–8.15) | 6.60 (4.73–8.00) | 0.0007 |
| ESR, mm/h | |||||
| n | 259 | 69 | 113 | 77 | |
| Median (IQR) | 23 (7–50) | 28 (11–58) | 24 (8–47) | 16 (5–40) | 0.0882 |
| CRP, mg/L | |||||
| n | 259 | 69 | 113 | 77 | |
| Median (IQR) | 0.80 (0.19–2.64) | 0.80 (0.27–2.72) | 0.81 (0.31–3.40) | 0.48 (0.08–1.60) | 0.0436 |
| Week 0 | Week 54 | Week 102 | Week 156 | Week 210 | |
|---|---|---|---|---|---|
| <4 mg/kg baseline dose group | |||||
| Overall | |||||
| n | 55 | 48 | 39 | 28 | 22 |
| Median (IQR) | 5.47 (0.58–7.20) | 0.88 (0.42–1.72) | 0.80 (0.30–1.64) | 0.49 (0.26–1.31) | 0.60 (0.22–1.00) |
| Naïve | |||||
| n | 30 | 24 | 17 | 12 | 8 |
| Median (IQR) | 7.00 (6.09–8.00) | 1.15 (0.73–1.93) | 1.30 (0.60–1.90) | 1.31 (0.30–1.78) | 0.97 (0.59–1.25) |
| Switched | |||||
| n | 25 | 24 | 22 | 16 | 14 |
| Median (IQR) | 0.57 (0.30–1.66) | 0.60 (0.30–1.10) | 0.45 (0.30–1.20) | 0.38 (0.26–0.62) | 0.41 (0.18–0.97) |
| ≥4–<5 mg/kg baseline dose group | |||||
| Overall | |||||
| n | 88 | 68 | 59 | 40 | 21 |
| Median (IQR) | 7.25 (4.80–8.15) | 2.00 (0.80–3.20) | 1.57 (0.79–2.60) | 1.40 (0.61–3.00) | 1.60 (0.60–3.00) |
| Naïve | |||||
| n | 67 | 49 | 41 | 26 | 11 |
| Median (IQR) | 7.50 (6.80–8.30) | 2.00 (0.90–3.20) | 1.30 (0.80–2.30) | 1.25 (0.76–1.80) | 1.60 (0.80–2.80) |
| Switched | |||||
| n | 21 | 19 | 18 | 14 | 10 |
| Median (IQR) | 1.01 (0.66–2.80) | 2.00 (0.49–3.40) | 2.33 (0.50–3.20) | 1.95 (0.43–3.20) | 1.72 (0.36–3.40) |
| ≥5 mg/kg baseline dose group | |||||
| Overall | |||||
| n | 69 | 53 | 45 | 34 | 24 |
| Median (IQR) | 6.60 (4.73–8.00) | 2.90 (1.40–4.40) | 3.00 (1.20–4.00) | 3.00 (2.00–4.20) | 2.85 (1.60–4.00) |
| Naïve | |||||
| n | 43 | 33 | 26 | 17 | 11 |
| Median (IQR) | 7.64 (6.50–9.10) | 2.70 (1.30–4.20) | 2.20 (0.94–3.80) | 2.54 (0.80–3.60) | 2.30 (0.80–3.60) |
| Switched | |||||
| n | 26 | 20 | 19 | 17 | 13 |
| Median (IQR) | 4.24 (2.86–5.10) | 3.30 (2.30–4.60) | 3.10 (2.40–4.60) | 3.10 (2.60–4.40) | 3.00 (2.60–4.20) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-S.; Kim, T.-H.; Park, W.; Song, Y.-W.; Suh, C.-H.; Kim, S.-K.; Yoo, D.-H. Impact of Infliximab Biosimilar CT-P13 Dose and Infusion Interval on Real-World Drug Survival and Effectiveness in Patients with Ankylosing Spondylitis. J. Clin. Med. 2021, 10, 4568. https://doi.org/10.3390/jcm10194568
Lee S-S, Kim T-H, Park W, Song Y-W, Suh C-H, Kim S-K, Yoo D-H. Impact of Infliximab Biosimilar CT-P13 Dose and Infusion Interval on Real-World Drug Survival and Effectiveness in Patients with Ankylosing Spondylitis. Journal of Clinical Medicine. 2021; 10(19):4568. https://doi.org/10.3390/jcm10194568
Chicago/Turabian StyleLee, Shin-Seok, Tae-Hwan Kim, Won Park, Yeong-Wook Song, Chang-Hee Suh, Soo-Kyoung Kim, and Dae-Hyun Yoo. 2021. "Impact of Infliximab Biosimilar CT-P13 Dose and Infusion Interval on Real-World Drug Survival and Effectiveness in Patients with Ankylosing Spondylitis" Journal of Clinical Medicine 10, no. 19: 4568. https://doi.org/10.3390/jcm10194568
APA StyleLee, S.-S., Kim, T.-H., Park, W., Song, Y.-W., Suh, C.-H., Kim, S.-K., & Yoo, D.-H. (2021). Impact of Infliximab Biosimilar CT-P13 Dose and Infusion Interval on Real-World Drug Survival and Effectiveness in Patients with Ankylosing Spondylitis. Journal of Clinical Medicine, 10(19), 4568. https://doi.org/10.3390/jcm10194568







