Why Did All Patients with Atrial Fibrillation and High Risk of Stroke Not Receive Oral Anticoagulants? Results of the Polish Atrial Fibrillation (POL-AF) Registry
Abstract
:1. Introduction
2. Methods and Materials
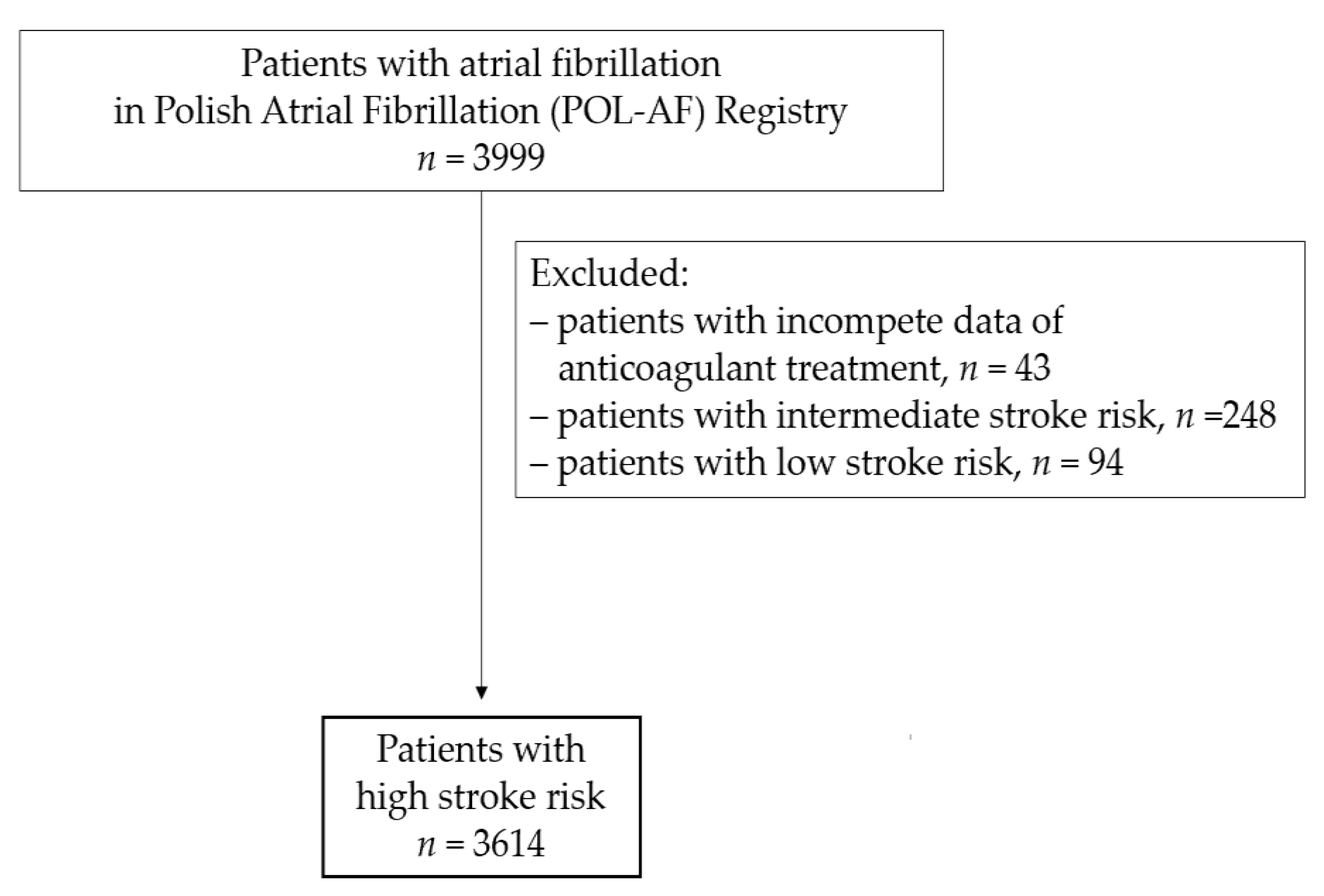
2.1. Study Population
2.2. Covariates
2.3. Stroke Risk Assessment
2.4. Stroke Prevention Assessment
2.5. Statistical Analyses
3. Results
3.1. Baseline Characteristics
3.2. Antithrombotic Therapy Use
3.3. Predictors of the Individual Stroke Prevention Use
4. Discussion
5. Study Strengths and Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wolf, P.A.; Abbott, R.D.; Kannel, W.B. Atrial fibrillation as an independent risk factor for stroke: The Framingham Study. Stroke 1991, 22, 983–988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freedman, J.F.; Gersh, B.J. Atrial fibrillation and stroke prevention in aging patients. Circulation 2014, 130, 129–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piccini, J.P.; Stevens, S.R.; Chang, Y.; Singer, D.E.; Lokhnygina, Y.; Go, A.S.; Patel, M.R.; Mahaffey, K.W.; Halperin, J.L.; Breithardt, G.; et al. Renal dysfunction as a predictor of stroke and systemic embolism in patients with nonvalvular atrial fibrillation: Validation of the R(2)CHADS(2) index in the ROCKET AF (Rivaroxaban Once-daily, oral, direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation) and ATRIA (AnTicoagulation and Risk factors In Atrial fibrillation) study cohorts. Circulation 2013, 127, 224–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gage, B.F.; Waterman, A.D.; Shannon, W.; Boechler, M.; Rich, M.W.; Radford, M.J. Validation of clinical classification schemes for predicting stroke: Results from the National Registry of Atrial Fibrillation. JAMA 2001, 285, 2864–2870. [Google Scholar] [CrossRef] [PubMed]
- Lip, G.Y.; Nieuwlaat, R.; Pisters, R.; Lane, D.A.; Crijns, H.J. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: The euro heart survey on atrial fibrillation. Chest 2010, 137, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, B.A.; Gao, H.; Shrader, P.; Pieper, K.; Thomas, L.; Camm, A.J.; Ezekowitz, M.D.; Fonarow, G.C.; Gersh, B.J.; Goldhaber, S.; et al. International trends in clinical characteristics and oral anticoagulation treatment for patients with atrial fibrillation: Results from the GARFIELD-AF, ORBIT-AF I and ORBIT-AF II registries. Am. Heart. J. 2017, 194, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Huiart, L.; Ferdynus, C.; Renoux, C.; Beaugrand, A.; Lafarge, S.; Bruneau, L.; Suissa, S.; Maillard, O.; Ranouil, X. Trends in initiation of direct oral anticoagulant therapies for atrial fibrillation in a national population-based cross-sectional study in the French health insurance databases. BMJ Open 2018, 30, e018180. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.R.; Choi, E.K.; Lee, S.Y.; Lee, E.; Han, K.D.; Cha, M.J.; Kwon, W.Y.; Shin, S.D.; Oh, S.; Lip, G. Temporal Trends of Emergency Department Visits of Patients with Atrial Fibrillation: A Nationwide Population-Based Study. J. Clin. Med. 2020, 9, 1485. [Google Scholar] [CrossRef]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur. Heart. J. 2020, 42, 373–498. [Google Scholar] [CrossRef]
- Pisters, R.; Lane, D.A.; Nieuwlaat, R.; de Vos, C.B.; Crijns, H.J.; Lip, G.Y.H. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: The Euro Heart Survey. Chest 2010, 138, 1093–1100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirchhof, P.; Benussi, S.; Kotecha, D.; Ahlsson, A.; Atar, D.; Casadei, B.; Castellá, M.; Diener, H.C.; Heidbuchel, H.; Hendriks, J.; et al. 2016 ESC Guidelines for the Management of Atrial Fibrillation Developed in Collaboration With EACTS. Europace 2016, 18, 1609–1678. [Google Scholar] [CrossRef]
- Heidbuchel, H.; Verhamme, P.; Alings, M.; Antz, M.; Diener, H.C.; Hacke, W.; Oldgren, J.; Sinnaeve, P.; Camm, A.J.; Kirchhof, P.; et al. Updated European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist anticoagulants in patients with non-valvular atrialfibrillation. Europace 2015, 17, 1467–1507. [Google Scholar] [CrossRef]
- Heidbuchel, H.; Verhamme, P.; Alings, M.; Antz, M.; Hacke, W.; Oldgren, J.; Sinnaeve, P.; Camm, A.J.; Kirchhof, P. European Heart Rhythm Association Practical Guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation. Europace 2013, 15, 625–651. [Google Scholar] [CrossRef]
- Steffel, J.; Verhamme, P.; Potpara, T.S.; Albaladejo, P.; Antz, M.; Desteghe, L.; Haeusler, K.G.; Oldgren, J.; Reinecke, H.; Roldan-Schilling, V.; et al. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation: Executive summary. Europace 2018, 20, 1231–1242. [Google Scholar] [CrossRef]
- Lubitz, S.A.; Khurshid, S.; Weng, L.C.; Doros, G.; Keach, J.W.; Gao, Q.; Gehi, A.K.; Hsu, J.C.; Reynolds, M.R.; Turakhia, M.P.; et al. Predictors of oral anticoagulant non-prescription in patients with atrial fibrillation and elevated stroke risk. Am. Heart J. 2018, 200, 24–31. [Google Scholar] [CrossRef]
- Gorczyca, I.; Wożakowska-Kapłon, B.; Starzyk, K.; Szpotowicz, A.; Stec, A. Evaluation of the recommended prevention of thrombosis in hospitalised patients with atrial fibrillation and high thromboembolism risk. Kardiol. Pol. 2018, 76, 625–632. [Google Scholar] [CrossRef] [Green Version]
- Granger, C.; Alexander, J.; McMurray, J.; Lopes, R.D.; Hylek, E.M.; Hanna, M.; Al-Khalidi, H.R.; Ansell, J.; Atar, D.; Avezum, A.; et al. Apixaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2011, 365, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Connolly, S.; Ezekowitz, M.; Yusuf, S.; Eikelboom, J.; Oldgren, J.; Parekh, A.; Pogue, J.; Reilly, P.A.; Themeles, E.; Varrone, J.; et al. Dabigatran versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2009, 361, 1139–1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, M.; Mahaffey, K.; Garg, J.; Pan, G.; Singer, D.E.; Hacke, W.; Breithardt, G.; Halperin, J.L.; Hankey, G.J.; Piccini, J.P.; et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N. Engl. J. Med. 2011, 365, 883–891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruff, C.T.; Giugliano, R.P.; Braunwald, E.; Hoffman, E.B.; Deenadayalu, N.; Ezekowitz, M.D.; Camm, A.J.; Weitz, J.I.; Lewis, B.S.; Parkhomenko, A.; et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: A meta-analysis of randomised trials. Lancet 2014, 383, 955–962. [Google Scholar] [CrossRef]
- Boriani, G.; Proietti, M.; Laroche, C.; Fauchier, L.; Marin, F.; Nabauer, M.; Potpara, T.; Dan, G.A.; Kalarus, Z.; Diemberger, I.; et al. Contemporary stroke prevention strategies in 11 096 European patients with atrial fibrillation: A report from the EURObservational Research Programme on Atrial Fibrillation (EORP-AF) Long-Term General Registry. Europace 2018, 20, 747–757. [Google Scholar] [CrossRef]
- Bassand, J.P.; Accetta, G.; Al Mahmeed, W.; Corbalan, R.; Eikelboom, J.; Fitzmaurice, D.A.; Fox, K.; Gao, H.; Goldhaber, S.Z.; Goto, S.; et al. Risk factors for death, stroke, and bleeding in 28,628 patients from the GARFIELD-AF registry: Rationale for comprehensive management of atrial fibrillation. PLoS ONE 2018, 13, e0191592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lip, G.Y.; Laroche, C.; Dan, G.A.; Santini, M.; Kalarus, Z.; Rasmussen, L.H.; Oliveira, M.M.; Mairesse, G.; Crijns, H.J.; Simantirakis, E.; et al. A prospective survey in European Society of Cardiology member countries of atrial fibrillation management: Baseline results of EURObservational Research Programme Atrial Fibrillation (EORP-AF) Pilot General Registry. Europace 2014, 16, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.R.; Choi, E.K.; Kwon, S.; Jung, J.H.; Han, K.D.; Cha, M.J.; Oh, S.; Lip, G. Oral Anticoagulation in Asian Patients with Atrial Fibrillation and a History of Intracranial Hemorrhage. Stroke 2020, 51, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Hess, P.L.; Kim, S.; Fonarow, G.C.; Thomas, L.; Singer, D.E.; Freeman, J.V.; Gersh, B.J.; Ansell, J.; Kowey, P.R.; Mahaffey, K.W.; et al. Absence of Oral Anticoagulation and Subsequent Outcomes Among Outpatients with Atrial Fibrillation. Am. J. Med. 2017, 130, 449–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Clinical Characteristic | All n = 3614 | OAC n = 3306 | No OAC n = 308 | ||
|---|---|---|---|---|---|
| APT n = 135 | Heparin n = 96 | Without any Stroke Prophylaxis n = 77 | |||
| Age mean (SD), years | 73.6 (10.3) | 73.5 (10.2) | 74.6 (10.3) | 72.6 (10.2) | 77.1 (12.1) |
| <65 | 598 (16.5) | 555 (16.8) | 18 (13.3) | 15 (15.9) | 10 (13.3) |
| 65–74 | 1285 (35.6) | 1188 (35.9) | 46 (34.1) | 39 (40.6) | 24 (31.2) |
| ≥75 | 1731 (47.9) | 1563 (47.3) | 71 (52.6) | 42 (43.8) | 43 (55.8) |
| Female | 1572 (43.5) | 1445 (43.7) | 60 (44.4) | 48 (50.0) | 32 (41.6) |
| Type of atrial fibrillation | |||||
| Paroxysmal | 1723 (47.7) | 1569 (47.5) | 80 (59.3) | 57 (59.4) | 35 (45.5) |
| Persistent | 803 (22.2) | 760 (23.0) | 14 (10.4) | 15 (15.6) | 15 (19.5) |
| Permanent | 1088 (30.1) | 977 (29.6) | 41 (30.4) | 24 (25.0) | 27 (35.1) |
| Medical history | |||||
| Hypertension | 3174 (87.4) | 2918 (88.3) | 112 (83.0) | 83 (86.5) | 63 (81.8) |
| Heart failure | 2529 (70.0) | 2311 (69.9) | 92 (68.1) | 60 (62.5) | 50 (64.9) |
| Vascular disease | 2207 (61.1) | 2005 (60.6) | 112 (83.0) | 64 (66.7) | 42 (54.5) |
| Coronary artery disease | 1979 (54.8) | 1796 (54.3) | 107 (79.3) | 59 (61.5) | 36 (46.8) |
| Previous myocardial infarction | 881 (24.4) | 782 (23.7) | 60 (44.4) | 25 (26.0) | 18 (23.4) |
| Peripheral artery disease | 564 (15.6) | 500 (15.1) | 32 (23.7) | 21 (21.9) | 11 (14.3) |
| Previous stroke/TIA/systemic embolism | 648 (17.9) | 599 (18.1) | 25 (18.5) | 28 (29.2) | 8 (10.4) |
| Diabetes mellitus | 1341 (37.1) | 1218 (36.8) | 53 (39.3) | 35 (36.5) | 32 (41.6) |
| Any previous bleeding | 118 (3.3) | 93 (2.8) | 14 (10.4) | 3 (3.1) | 4 (5.2) |
| Intracranial bleeding | 29 (0.8) | 18 (0.5) | 4 (3.0) | 0 (0.0) | 2 (2.6) |
| Gastrointestinal bleeding | 149 (4.1) | 138 (4.2) | 4 (3.0) | 2 (2.1) | 4 (5.2) |
| Cancer | 186 (5.1) | 149 (4.5) | 13 (9.6) | 8 (8.3) | 5 (6.5) |
| Hemoglobin < 12 g/dL | 872 (24.1) | 751 (22.7) | 45 (33.3) | 25 (26.0) | 30 (39.0) |
| eGFR < 60 mL/min/1.73 m2 | 1731 (47.9) | 1555 (47.0) | 80 (59.3) | 40 (41.7) | 40 (51.9) |
| eGFR < 30 mL/min/1.73 m2 | 255 (6.2) | 178 (5.4) | 24 (17.8) | 11 (11.5) | 12 (15.6) |
| Thromboembolic risk | |||||
| CHA2DS2VASc score mean (SD) | 4.7 (1.6) | 4.7 (1.6) | 4.9 (1.6) | 4.9 (1.6) | 4.5 (1.4) |
| ≥3 | 3331 (92.2) | 3047 (92.2) | 128 (94.8) | 90 (92.8) | 72 (93.5) |
| ≥5 | 1909 (52.8) | 1737 (52.5) | 80 (59.3) | 57 (59.4) | 39 (50.6) |
| Bleeding risk | |||||
| HAS-BLED score mean (SD) | 2.2 (0.8) | 2.2 (0.8) | 2.3 (0.9) | 2.21 (0.9) | 2.2 (0.8) |
| ≥3 | 1208 (33.4) | 1078 (32.6) | 58 (43.0) | 33 (34.4) | 27 (35.1) |
| ≥5 | 13 (0.4) | 11 (0.3) | 1 (0.7) | 0 (0.0) | 0 (0.0) |
| Reason for hospitalization | |||||
| Electrical cardioversion | 796 (22.0) | 784 (23.7) | 5 (3.7) | 21 (21.9) | 4 (5.2) |
| Planned coronarography/PCI | 372 (10.3) | 338 (10.2) | 21 (15.6) | 7 (7.3) | 5 (6.5) |
| CIED implantation/reimplantation | 346 (9.6) | 329 (10.0) | 5 (3.7) | 11 (11.5) | 5 (6.5) |
| Acute coronary syndrome | 240 (6.6) | 197 (6.0) | 38 (28.1) | 7 (7.3) | 4 (5.2) |
| Heart failure | 788 (21.8) | 714 (21.6) | 24 (17.8) | 16 (16.7) | 23 (29.9) |
| Ablation other than AF | 189 (5.2) | 172 (5.2) | 8 (5.9) | 7 (7.3) | 7 (9.1) |
| AF without any procedures | 191 (5.3) | 180 (5.4) | 4 (3.0) | 5 (5.2) | 3 (3.9) |
| Factors | OAC Versus No OAC | ||
|---|---|---|---|
| OR | 95%CI | p | |
| Hospitalization due to electrical cardioversion | 6.02 | 3.32–10.89 | <0.001 |
| Hypertension | 1.40 | 1.01–1.95 | 0.049 |
| Age ≥ 75 | 1.06 | 0.82–1.36 | 0.701 |
| Myocardial infarction | 0.89 | 0.68–1.17 | 0.400 |
| Peripheral artery disease | 0.88 | 0.64–1.21 | 0.411 |
| Intracranial bleeding | 0.15 | 0.07–0.35 | <0.001 |
| Gastrointestinal bleeding | 0.25 | 0.17–0.37 | <0.001 |
| Cancer | 0.37 | 0.25–0.55 | <0.001 |
| Hospitalization due to acute coronary syndrome | 0.48 | 0.33–0.69 | <0.001 |
| Hemoglobin < 12 g/dL | 0.62 | 0.48–0.81 | <0.001 |
| eGFR < 60 mL/min/1.73 m2 | 0.86 | 0.67–1.11 | 0.238 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szpotowicz, A.; Gorczyca, I.; Jelonek, O.; Uziębło-Życzkowska, B.; Maciorowska, M.; Wójcik, M.; Błaszczyk, R.; Kapłon-Cieślicka, A.; Gawałko, M.; Budnik, M.; et al. Why Did All Patients with Atrial Fibrillation and High Risk of Stroke Not Receive Oral Anticoagulants? Results of the Polish Atrial Fibrillation (POL-AF) Registry. J. Clin. Med. 2021, 10, 4611. https://doi.org/10.3390/jcm10194611
Szpotowicz A, Gorczyca I, Jelonek O, Uziębło-Życzkowska B, Maciorowska M, Wójcik M, Błaszczyk R, Kapłon-Cieślicka A, Gawałko M, Budnik M, et al. Why Did All Patients with Atrial Fibrillation and High Risk of Stroke Not Receive Oral Anticoagulants? Results of the Polish Atrial Fibrillation (POL-AF) Registry. Journal of Clinical Medicine. 2021; 10(19):4611. https://doi.org/10.3390/jcm10194611
Chicago/Turabian StyleSzpotowicz, Anna, Iwona Gorczyca, Olga Jelonek, Beata Uziębło-Życzkowska, Małgorzata Maciorowska, Maciej Wójcik, Robert Błaszczyk, Agnieszka Kapłon-Cieślicka, Monika Gawałko, Monika Budnik, and et al. 2021. "Why Did All Patients with Atrial Fibrillation and High Risk of Stroke Not Receive Oral Anticoagulants? Results of the Polish Atrial Fibrillation (POL-AF) Registry" Journal of Clinical Medicine 10, no. 19: 4611. https://doi.org/10.3390/jcm10194611
APA StyleSzpotowicz, A., Gorczyca, I., Jelonek, O., Uziębło-Życzkowska, B., Maciorowska, M., Wójcik, M., Błaszczyk, R., Kapłon-Cieślicka, A., Gawałko, M., Budnik, M., Tokarek, T., Rajtar-Salwa, R., Bil, J., Wojewódzki, M., Bednarski, J., Bakuła-Ostalska, E., Tomaszuk-Kazberuk, A., Szyszkowska, A., Wełnicki, M., ... Wożakowska-Kapłon, B. (2021). Why Did All Patients with Atrial Fibrillation and High Risk of Stroke Not Receive Oral Anticoagulants? Results of the Polish Atrial Fibrillation (POL-AF) Registry. Journal of Clinical Medicine, 10(19), 4611. https://doi.org/10.3390/jcm10194611









