The Human Cutaneous Sensory Corpuscles: An Update
Abstract
:1. Introduction
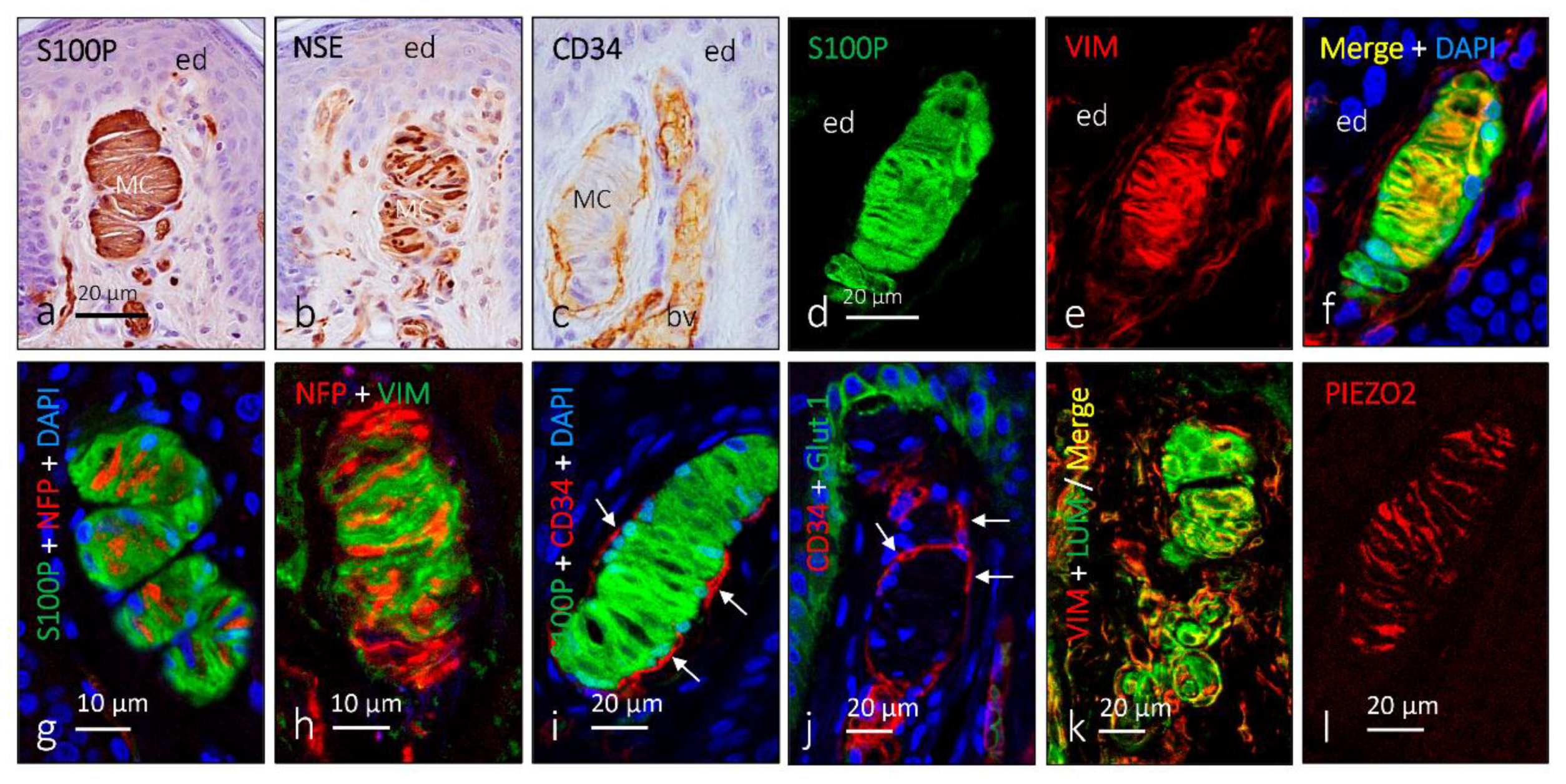
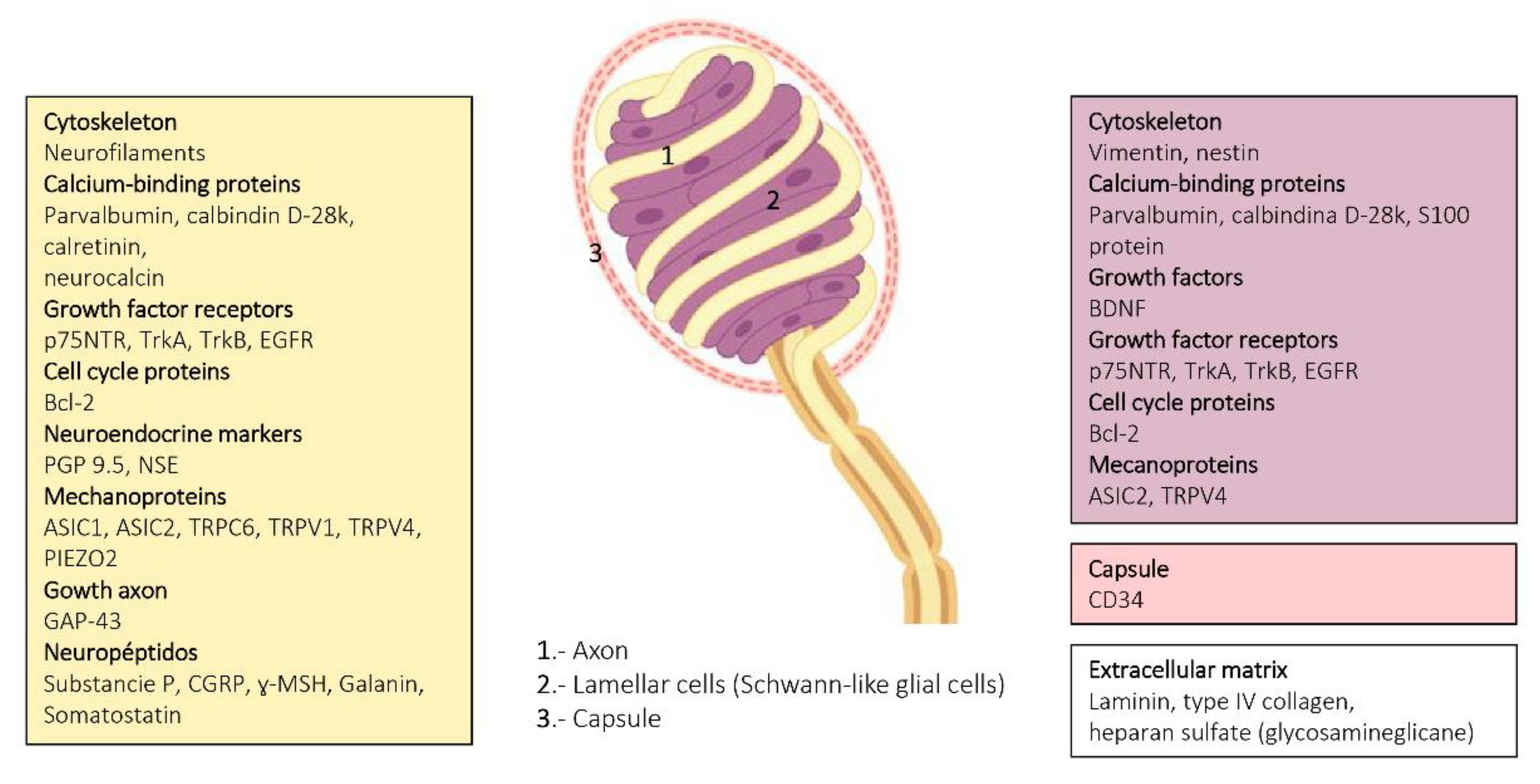
2. Meissner Corpuscles
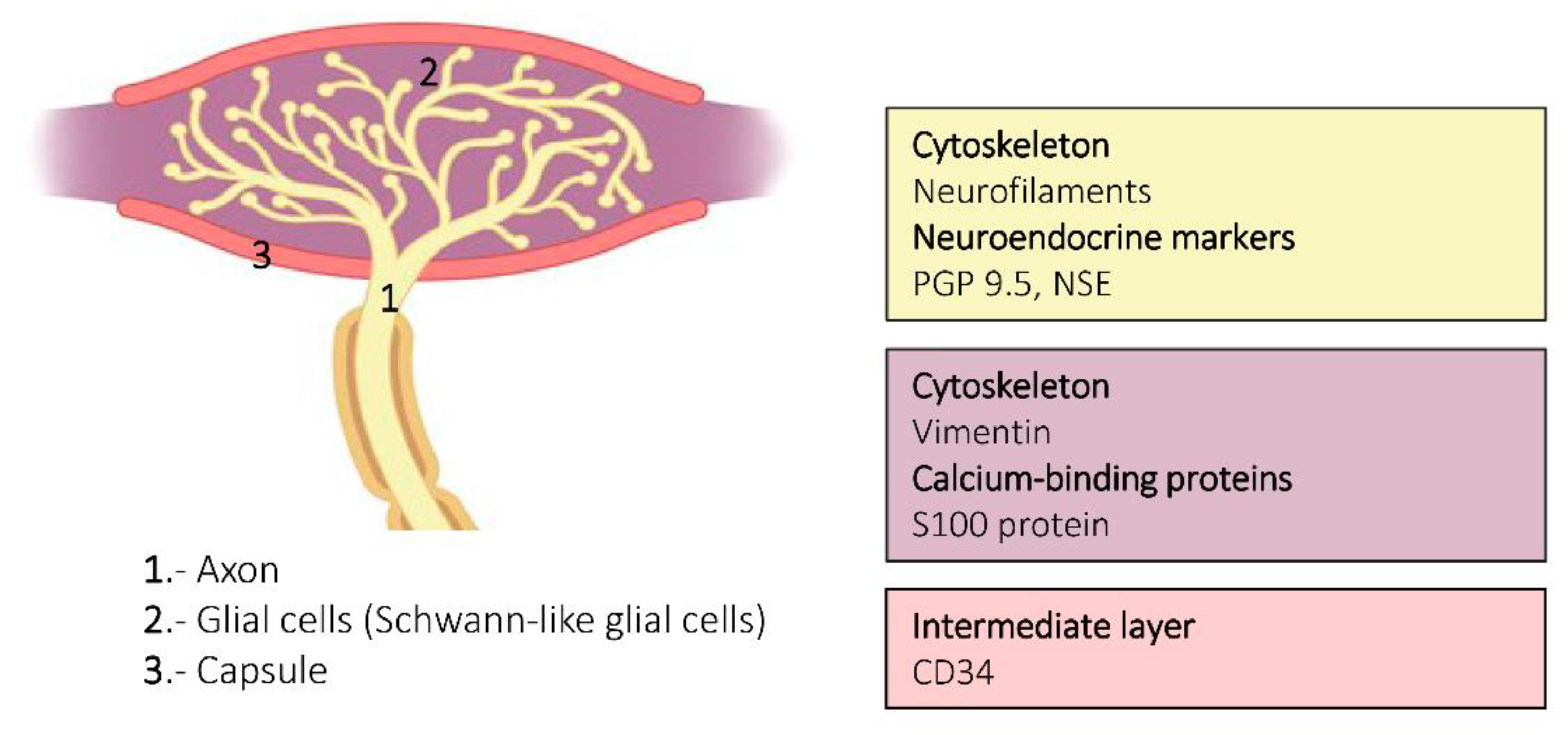
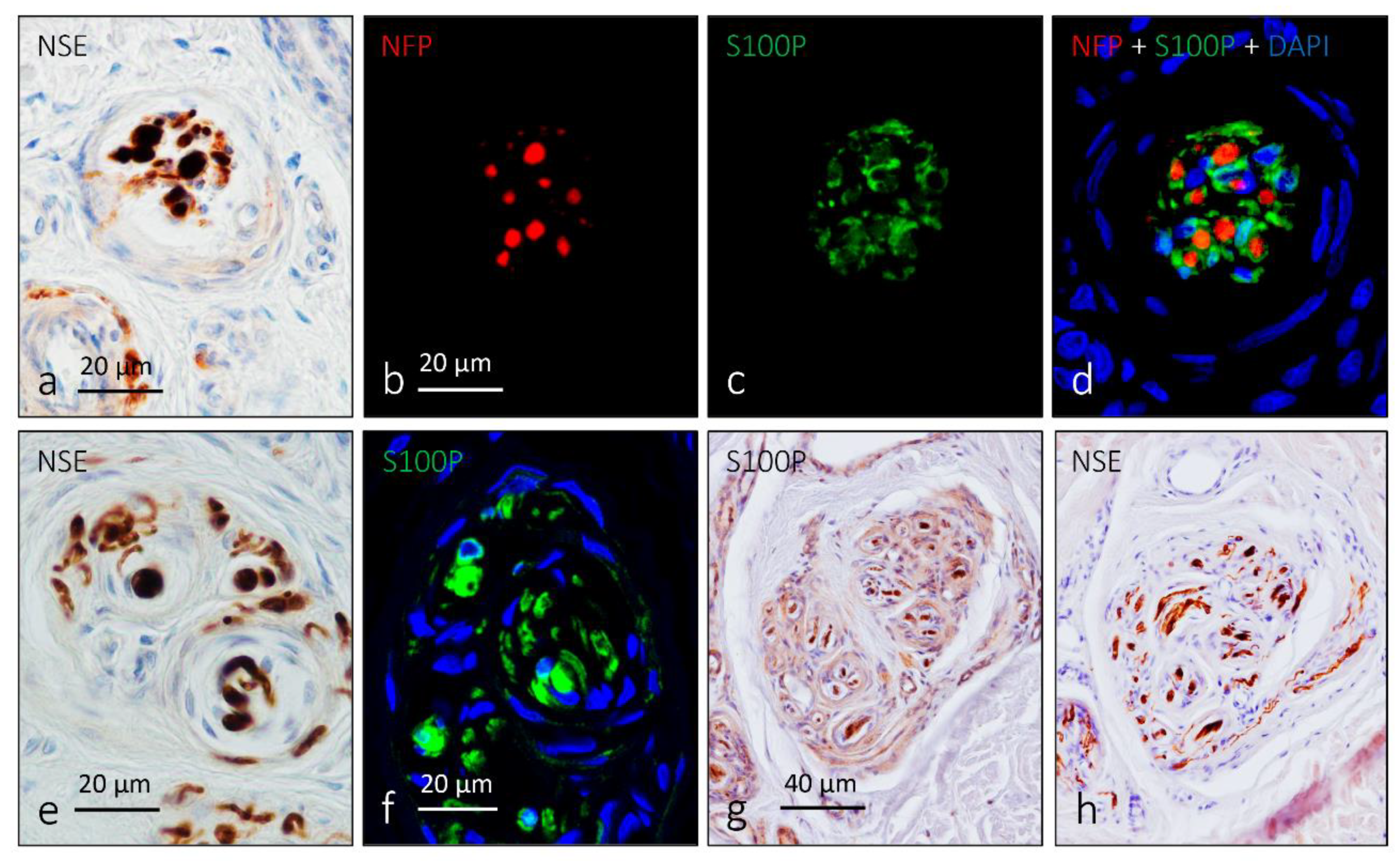
3. Ruffini Corpuscles
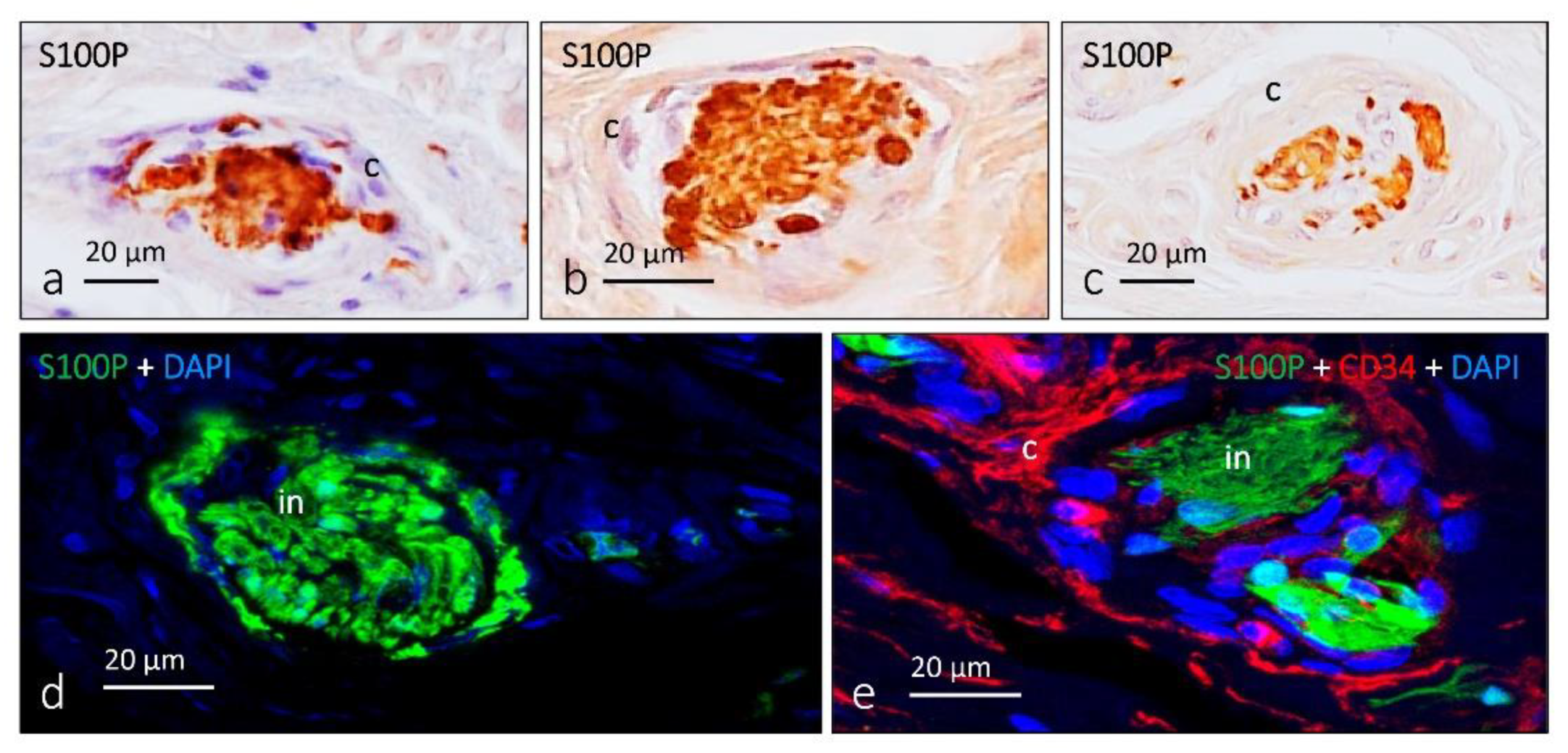
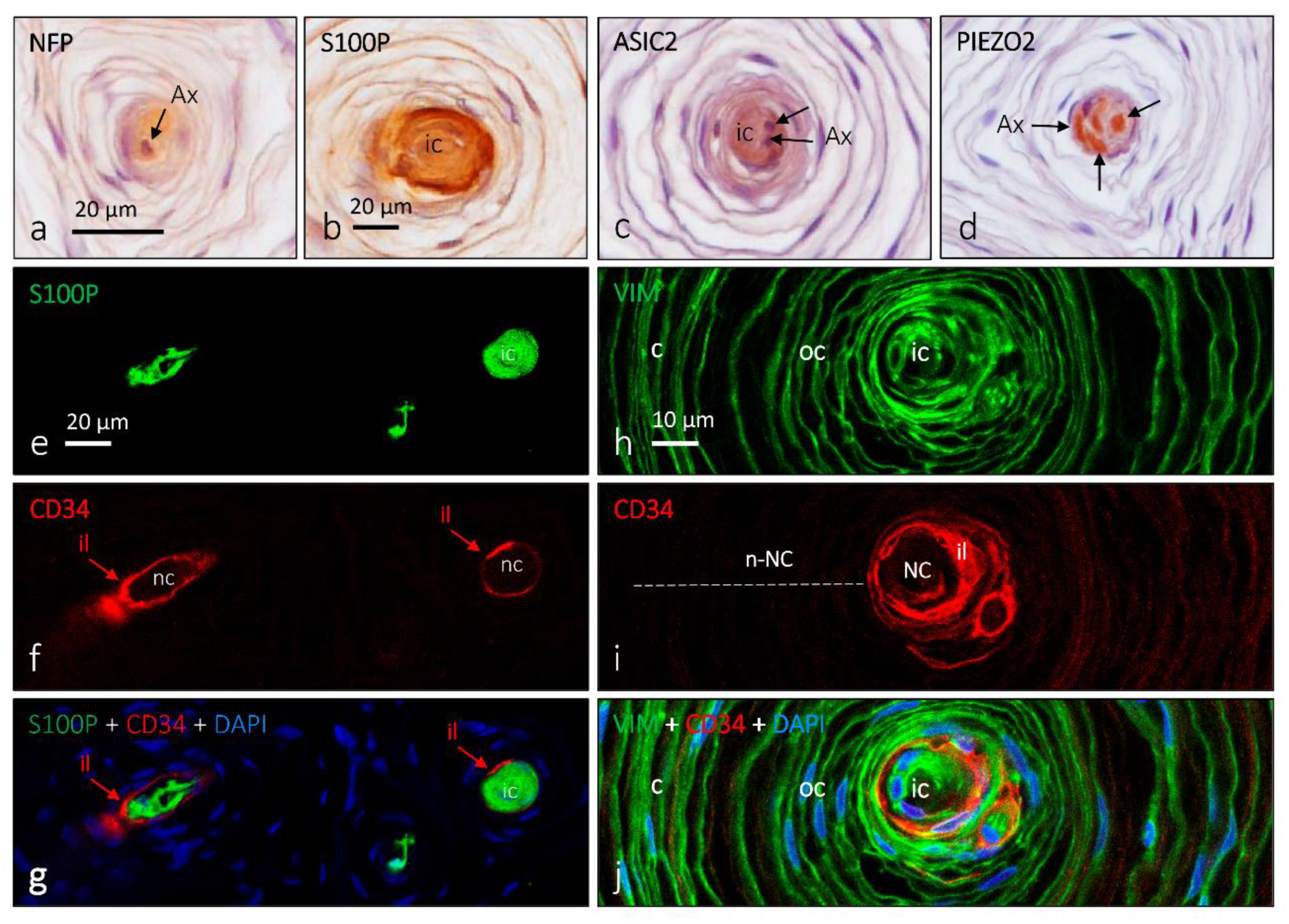
4. Pacini (Pacinian) Corpuscles
5. Other Morphotypes of Sensory Corpuscles
6. Proteins in Human Sensory Corpuscles
7. Clinical and Pathological Interest of Cutaneous Sensory Corpuscles and Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zimmerman, A.; Bai, L.; Ginty, D.D. The gentle touch receptors of mammalian skin. Science 2014, 346, 950–954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munger, B.L.; Ide, C. The structure and function of cutaneous sensory receptors. Arch. Histol. Cytol. 1988, 51, 1–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rice, F.L.; Albrecht, P.J. Cutaneous Mechanisms of Tactile Perception: Morphological and Chemical Organization of the Innervation to the Skin. In The Senses: A Comprehensive Reference, Volume 6, Somatosensation; Gardner, E., Kaas, J.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 1–31. [Google Scholar]
- McGlone, F.; Reilly, D. The cutaneous sensory system. Neurosci. Biobehav. Rev. 2010, 34, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Abraira, V.E.; Ginty, D.D. The sensory neurons of touch. Neuron 2013, 79, 618–639. [Google Scholar] [CrossRef] [Green Version]
- Jones, L.A.; Smith, A.M. Tactile sensory system: Encoding from the periphery to the cortex. Wiley Interdiscip. Rev. Syst. Biol. Med. 2014, 6, 279–287. [Google Scholar] [CrossRef]
- Olson, W.; Dong, P.; Fleming, M.; Luo, W. The specification and wiring of mammalian cutaneous low-threshold mechanoreceptors. Wiley Interdiscip. Rev. Dev. Biol. 2016, 5, 389–404. [Google Scholar] [CrossRef] [Green Version]
- Zelenà, J. Nerves and Mechanoreceptors: The Role of Innervation in the Development and Maintenance of Mammalian Mechanoreceptors; Chapman & Hall: New York, NY, USA, 1994. [Google Scholar]
- Vega, J.A.; Haro, J.J.; Del Valle, M.E. Immunohistochemistry of human cutaneous Meissner and Pacinian corpuscles. Microsc. Res. Tech. 1996, 34, 351–361. [Google Scholar] [CrossRef]
- Vega, J.A.; García-Suárez, O.; Montaño, J.A.; Pardo, B.; Cobo, J.M. The Meissner and Pacinian sensory corpuscles revisited: New data from the last decade. Microsc. Res. Tech. 2009, 72, 299–309. [Google Scholar] [CrossRef]
- García-Piqueras, J.; García-Suárez, O.; Rodríguez-González, M.C.; Cobo, J.L.; Cabo, R.; Vega, J.A.; Feito, J. Endoneurial-CD34 positive cells define an intermediate layer in human digital Pacinian corpuscles. Ann. Anat. 2017, 211, 55–60. [Google Scholar] [CrossRef]
- García-Piqueras, J.; Cobo, R.; Cárcaba, L.; García-Mesa, Y.; Feito., J.; Cobo, J.; García-Suárez, O.; Vega, J.A. The capsule of human Meissner corpuscles: Immunohistochemical evidence. J. Anat. 2020, 236, 54–61. [Google Scholar] [CrossRef]
- Feito, J.; Ramos-García, J.L.; Gago, A.; Cobo, J.L.; García-Suárez, O.; Junquera, L.M.; Vega, J.A. Pacinian Corpuscles in a Cervical Chondrocutaneous Remnant: A Case Report and Update of Pacinian Corpuscles. Am. J. Dermatopathol. 2016, 38, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Vega, J.A.; Esteban, I.; Naves, F.J.; Del Valle, M.E.; Malinovsky, L. Immunohistochemical localization of laminin and type IV collagen in human cutaneous sensory nerve formations. Anat. Embryol. (Berl.) 1995, 191, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Dubový, P.; Bednárová, J. The extracellular matrix of rat pacinian corpuscles: An analysis of its fine structure. Anat. Embryol. 1999, 200, 615–623. [Google Scholar] [CrossRef]
- García-Piqueras, J.; Carcaba, L.; García-Mesa, Y.; Feito, J.; García, B.; Viña, E.; Suárez-Quintanilla, J.; Cobo, J.; Vega, J.A.; García-Suárez, O. Chondroitin Sulfate in Human Cutaneous Meissner and Pacinian Sensory Corpuscles. Anat. Rec. (Hoboken) 2019, 302, 325–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Piqueras, J.; García-Mesa, Y.; Feito, J.; García, B.; Quiros, L.M.; Martín-Biedma, B.; Cobo, T.; Vega, J.A.; García-Suárez, O. Class I and Class II small leucine-rich proteoglycans in human cutaneous pacinian corpuscles. Ann. Anat. 2019, 224, 62–72. [Google Scholar] [CrossRef]
- García-Piqueras, J.; García-Suárez, O.; García-Mesa, Y.; García-Fernandez, B.; Quirós, L.M.; Cobo, R.; Martín-Biedma, B.; Feito, J.; Vega, J.A. Heparan sulfate in human cutaneous Meissner’s and Pacinian corpuscles. Anat. Rec. (Hoboken) 2020, 303, 2262–2273. [Google Scholar] [CrossRef]
- Vega, J.A.; López-Muñiz, A.; Calavia, M.G.; García-Suárez, O.; Cobo, J.; Otero, J.; Arias-Carrión, O.; Pérez-Piñera, P.; Menéndez-González, M. Clinical implication of Meissner’s corpuscles. CNS Neurol. Disord. Drug Targets 2012, 11, 856–868. [Google Scholar] [CrossRef]
- García-Suarez, O.; García-Mesa, Y.; García-Piqueras, J.; Salvo, G.; Cobo, J.L.; Alba, E.; Cobo, R.; Feito, J.; Vega, J.A. The Cutaneous Biopsy for the Diagnosis of Peripheral Neuropathies: Meissner’s Corpuscles and Merkel’s Cells. In Desmystifying Polyneuropathy-Recent Adavances and New Directions; Bozzetto-Ambrosi, P., Ed.; IntechOpen: London, UK, 2019; pp. 267–278. [Google Scholar]
- Dyck, P.J. Enumerating Meissner corpuscles: Future gold standard of large fiber sensorimotor polyneuropathy? Neurology 2007, 69, 2116–2118. [Google Scholar] [CrossRef]
- Herrmann, D.N.; Boger, J.N.; Jansen, C.; Alessi-Fox, C. In vivo confocal microscopy of Meissner corpuscles as a measure of sensory neuropathy. Neurology 2007, 69, 2121–2127. [Google Scholar] [CrossRef]
- Nolano, M.; Provitera, V.; Estraneo, A.; Selim, M.M.; Caporaso, G.; Stancanelli, A.; Saltalamacchia, A.M.; Lanzillo, B.; Santoro, L. Sensory deficit in Parkinson’s disease: Evidence of a cutaneous denervation. Brain 2008, 131, 1903–1911. [Google Scholar] [CrossRef] [Green Version]
- Feito, J.; García-Suárez, O.; García-Piqueras, J.; García-Mesa, Y.; Pérez-Sánchez, A.; Suazo, I.; Cabo, R.; Suárez-Quintanilla, J.; Cobo, J.; Vega, J.A. The development of human digital Meissner’s and Pacinian corpuscles. Ann. Anat. 2018, 219, 8–24. [Google Scholar] [CrossRef] [PubMed]
- García-Piqueras, J.; García-Mesa, Y.; Cárcaba, L.; Feito, J.; Torres-Parejo, I.; Martín-Biedma, B.; Cobo, J.; García-Suárez, O.; Vega, J.A. Ageing of the somatosensory system at the periphery: Age-related changes in cutaneous mechanoreceptors. J. Anat. 2019, 234, 839–852. [Google Scholar] [CrossRef] [PubMed]
- Paré, M.; Elde, R.; Mazurkiewicz, J.E.; Smith, A.M.; Rice, F.L. The Meissner corpuscle revised: A multiafferented mechanoreceptor with nociceptor immunochemical properties. J. Neurosci. 2001, 21, 7236–7246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neubarth, N.L.; Emanuel, A.J.; Liu, Y.; Springel, M.W.; Handler, A.; Zhang, Q.; Lehnert, B.P.; Guo, C.; Orefice, L.L.; Abdelaziz, A.; et al. Meissner corpuscles and their spatially intermingled afferents underlie gentle touch perception. Science 2020, 368, 1311–1312. [Google Scholar] [CrossRef] [PubMed]
- García-Suárez, O.; Montaño, J.A.; Esteban, I.; González-Martínez, T.; Alvarez-Abad, C.; López-Arranz, E.; Cobo, J.; Vega, J.A. Myelin basic protein-positive nerve fibres in human Meissner corpuscles. J. Anat. 2009, 214, 888–893. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.O.; Yoshioka, T.; Vega-Bermudez, F. Tactile functions of mechanoreceptive afferents innervating the hand. J. Clin. Neurophysiol. 2000, 17, 539–558. [Google Scholar] [CrossRef] [PubMed]
- Nikolaev, Y.A.; Feketa, V.V.; Anderson, E.O.; Gracheva, E.O.; Bagriantsev, S.N. Lamellar cells in Pacinian and Meissner corpuscles are touch sensors. bioXriv 2020. [Google Scholar] [CrossRef]
- Cobo, R.; García-Mesa, Y.; Cárcaba, L.; Martín-Cruces, J.; Feito, J.; García-Suárez, O.; Cobo, J.; García-Piqueras, J.; Vega, J.A. Verification and characterisation of human digital Ruffini’s sensory corpuscles. J. Anat. 2020. [Google Scholar] [CrossRef]
- Halata, Z. Sensory innervation of the hairy skin (light- and electron-microscopic study). J. Investig. Dermatol. 1993, 101, 75S–81S. [Google Scholar] [CrossRef]
- Paré, M.; Behets, C.; Cornu, O. Paucity of presumptive Ruffini corpuscles in the index finger pad of humans. J. Comp. Neurol. 2003, 456, 260–266. [Google Scholar] [CrossRef]
- Fleming, M.S.; Luo, W. The anatomy, function, and development of mammalian Aβ low-threshold mechanoreceptors. Front. Biol. (Beijing) 2013, 8, 408–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell, J.; Bolanowski, S.; Holmes, M.H. The structure and function of Pacinian corpuscles: A review. Prog. Neurobiol. 1994, 42, 79–128. [Google Scholar] [CrossRef]
- García-Suárez, O.; Calavia, M.G.; Pérez-Moltó, F.J.; Alvarez-Abad, C.; Pérez-Piñera, P.; Cobo, J.M.; Vega, J.A. Immunohistochemical profile of human pancreatic pacinian corpuscles. Pancreas 2010, 39, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Cobo, R.; García-Mesa, Y.; García-Piqueras, J.; Feito, J.; Martín-Cruces, J.; García-Suárez, O.; Vega, J.A. The Glial Cell of Human Cutaneous Sensory Corpuscles: Origin, Characterization, and Putative Roles. In Somatosensory and Motor Research; Suzuki, T., Ed.; InTechOpen: London, UK, 2020. [Google Scholar] [CrossRef] [Green Version]
- Cobo, R.; García-Piqueras, J.; García-Mesa, Y.; Feito, J.; García-Suárez, O.; Vega, J.A. Peripheral Mechanobiology of Touch-Studies on Vertebrate Cutaneous Sensory Corpuscles. Int. J. Mol. Sci. 2020, 21, 6221. [Google Scholar] [CrossRef]
- Márquez, J.; Pérez-Pérez, M.; Naves, F.J.; Vega, J.A. Effect of spinal cord and peripheral nerve injury on human cutaneous sensory corpuscles. An immunohistochemical study. J. Peripher. Nerv. Syst. 1997, 2, 49–59. [Google Scholar]
- Fassola, I.; Wenzke, L.; Ertel, W.; Krasnici, S. Pacinian neuromas and neurofibromas of the hands and fingers: A systematic review. J. Hand Surg. Eur. 2019, 44, 925–931. [Google Scholar] [CrossRef]
- Jiménez, I.; Marcos-García, A.; Muratore, G.; Medina, J. Pacinian Corpuscles Neuroma. An Exceptional Cause of Pain in the Hand. J. Hand Surg. Asian Pac. 2017, 22, 229–231. [Google Scholar] [CrossRef]
- Kumar, A.; Darby, A.J.; Kelly, C.P. Pacinian corpuscles hyperplasia-an uncommon cause of digital pain. Acta Orthop. Belg. 2003, 69, 74–76. [Google Scholar]
- Yan, S.; Horangic, N.J.; Harris, B.T. Hypertrophy of Pacinian corpuscles in a young patient with neurofibromatosis. Am. J. Dermatopathol. 2006, 28, 202–204. [Google Scholar] [CrossRef]
- García, F.C.; Acosta, D.R.; Diaz González, J.M.; Lima, M.S. Hyperplasia and Hypertrophy of Pacinian Corpuscles: A Case Report. Am. J. Dermatopathol. 2015, 37, e100–e101. [Google Scholar] [CrossRef]
- Krivošíková, L.; Janega, P.; Babala, J.; Babál, P. Pacinian collagenoma: A distinct form of sclerotic fibroma. J. Cutan. Pathol. 2020, 47, 291–294. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cobo, R.; García-Piqueras, J.; Cobo, J.; Vega, J.A. The Human Cutaneous Sensory Corpuscles: An Update. J. Clin. Med. 2021, 10, 227. https://doi.org/10.3390/jcm10020227
Cobo R, García-Piqueras J, Cobo J, Vega JA. The Human Cutaneous Sensory Corpuscles: An Update. Journal of Clinical Medicine. 2021; 10(2):227. https://doi.org/10.3390/jcm10020227
Chicago/Turabian StyleCobo, Ramón, Jorge García-Piqueras, Juan Cobo, and José A. Vega. 2021. "The Human Cutaneous Sensory Corpuscles: An Update" Journal of Clinical Medicine 10, no. 2: 227. https://doi.org/10.3390/jcm10020227
APA StyleCobo, R., García-Piqueras, J., Cobo, J., & Vega, J. A. (2021). The Human Cutaneous Sensory Corpuscles: An Update. Journal of Clinical Medicine, 10(2), 227. https://doi.org/10.3390/jcm10020227




