The Neurosurgeon’s Armamentarium for Gliomas: An Update on Intraoperative Technologies to Improve Extent of Resection
Abstract
:1. Introduction
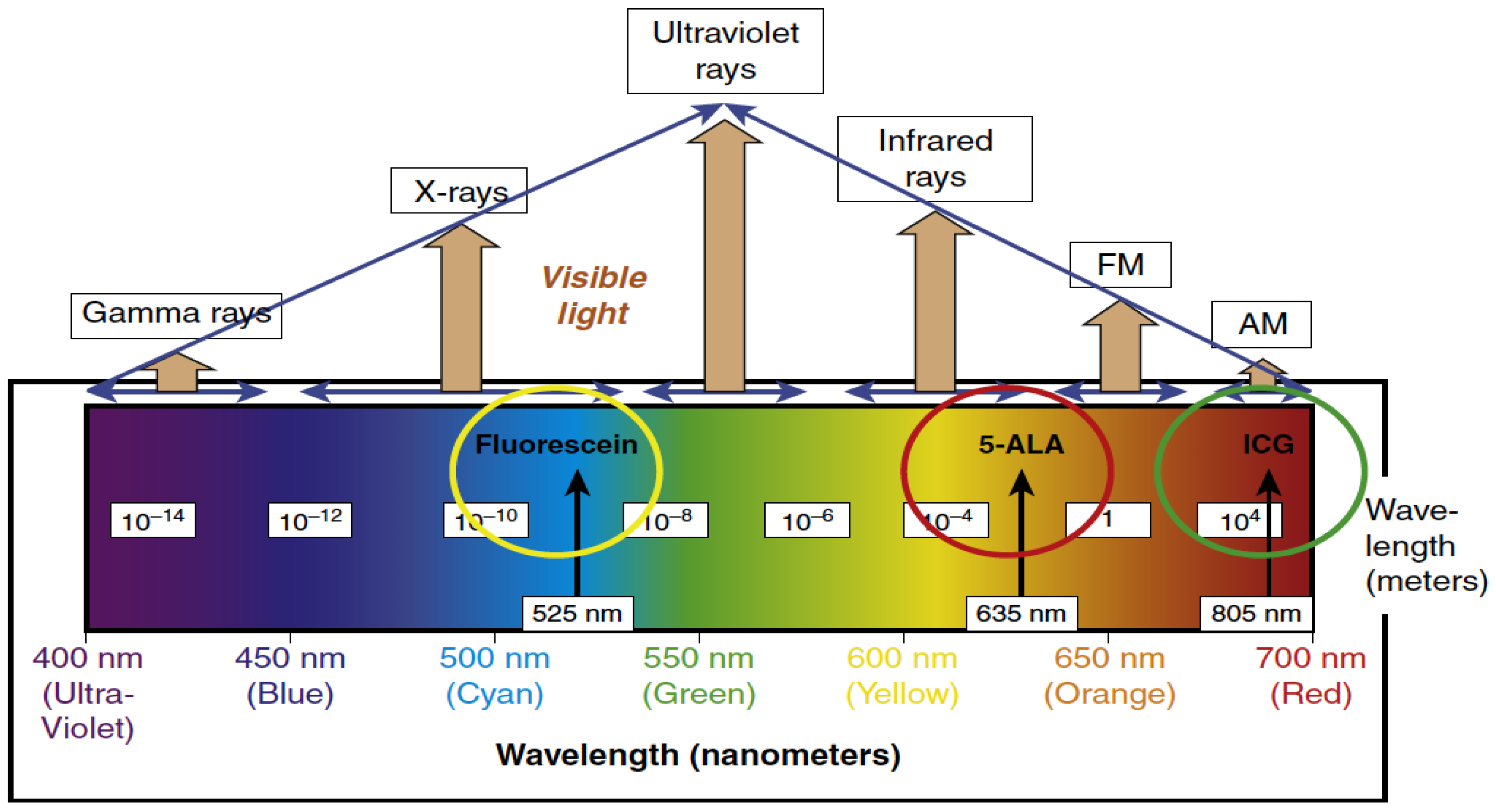
2. Fluorescence-Guided Surgery (FGS)
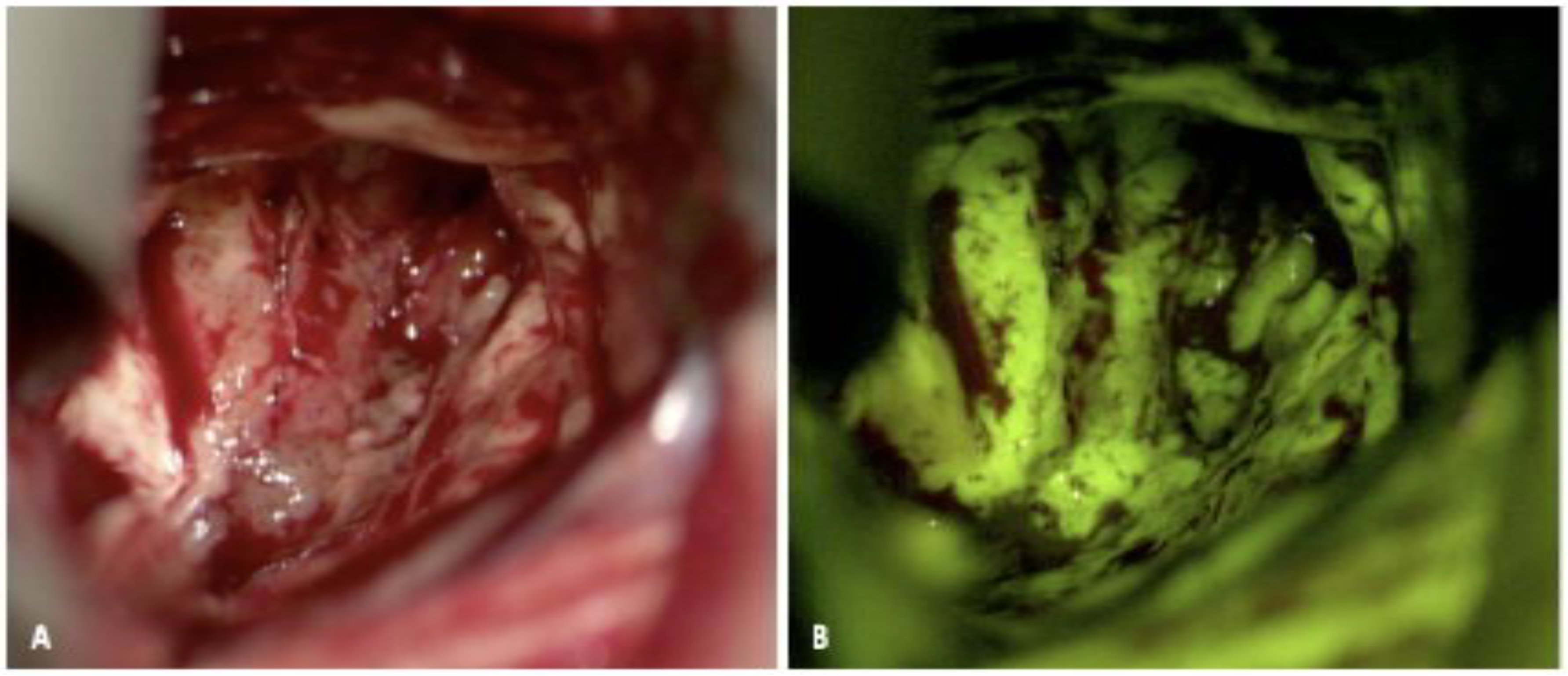
2.1. 5-Aminolevulinic Acid (5-ALA)
2.1.1. 5-ALA: Background and Mechanism of Action
2.1.2. 5-ALA: Limitations
2.1.3. 5-ALA: Evidence for Use
2.2. Fluorescein
2.2.1. Fluorescein: Background and Mechanism of Action
2.2.2. Fluorescein: Evidence for Use
2.2.3. Fluorescein: Limitations
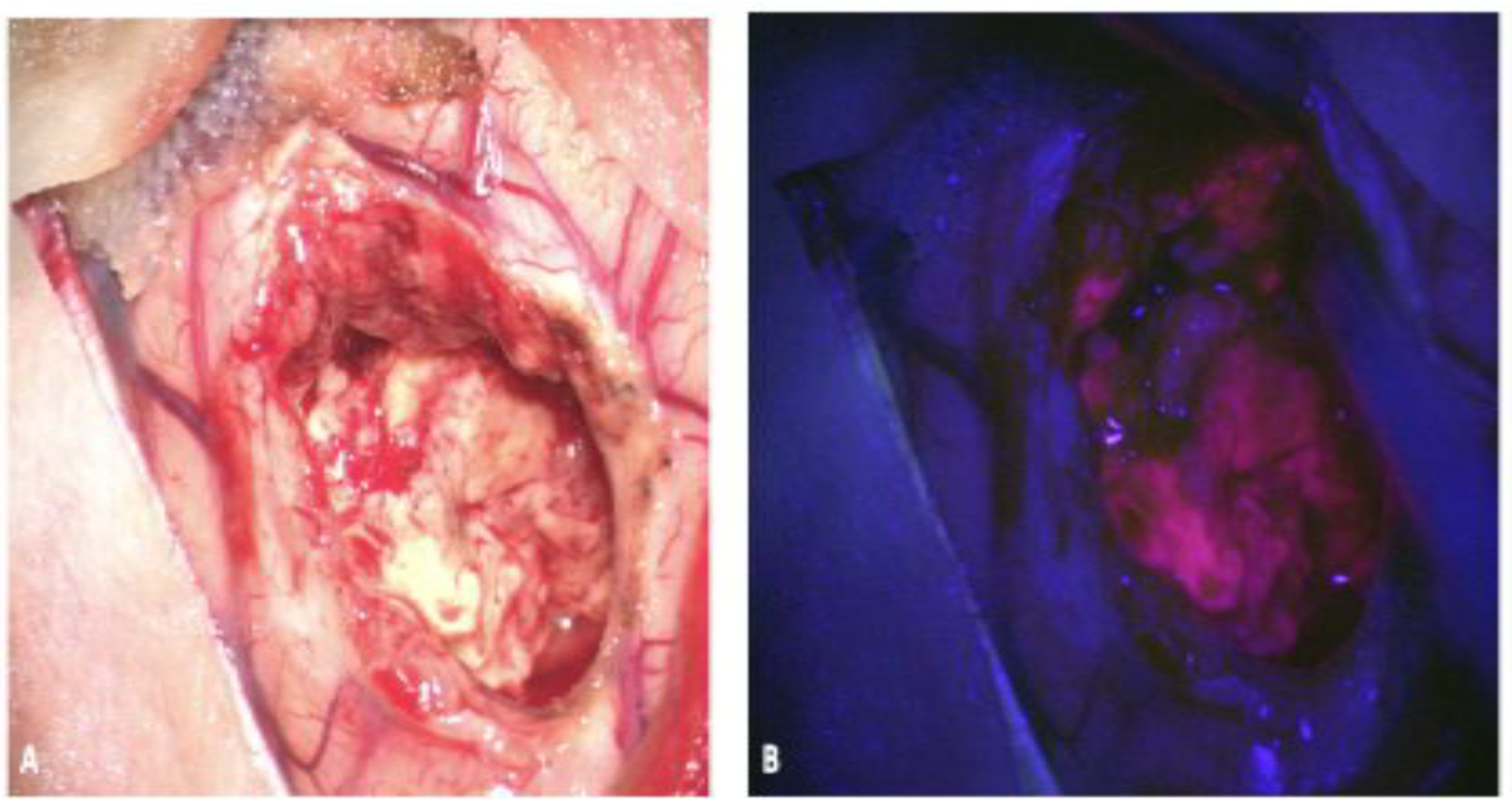
2.3. ICG
2.3.1. ICG: Background and Mechanism of Action
2.3.2. ICG: Evidence for Use
2.3.3. ICG: Limitations
2.4. Future Targets
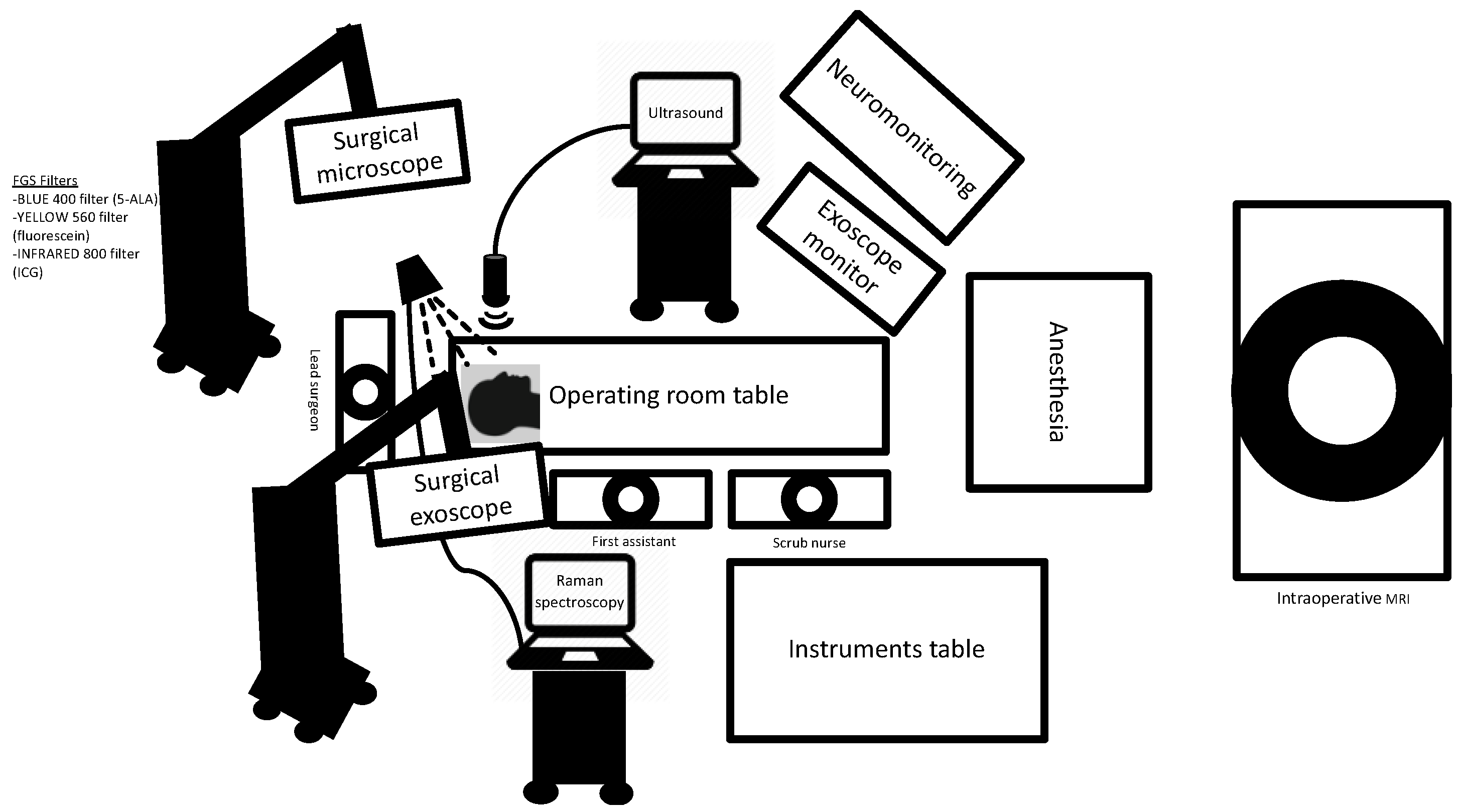
3. Image Guidance
3.1. Neuronavigation
3.2. Intraoperative MRI
3.3. Intraoperative MRI and 5-ALA
3.4. Exoscope
3.5. Intraoperative Ultrasound
3.6. Intraoperative Mapping and Neuromonitoring
3.6.1. Intraoperative Mapping
3.6.2. Intraoperative Neurophysiologic Monitoring (IONM)
3.7. Intraoperative Histopathology and Imaging Probe Devices
3.8. Raman Microscopy
3.8.1. Probe-Based Microscopy
3.8.2. Wide-Field Endomicroscopy
4. Summary
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lacroix, M.; Abi-Said, D.; Fourney, D.R.; Gokaslan, Z.L.; Shi, W.; DeMonte, F.; Lang, F.F.; McCutcheon, I.E.; Hassenbusch, S.J.; Holland, E.; et al. A multivariate analysis of 416 patients with glioblastoma multiforme: Prognosis, extent of resection, and survival. J. Neurosurg. 2001, 95, 190–198. [Google Scholar] [CrossRef] [Green Version]
- McGirt, M.J.; Chaichana, K.L.; Gathinji, M.; Attenello, F.J.; Than, K.; Olivi, A.; Weingart, J.D.; Brem, H.; Quiñones-Hinojosa, A.R. Independent association of extent of resection with survival in patients with malignant brain astrocytoma. J. Neurosurg. 2009, 110, 156–162. [Google Scholar] [CrossRef] [Green Version]
- Glenn, C.A.; Baker, C.M.; Conner, A.K.; Burks, J.D.; Bonney, P.A.; Briggs, R.G.; Smitherman, A.D.; Battiste, J.D.; Sughrue, M.E. An Examination of the Role of Supramaximal Resection of Temporal Lobe Glioblastoma Multiforme. World Neurosurg. 2018, 114, e747–e755. [Google Scholar] [CrossRef]
- Yong, R.L.; Lonser, R.R. Surgery for glioblastoma multiforme: Striking a balance. World Neurosurg. 2011, 76, 528–530. [Google Scholar] [CrossRef] [Green Version]
- Hadjipanayis, C.G.; Stummer, W. Fluorescence-Guided Neurosurgery: Neuro-Oncology and Cerebrovascular Applications; Thieme: New York, NY, USA, 2019. [Google Scholar]
- Stummer, W.; Novotny, A.; Stepp, H.; Goetz, C.; Bise, K.; Reulen, H.J. Fluorescence-guided resection of glioblastoma multiforme by using 5-aminolevulinic acid-induced porphyrins: A prospective study in 52 consecutive patients. J. Neurosurg. 2000, 93, 1003–1013. [Google Scholar] [CrossRef]
- Stummer, W.; Pichlmeier, U.; Meinel, T.; Wiestler, O.D.; Zanella, F.; Reulen, H.J.; ALA-Glioma Study Group. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. Lancet Oncol. 2006, 7, 392–401. [Google Scholar] [CrossRef]
- Eljamel, M.S.; Goodman, C.; Moseley, H. ALA and Photofrin fluorescence-guided resection and repetitive PDT in glioblastoma multiforme: A single centre Phase III randomised controlled trial. Lasers Med. Sci. 2008, 23, 361–367. [Google Scholar] [CrossRef]
- Nabavi, A.; Thurm, H.; Zountsas, B.; Pietsch, T.; Lanfermann, H.; Pichlmeier, U.; Mehdorn, M.; 5-ALA Recurrent Glioma Study Group. Five-aminolevulinic acid for fluorescence-guided resection of recurrent malignant gliomas: A phase ii study. Neurosurgery 2009, 65, 1070–1076; discussion 1076–1077. [Google Scholar] [CrossRef] [PubMed]
- Díez Valle, R.; Tejada Solis, S.; Idoate Gastearena, M.A.; García de Eulate, R.; Domínguez Echávarri, P.; Aristu Mendiroz, J. Surgery guided by 5-aminolevulinic fluorescence in glioblastoma: Volumetric analysis of extent of resection in single-center experience. J. Neurooncol. 2011, 102, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Stummer, W.; Nestler, U.; Stockhammer, F.; Krex, D.; Kern, B.C.; Mehdorn, H.M.; Vince, G.H.; Pichlmeier, U. Favorable outcome in the elderly cohort treated by concomitant temozolomide radiochemotherapy in a multicentric phase II safety study of 5-ALA. J. Neurooncol. 2011, 103, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Díez Valle, R.; Slof, J.; Galván, J.; Arza, C.; Romariz, C.; Vidal, C.; VISIONA Study Researchers. Observational, retrospective study of the effectiveness of 5-aminolevulinic acid in malignant glioma surgery in Spain (The VISIONA study). Neurologia 2014, 29, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Eljamel, S. 5-ALA Fluorescence Image Guided Resection of Glioblastoma Multiforme: A Meta-Analysis of the Literature. Int. J. Mol. Sci. 2015, 16, 10443–10456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teixidor, P.; Arráez, M.Á.; Villalba, G.; Garcia, R.; Tardáguila, M.; González, J.J.; Rimbau, J.; Vidal, X.; Montané, E. Safety and Efficacy of 5-Aminolevulinic Acid for High Grade Glioma in Usual Clinical Practice: A Prospective Cohort Study. PLoS ONE 2016, 11, e0149244. [Google Scholar] [CrossRef] [Green Version]
- Koc, K.; Anik, I.; Cabuk, B.; Ceylan, S. Fluorescein sodium-guided surgery in glioblastoma multiforme: A prospective evaluation. Br. J. Neurosurg. 2008, 22, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Acerbi, F.; Broggi, M.; Eoli, M.; Anghileri, E.; Cavallo, C.; Boffano, C.; Cordella, R.; Cuppini, L.; Pollo, B.; Schiariti, M.; et al. Is fluorescein-guided technique able to help in resection of high-grade gliomas? Neurosurg. Focus 2014, 36, E5. [Google Scholar] [CrossRef]
- Martirosyan, N.L.; Eschbacher, J.M.; Kalani, M.Y.; Turner, J.D.; Belykh, E.; Spetzler, R.F.; Nakaji, P.; Preul, M.C. Prospective evaluation of the utility of intraoperative confocal laser endomicroscopy in patients with brain neoplasms using fluorescein sodium: Experience with 74 cases. Neurosurg. Focus 2016, 40, E11. [Google Scholar] [CrossRef] [Green Version]
- Acerbi, F.; Broggi, M.; Schebesch, K.M.; Höhne, J.; Cavallo, C.; De Laurentis, C.; Eoli, M.; Anghileri, E.; Servida, M.; Boffano, C.; et al. Fluorescein-Guided Surgery for Resection of High-Grade Gliomas: A Multicentric Prospective Phase II Study (FLUOGLIO). Clin. Cancer Res. 2018, 24, 52–61. [Google Scholar] [CrossRef] [Green Version]
- Cho, S.S.; Salinas, R.; De Ravin, E.; Teng, C.W.; Li, C.; Abdullah, K.G.; Buch, L.; Hussain, J.; Ahmed, F.; Dorsey, J.; et al. Near-Infrared Imaging with Second-Window Indocyanine Green in Newly Diagnosed High-Grade Gliomas Predicts Gadolinium Enhancement on Postoperative Magnetic Resonance Imaging. Mol. Imaging Biol. 2020, 22, 1427–1437. [Google Scholar] [CrossRef]
- Kaneko, S.; Suero Molina, E.; Ewelt, C.; Warneke, N.; Stummer, W. Fluorescence-Based Measurement of Real-Time Kinetics of Protoporphyrin IX After 5-Aminolevulinic Acid Administration in Human in Situ Malignant Gliomas. Neurosurgery 2019, 85, E739–E746. [Google Scholar] [CrossRef]
- Hadjipanayis, C.G.; Schupper, A.J.; Schipmann, S.; Stummer, W. Fluorescence Guided Brain Tumor Surgery. Youmans & Winn Neurological Surgery, 8th ed.; Chapter 157B; Elsevier: New York, NY, USA, 2021. [Google Scholar]
- Senders, J.T.; Muskens, I.S.; Schnoor, R.; Karhade, A.V.; Cote, D.J.; Smith, T.R.; Broekman, M.L. Agents for fluorescence-guided glioma surgery: A systematic review of preclinical and clinical results. Acta Neurochir. (Wien) 2017, 159, 151–167. [Google Scholar] [CrossRef] [Green Version]
- Gessler, F.; Forster, M.-T.; Dützmann, S.; Mittelbronn, M.; Hattingen, E.; Franz, K.; Seifert, V.; Senft, C. Combination of Intraoperative Magnetic Resonance Imaging and Intraoperative Fluorescence to Enhance the Resection of Contrast Enhancing Gliomas. Neurosurgery 2015, 77, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Hadjipanayis, C.G.; Stummer, W. 5-ALA and FDA approval for glioma surgery. J. Neurooncol. 2019, 141, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Valdés, P.A.; Roberts, D.W.; Lu, F.K.; Golby, A. Optical technologies for intraoperative neurosurgical guidance. Neurosurg. Focus 2016, 40, 1–18. [Google Scholar] [CrossRef] [Green Version]
- La Rocca, G.; Sabatino, G.; Menna, G.; Altieri, R.; Ius, T.; Marchese, E.; Olivi, A.; Barresi, V.; Della Pepa, G.M. 5-Aminolevulinic Acid False Positives in Cerebral Neuro-Oncology: Not All That Is Fluorescent Is Tumor. A Case-Based Update and Literature Review. World Neurosurg. 2020, 137, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Knipps, J.; Fischer, I.; Neumann, L.M.; Rapp, M.; Dibué-Adjei, M.; Freiin von Saß, C.; Placke, J.M.; Mijderwijk, H.J.; Steiger, H.J.; Sabel, M.; et al. Quantification of PpIX-fluorescence of cerebral metastases: A pilot study. Clin. Exp. Metastasis 2019, 36, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Lau, D.; Hervey-Jumper, S.L.; Chang, S.; Molinaro, A.M.; McDermott, M.W.; Phillips, J.J.; Berger, M.S. A prospective Phase II clinical trial of 5-aminolevulinic acid to assess the correlation of intraoperative fluorescence intensity and degree of histologic cellularity during resection of high-grade gliomas. J. Neurosurg. 2016, 124, 1300–1309. [Google Scholar] [CrossRef] [Green Version]
- Roberts, D.W.; Valdés, P.A.; Harris, B.T.; Fontaine, K.M.; Hartov, A.; Fan, X.; Ji, S.; Lollis, S.S.; Pogue, B.W.; Leblond, F.; et al. Coregistered fluorescence-enhanced tumor resection of malignant glioma: Relationships between δ- aminolevulinic acid-induced protoporphyrin IX fluorescence, magnetic resonance imaging enhancement, and neuropathological parameters. J. Neurosurg. 2011, 114, 595–603. [Google Scholar]
- Idoate, M.A.; Díez Valle, R.; Echeveste, J.; Tejada, S. Pathological characterization of the glioblastoma border as shown during surgery using 5-aminolevulinic acid-induced fluorescence. Neuropathology 2011, 31, 575–582. [Google Scholar] [CrossRef]
- Müther, M.; Stummer, W. Ependymal fluorescence in fluorescence-guided resection of malignant glioma: A systematic review. Acta Neurochir. (Wien) 2020, 162, 365–372. [Google Scholar] [CrossRef]
- Stummer, W.; Susanne, S.; Simon, W.; Herbert, S.; Clemens, F.; Claudia, G.; Alwin, E.G.; Rainer, K.; Hans, J.R. Intraoperative detection of malignant gliomas by 5-aminolevulinic acid-induced porphyrin fluorescence. Neurosurgery 1998, 42, 518–525; discussion 525–526. [Google Scholar]
- Chohan, M.O.; Berger, M.S. 5-Aminolevulinic acid fluorescence guided surgery for recurrent high-grade gliomas. J. Neurooncol. 2019, 141, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, S.; Brokinkel, B.; Suero Molina, E.; Warneke, N.; Holling, M.; Bunk, E.C.; Hess, K.; Senner, V.; Paulus, W.; Stummer, W. Real-time in vivo kinetics of protoporphyrin IX after administration of 5-aminolevulinic acid in meningiomas and comparative analyses with glioblastomas. Acta Neurochir. (Wien) 2020, 162, 2197–2202. [Google Scholar] [CrossRef] [PubMed]
- Kamp, M.A.; Grosser, P.; Felsberg, J.; Slotty, P.J.; Steiger, H.-J.; Reifenberger, G.; Sabel, M. 5-Aminolevulinic acid (5-ALA)-induced fluorescence in intracerebral metastases: A retrospective study. Acta Neurochir. 2011, 154, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, P.; Barbero, P.; Monedero, G.; Presti, A.L.; Bejarano, B.; Penanes, J.R. Primary central nervous system lymphoma and 5-aminolevulinic acid. Surg. Neurol. Int. 2020, 11, 122. [Google Scholar] [CrossRef] [PubMed]
- Utsuki, S.; Oka, H.; Sato, K.; Shimizu, S.; Suzuki, S.; Fujii, K. Fluorescence diagnosis of tumor cells in hemangioblastoma cysts with 5-aminolevulinic acid. J. Neurosurg. 2010, 112, 130–132. [Google Scholar] [CrossRef] [PubMed]
- Labuschagne, J.J. The Use of 5-Aminolevulinic Acid to Assist Gross Total Resection of Paediatric Posterior Fossa. Tumours Pediatr. Neurosurg. 2020, 55, 268–279. [Google Scholar] [CrossRef]
- Bernal García, L.M.; Cabezudo Artero, J.M.; Marcelo Zamorano, M.B.; Gilete Tejero, I. Fluorescence-guided resection with 5-aminolevulinic Acid of subependymomas of the fourth ventricle: Report of 2 cases: Technical case report. Neurosurgery 2015, 11 (Suppl. 2), E364–E371; discussion E371. [Google Scholar] [CrossRef]
- Schwake, M.; Günes, D.; Köchling, M.; Brentrup, A.; Schroeteler, J.; Hotfilder, M.; Fruehwald, M.C.; Stummer, W.; Ewelt, C. Kinetics of porphyrin fluorescence accumulation in pediatric brain tumor cells incubated in 5-aminolevulinic acid. Acta Neurochir. 2014, 156, 1077–1084. [Google Scholar] [CrossRef]
- Jaber, M.; Wölfer, J.; Ewelt, C.; Holling, M.; Hasselblatt, M.; Niederstadt, T.; Zoubi, T.; Weckesser, M.; Stummer, W. The value of 5-aminolevulinic acid in low grade gliomas and high-grade gliomas lacking glioblastoma imaging features: An analysis based on fluorescence, magnetic resonance imaging, 18F-fluoroethyl tyrosine positron emission tomography, and tumor molecular factors. Neurosurgery 2016, 78, 401–411; discussion 411. [Google Scholar]
- Moore, G.E. Fluorescein as an Agent in the Differentiation of Normal and Malignant Tissues. Science 1947, 106, 130–131. [Google Scholar] [CrossRef]
- Shinoda, J.; Yano, H.; Yoshimura, S.I.; Okumura, A.; Kaku, Y.; Iwama, T.; Sakai, N. Fluorescence-guided resection of glioblastoma multiforme by using high-dose fluorescein sodium. Technical note. J. Neurosurg. 2003, 99, 597–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okuda, T.; Yoshioka, H.; Kato, A. Fluorescence-guided surgery for glioblastoma multiforme using high-dose fluorescein sodium with excitation and barrier filters. J. Clin. Neurosci. 2012, 19, 1719–1722. [Google Scholar] [PubMed]
- Schebesch, K.M.; Brawanski, A.; Hohenberger, C.; Hohne, J. Fluorescein Sodium-Guided Surgery of Malignant Brain Tumors: History, Current Concepts, and Future Project. Turk. Neurosurg. 2016, 26, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Wang, H.; Ge, P.; Zhao, J.; Li, W.; Gu, H.; Wang, G.; Luo, Y.; Chen, D. Gross total resection of glioma with the intraoperative fluorescence-guidance of fluorescein sodium. Int. J. Med. Sci. 2012, 9, 708–714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neira, J.A.; Ung, T.H.; Sims, J.S.; Malone, H.R.; Chow, D.S.; Samanamud, J.L.; Zanazzi, G.J.; Guo, X.; Bowden, S.G.; Zhao, B.; et al. Aggressive resection at the infiltrative margins of glioblastoma facilitated by intraoperative fluorescein guidance. J. Neurosurg. 2017, 127, 111–122. [Google Scholar] [CrossRef] [Green Version]
- Diaz, R.J.; Dios, R.R.; Hattab, E.M.; Burrell, K.; Rakopoulos, P.; Sabha, N.; Hawkins, C.; Zadeh, G.; Rutka, J.T.; Cohen-Gadol, A.A. Study of the biodistribution of fluorescein in glioma-infiltrated mouse brain and histopathological correlation of intraoperative findings in high-grade gliomas resected under fluorescein fluorescence guidance. J. Neurosurg. 2015, 122, 1360–1369. [Google Scholar]
- Suero Molina, E.; Wolfer, J.; Ewelt, C.; Ehrhardt, A.; Brokinkel, B.; Stummer, W. Dual-labeling with 5-aminolevulinic acid and fluorescein for fluorescence-guided resection of high-grade gliomas: Technical note. J. Neurosurg. 2018, 128, 399–405. [Google Scholar]
- Hansen, R.W.; Pedersen, C.B.; Halle, B.; Korshoej, A.R.; Schulz, M.K.; Kristensen, B.W.; Poulsen, F.R. Comparison of 5-aminolevulinic acid and sodium fluorescein for intraoperative tumor visualization in patients with high-grade gliomas: A single-center retrospective study. J. Neurosurg. 2019, 4, 1–8. [Google Scholar] [CrossRef]
- Stummer, W.; Gotz, C.; Hassan, A.; Heimann, A.; Kempski, O. Kinetics of Photofrin II in perifocal brain edema. Neurosurgery 1993, 33, 1075–1081; discussion 1081–1072. [Google Scholar]
- Stummer, W. Poor man’s fluorescence? Acta Neurochir. (Wien.) 2015, 157, 1379–1381. [Google Scholar]
- Raabe, A.; Beck, J.; Gerlach, R.; Zimmermann, M.; Seifert, V. Near-infrared indocyanine green video angiography: A new method for intraoperative assessment of vascular flow. Neurosurgery 2003, 52, 132–139, discussion 139. [Google Scholar] [PubMed]
- Reinhart, M.B.; Huntington, C.R.; Blair, L.J.; Heniford, B.T.; Augenstein, V.A. Indocyanine Green: Historical Context, Current Applications, and Future Considerations. Surg. Innov. 2016, 23, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.S.; Salinas, R.; Lee, J.Y.K. Indocyanine-Green for Fluorescence-Guided Surgery of Brain Tumors: Evidence, Techniques, and Practical Experience. Front. Surg. 2019, 6, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.Y.K.; Thawani, J.P.; Pierce, J.; Zeh, R.; Martinez-Lage, M.; Chanin, M.; Venegas, O.; Nims, S.; Learned, K.; Keating, J.; et al. Intraoperative Near-Infrared Optical Imaging Can Localize Gadolinium-Enhancing Gliomas During Surgery. Neurosurgery 2016, 79, 856–871. [Google Scholar] [CrossRef] [Green Version]
- Haglund, M.M.; Berger, M.S.; Hochman, D.W. Enhanced optical imaging of human gliomas and tumor margins. Neurosurgery 1996, 38, 308–317. [Google Scholar] [CrossRef]
- Eyupoglu, I.Y.; Hore, N.; Fan, Z.; Buslei, R.; Merkel, A.; Buchfelder, M.; Savaskan, N.E. Intraoperative vascular DIVA surgery reveals angiogenic hotspots in tumor zones of malignant gliomas. Sci. Rep. 2015, 5, 7958. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Buch, L.; Cho, S.; Lee, J.Y.K. Near-infrared intraoperative molecular imaging with conventional neurosurgical microscope can be improved with narrow band “boost” excitation. Acta Neurochir. 2019, 161, 2311–2318. [Google Scholar] [CrossRef]
- Dardevet, L.; Rani, D.; Aziz, T.A.; Bazin, I.; Sabatier, J.M.; Fadl, M.; Brambilla, E.; De Waard, M. Chlorotoxin: A helpful natural scorpion peptide to diagnose glioma and fight tumor invasion. Toxins 2015, 7, 1079–1101. [Google Scholar] [CrossRef]
- Patil, C.G.; Walker, D.G.; Miller, D.M.; Butte, P.; Morrison, B.; Kittle, D.S.; Hansen, S.J.; Nufer, K.L.; Byrnes-Blake, K.A.; Yamada, M.; et al. Phase 1 Safety, Pharmacokinetics, and Fluorescence Imaging Study of Tozuleristide (BLZ-100) in Adults With Newly Diagnosed or Recurrent Gliomas. Neurosurgery 2019, 85, E641–E649. [Google Scholar] [CrossRef] [PubMed]
- Weichert, J.P.; Clark, P.A.; Kandela, I.K.; Vaccaro, A.M.; Clarke, W.; Longino, M.A.; Pinchuk, A.N.; Farhoud, M.; Swanson, K.I.; Floberg, J.M.; et al. Alkylphosphocholine analogs for broad-spectrum cancer imaging and therapy. Sci. Transl. Med. 2014, 6, 240ra275. [Google Scholar] [CrossRef] [Green Version]
- Swanson, K.I.; Clark, P.A.; Zhang, R.R.; Kandela, I.K.; Farhoud, M.; Weichert, J.P.; Kuo, J.S. Fluorescent cancer-selective alkylphosphocholine analogs for intraoperative glioma detection. Neurosurgery 2015, 76, 115–123; discussion 123–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weller, M.; Butowski, N.; Tran, D.D.; Recht, L.D.; Lim, M.; Hirte, H.; Ashby, L.; Mechtler, L.; A Goldlust, S.; Iwamoto, F.; et al. Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): A randomised, double-blind, international phase 3 trial. Lancet Oncol. 2017, 18, 1373–1385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warram, J.M.; de Boer, E.; Korb, M.; Hartman, Y.; Kovar, J.; Markert, J.M.; Gillespie, G.Y.; Rosenthal, E.L. Fluorescence-guided resection of experimental malignant glioma using cetuximab-IRDye 800CW. Br. J. Neurosurg. 2015, 29, 850–858. [Google Scholar] [CrossRef] [Green Version]
- Samkoe, K.S.; Gunn, J.R.; Marra, K.; Hull, S.M.; Moodie, K.L.; Feldwisch, J.; Strong, T.V.; Draney, D.R.; Hoopes, P.J.; Roberts, D.W.; et al. Toxicity and Pharmacokinetic Profile for Single-Dose Injection of ABY-029: A Fluorescent Anti-EGFR Synthetic Affibody Molecule for Human Use. Mol. Imaging Biol. 2017, 19, 512–521. [Google Scholar] [PubMed]
- Willems, P.W.; Taphoorn, M.J.; Burger, H.; Berkelbach van der Sprenkel, J.W.; Tulleken, C.A. Effectiveness of neuronavigation in resecting solitary intracerebral contrast-enhancing tumors: A randomized controlled trial. J. Neurosurg. 2006, 104, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Senft, C.; Bink, A.; Franz, K.; Vatter, H.; Gasser, T.; Seifert, V. Intraoperative MRI guidance and 580 extent of resection in glioma surgery: A randomised, controlled trial. Lancet Oncol. 2011, 12, 997–1003. [Google Scholar] [CrossRef]
- Roder, C.; Bisdas, S.; Ebner, F.H.; Honegger, J.; Naegele, T.; Ernemann, U.; Tatagiba, M. Maximizing the extent of resection and survival benefit of patients in glioblastoma surgery: High-field iMRI versus conventional and 5-ALA-assisted surgery. Eur. J. Surg. Oncol. 2014, 40, 297–304. [Google Scholar] [CrossRef]
- Kubben, P.L.; Scholtes, F.; Schijns, O.E.; Ter Laak-Poort, M.P.; Teernstra, O.P.; Kessels, A.G.; van Overbeeke, J.J.; Martin, D.H.; van Santbrink, H. Intraoperative magnetic resonance imaging versus standard neuronavigation for the neurosurgical treatment of glioblastoma: A randomized controlled trial. Surg. Neurol. Int. 2014, 5, 70. [Google Scholar] [CrossRef]
- Wu, J.S.; Gong, X.; Song, Y.Y.; Zhuang, D.X.; Yao, C.J.; Qiu, T.M.; Lu, J.F.; Zhang, J.; Zhu, W.; Mao, Y.; et al. 3.0-T intraoperative magnetic resonance imaging-guided resection in cerebral glioma surgery: Interim analysis of a prospective, randomized, triple-blind, parallel-controlled trial. Neurosurgery 2014, 61 (Suppl. S1), 145–154. [Google Scholar] [CrossRef]
- Coburger, J.; Hagel, V.; Wirtz, C.R.; König, R. Surgery for glioblastoma: Impact of the combined use of 5-aminolevulinic acid and intraoperative MRI on extent of resection and FGS and Intraoperative Adjuvants to Maximize EOR. PLoS ONE 2015, 10, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Schatlo, B.; Fandino, J.; Smoll, N.R.; Wetzel, O.; Remonda, L.; Marbacher, S.; Perrig, W.; Landolt, H.; Fathi, A.-R. Outcomes after combined use of intraoperative MRI and 5-aminolevulinic acid in high-grade glioma surgery. Neuro-Oncology 2015, 17, 1560–1567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neidert, M.C.; Hostettler, I.C.; Burkhardt, J.K.; Mohme, M.; Held, U.; Kofmehl, R.; Eisele, G.; Woernle, C.M.; Regli, L.; Bozinov, O. The influence of intraoperative resection control modalities on survival following gross total resection of glioblastoma. Neurosurg. Rev. 2016, 39, 401–409. [Google Scholar] [CrossRef]
- Golub, D.; Hyde, J.; Dogra, S.; Nicholson, J.; Kirkwood, K.A.; Gohel, P.; Loftus, S.; Schwartz, T.H. Intraoperative MRI versus 5-ALA in high-grade glioma resection: A network meta-analysis. J. Neurosurg. 2020, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Omay, S.B.; Barnett, G.H. Surgical navigation for meningioma surgery. J. Neurooncol. 2010, 99, 357–364. [Google Scholar] [CrossRef]
- Czyz, M.; Tabakow, P.; Lechowicz-Głogowska, B.; Jarmundowicz, W. Prospective study on the efficacy of low-field intraoperative magnetic resonance imaging in neurosurgical operations. Neurol. Neurochir. Pol. 2011, 45, 226–234. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, X.; Zhao, Y.; Wang, F.; Li, F.; Xu, B. Impact of intraoperative magnetic resonance imaging and functional neuronavigation on surgical outcome in patients with gliomas involving language areas. Neurosurg. Rev. 2015, 38, 319–330; discussion 330. [Google Scholar] [CrossRef]
- Wirtz, C.R.; Bonsanto, M.M.; Knauth, M.; Tronnier, V.M.; Albert, F.K.; Staubert, A.; Kunze, S. Intraoperative Magnetic Resonance Imaging to Update Interactive Navigation in Neurosurgery: Method and Preliminary Experience. Comput. Aided Surg. 1997, 2, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Knauth, M.; Wirtz, C.R.; Tronnier, V.M.; Aras, N.; Kunze, S.; Sartor, K. Intraoperative MR imaging increases the extent of tumor resection in patients with high-grade gliomas. Am. J. Neuroradiol. 1999, 20, 1642–1646. [Google Scholar]
- Krivosheya, D.; Rao, G.; Tummala, S.; Kumar, V.; Suki, D.; Bastos, D.C.A.; Prabhu, S.S. Impact of Multi-modality Monitoring Using Direct Electrical Stimulation to Determine Corticospinal Tract Shift and Integrity in Tumors using the Intraoperative MRI. J. Neurol. Surg. A Cent. Eur. Neurosurg. 2019. [Google Scholar] [CrossRef]
- Kuhnt, D.; Becker, A.; Ganslandt, O.; Bauer, M.; Buchfelder, M.; Nimsky, C. Correlation of the extent of tumor volume resection and patient survival in surgery of glioblastoma multiforme with high-field intraoperative MRI guidance. Neuro Oncol. 2011, 13, 1339–1348. [Google Scholar] [CrossRef] [Green Version]
- Shah, A.S.; Sylvester, P.T.; Yahanda, A.T.; Vellimana, A.K.; Dunn, G.P.; Evans, J.; Rich, K.M.; Dowling, J.L.; Leuthardt, E.C.; Dacey, R.G.; et al. Intraoperative MRI for newly diagnosed supratentorial glioblastoma: A multicenter-registry comparative study to conventional surgery. J. Neurosurg. 2020, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Eljamel, M.S.; Mahboob, S.O. The effectiveness and cost-effectiveness of intraoperative imaging in high-grade glioma resection; a comparative review of intraoperative ALA, fluorescein, ultrasound and MRI. Photodiagn. Photodyn. Ther. 2016, 16, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Phan, K.; Kim, J.S.; Kim, J.H.; Somani, S.; Di’Capua, J.; Dowdell, J.E.; Cho, S.K. Anesthesia Duration as an Independent Risk Factor for Early Postoperative Complications in Adults Undergoing Elective ACDF. Glob. Spine J. 2017, 7, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Abraham, P.; Sarkar, R.; Brandel, M.G.; Wali, A.R.; Rennert, R.C.; Lopez Ramos, C.; Padwal, J.; Steinberg, J.A.; Santiago-Dieppa, D.R.; Cheung, V.; et al. Cost-effectiveness of Intraoperative MRI for Treatment of High-Grade Gliomas. Radiology 2019, 291, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Eyüpoglu, I.Y.; Hore, N.; Merkel, A.; Buslei, R.; Buchfelder, M.; Savaskan, N. Supra-complete surgery via dual intraoperative visualization approach (DiVA) prolongs patient survival in glioblastoma. Oncotarget 2016, 7, 25755–25768. [Google Scholar] [CrossRef] [PubMed]
- Coburger, J.; Engelke, J.; Scheuerle, A.; Thal, D.R.; Hlavac, M.; Wirtz, C.R.; König, R. Tumor detection with 5-aminolevulinic acid fluorescence and Gd-DTPA-enhanced intraoperative MRI at the border of contrast-enhancing lesions: A prospective study based on histopathological assessment. Neurosurg. Focus 2014, 36, E3. [Google Scholar] [CrossRef] [PubMed]
- Langer, D.J.; White, T.G.; Schulder, M.; Boockvar, J.A.; Labib, M.; Lawton, M.T. Advances in Intraoperative Optics: A Brief Review of Current Exoscope Platforms. Oper. Neurosurg. 2020, 19, 84–93. [Google Scholar] [CrossRef]
- Baron, R.B.; Lakomkin, N.; Schupper, A.J.; Nistal, D.; Nael, K.; Price, G.; Hadjipanayis, C.G. Postoperative outcomes following glioblastoma resection using a robot-assisted digital surgical exoscope: A case series. J. Neurooncol. 2020, 148, 519–527. [Google Scholar] [CrossRef]
- Morin, F.; Courtecuisse, H.; Reinertsen, I.; Le Lann, F.; Palombi, O.; Payan, Y.; Chabanas, M. Brain-shift compensation using intraoperative ultrasound and constraint-based biomechanical simulation. Med Image Anal. 2017, 40, 133–153. [Google Scholar] [CrossRef] [Green Version]
- Sweeney, J.F.; Smith, H.; Taplin, A.L.; Perloff, E.; Adamo, M.A. Effcacy of intraoperative ultrasonography in neurosurgical tumor resection. J. Neurosurg. Pediatr. 2018, 21, 504–510. [Google Scholar] [CrossRef]
- Della Pepa, G.M.; Menna, G.; Stifano, V.; Pezzullo, A.M.; Auricchio, A.M.; Rapisarda, A.; Caccavella, V.M.; La Rocca, G.; Sabatino, G.; Marchese, E.; et al. Predicting meningioma consistency and brain-meningioma interface with intraoperative strain ultrasound elastography: A novel application to guide surgical strategy. Neurosurg. Focus 2021, 50, E15. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Feng, F.; Cao, J.; Chen, Z.; He, B.; Kang, Z.; He, L.; Wu, W.; Tan, L.; Li, K.; et al. Real-time ultrasonography-magnetic resonance image fusion navigation for percutaneous transforaminal endoscopic discectomy. J. Neurosurg. Spine 2020, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Prada, F.; Perin, A.; Martegani, A.; Aiani, L.; Solbiati, L.; Lamperti, M.; Casali, C.; Legnani, F.; Mattei, L.; Saladino, A.; et al. Intraoperative contrast-enhanced ultrasound for brain tumor surgery. Neurosurgery 2014, 74, 542–552; discussion 552. [Google Scholar] [CrossRef] [Green Version]
- Prada, F.; Bene, M.D.; Fornaro, R.; Vetrano, I.G.; Martegani, A.; Aiani, L.; Sconfienza, L.M.; Mauri, G.; Solbiati, L.; Pollo, B.; et al. Identification of residual tumor with intraoperative contrast-enhanced ultrasound during glioblastoma resection. Neurosurg. Focus 2016, 40, E7. [Google Scholar] [CrossRef] [PubMed]
- De Witt Hamer, P.C.; Robles, S.G.; Zwinderman, A.H.; Duffau, H.; Berger, M.S. Impact of intraoperative stimulation brain mapping on glioma surgery outcome: A meta-analysis. J. Clin. Oncol. 2012, 30, 2559–2565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morshed, R.A.; Young, J.S.; Lee, A.T.; Hervey-Jumper, S.L. Functional Mapping for Glioma Surgery, Part 2: Intraoperative Mapping Tools. Neurosurg. Clin. N. Am. 2021, 32, 75–81. [Google Scholar] [CrossRef]
- De Benedictis, A.; Moritz-Gasser, S.; Duffau, H. Awake mapping optimizes the extent of resection for low-grade gliomas in eloquent areas. Neurosurgery 2010, 66, 1074–1084. [Google Scholar] [CrossRef]
- Feigl, G.C.; Ritz, R.; Moraes, M.; Klein, J.; Ramina, K.; Gharabaghi, A.; Krischek, B.; Danz, S.; Bornemann, A.; Liebsch, M.; et al. Resection of malignant brain tumors in eloquent cortical areas: A new multimodal approach combining 5-aminolevulinic acid and intraoperative monitoring. J. Neurosurg. 2010, 113, 352–357. [Google Scholar] [CrossRef]
- Schucht, P.; Beck, J.; Abu-Isa, J.; Andereggen, L.; Murek, M.; Seidel, K.; Stieglitz, L.; Raabe, A. Gross Total Resection Rates in Contemporary Glioblastoma Surgery. Neurosurg. 2012, 71, 927–936. [Google Scholar] [CrossRef] [Green Version]
- Leuthardt, E.C.; Lim, C.C.H.; Shah, M.N.; A Evans, J.; Rich, K.M.; Dacey, R.G.; Tempelhoff, R.; Chicoine, M.R. Use of Movable High-Field-Strength Intraoperative Magnetic Resonance Imaging With Awake Craniotomies for Resection of Gliomas: Preliminary Experience. Neurosurg. 2011, 69, 194–206. [Google Scholar] [CrossRef]
- Tuominen, J.; Yrjänä, S.; Ukkonen, A.; Koivukangas, J. Awake craniotomy may further improve neurological outcome of intraoperative MRI-guided brain tumor surgery. Acta Neurochir. (Wien) 2013, 155, 1805–1812. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.Y.; Chen, J.P.; Cheng, W.Y.; Lee, H.T.; Shen, C.C. The role of tailored intraoperative neurophysiological monitoring in glioma surgery: A single institute experience. J. Neurooncol. 2020, 146, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Krieg, S.M.; Shiban, E.; Droese, D.; Gempt, J.; Buchmann, N.; Pape, H.; Ryang, Y.M.; Meyer, B.; Ringel, F. Predictive value and safety of intraoperative neurophysiological monitoring with motor evoked potentials in glioma surgery. Neurosurgery 2012, 70, 1060–1070; discussion 1070–1071. [Google Scholar] [CrossRef] [PubMed]
- Krieg, S.M.; Schäffner, M.; Shiban, E.; Droese, D.; Obermüller, T.; Gempt, J.; Meyer, B.; Ringel, F. Reliability of intraoperative neurophysiological monitoring using motor evoked potentials during resection of metastases in motor-eloquent brain regions: Clinical article. J. Neurosurg. 2013, 118, 1269–1278. [Google Scholar] [CrossRef]
- Eisenhardt, L.; Cushing, H. Diagnosis of Intracranial Tumors by Supravital Technique. Am. J. Pathol. 1930, 6, 541–552.e7. [Google Scholar]
- Freudiger, C.W.; Min, W.; Saar, B.G.; Lu, S.; Holtom, G.R.; He, C.; Tsai, J.C.; Kang, J.X.; Xie, X.S. Label-free biomedical imaging with high sensitivity by stimulated Raman scattering microscopy. Science 2008, 322, 1857–1861. [Google Scholar] [CrossRef] [Green Version]
- Cui, S.; Zhang, S.; Yue, S. Raman Spectroscopy and Imaging for Cancer Diagnosis. J. Healthc. Eng. 2018, 2018, 8619342. [Google Scholar] [CrossRef]
- Orringer, D.A.; Pandian, B.; Niknafs, Y.S.; Hollon, T.C.; Boyle, J.; Lewis, S.; Garrard, M.; Hervey-Jumper, S.L.; Garton, H.J.L.; Maher, C.O.; et al. Rapid intraoperative histology of unprocessed surgical specimens via fibre-laser-based stimulated Raman scattering microscopy. Nat. Biomed. Eng. 2017, 1, 1–13. [Google Scholar] [CrossRef]
- Ji, M.; Lewis, S.; Camelo-Piragua, S.; Ramkissoon, S.H.; Snuderl, M.; Venneti, S.; Fisher-Hubbard, A.; Garrard, M.; Fu, D.; Wang, A.C.; et al. Detection of human brain tumor infiltration with quantitative stimulated Raman scattering microscopy. Sci. Transl. Med. 2015, 7, 309ra163. [Google Scholar] [CrossRef] [Green Version]
- Ji, M.; Orringer, D.A.; Freudiger, C.W.; Ramkissoon, S.; Liu, X.; Lau, D.; Golby, A.J.; Norton, I.; Hayashi, M.; Agar, N.Y.R.; et al. Rapid, Label-Free Detection of Brain Tumors with Stimulated Raman Scattering Microscopy. Sci. Transl. Med. 2013, 5, 201ra119. [Google Scholar] [CrossRef] [Green Version]
- Hollon, T.C.; Pandian, B.; Adapa, A.R.; Urias, E.; Save, A.V.; Khalsa, S.S.S.; Eichberg, D.G.; D’Amico, R.S.; Farooq, Z.U.; Lewis, S.; et al. Near real-time intraoperative brain tumor diagnosis using stimulated Raman histology and deep neural networks. Nat. Med. 2020, 26, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.L.; Beroukhim, R. Beating the odds: Extreme long-term survival with glioblastoma. Neuro Oncol. 2014, 16, 1159–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGirt, M.J.; Chaichana, K.L.; Attenello, F.J.; Weingart, J.D.; Than, K.; Burger, P.C.; Olivi, A.; Brem, H.; Quinoñes-Hinojosa, A. Extent of surgical resection is independently associated with survival in patients with hemispheric infiltrating low-grade gliomas. Neurosurgery 2008, 63, 700–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desroches, J.; Jermyn, M.; Mok, K.; Lemieux-Leduc, C.; Mercier, J.; St-Arnaud, K.; Urmey, K.; Guiot, M.-C.; Marple, E.T.; Petrecca, K.; et al. Characterization of a Raman spectroscopy probe system for intraoperative brain tissue classification. Biomed. Opt. Express 2015, 6, 2380–2397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desroches, J.; Laurence, A.; Jermyn, M.; Pinto, M.; Tremblay, M.A.; Petrecca, K.; Leblond, F. Raman spectroscopy in microsurgery: Impact of operating microscope illumination sources on data quality and tissue classification. Analyst 2017, 142, 1185–1191. [Google Scholar] [CrossRef]
- Lakomkin, N.; Hadjipanayis, C.G. The Use of Spectroscopy Handheld Tools in Brain Tumor Surgery: Current Evidence and Techniques. Front. Surg. 2019, 6, 30. [Google Scholar] [CrossRef]
- Jermyn, M.; Mok, K.; Mercier, J.; Desroches, J.; Pichette, J.; Saint-Arnaud, K.; Bernstein, L.; Guiot, M.C.; Petrecca, K.; Leblond, F. Intraoperative brain cancer detection with Raman spectroscopy in humans. Sci. Transl. Med. 2015, 7, 274ra19. [Google Scholar] [CrossRef]
- Jermyn, M.; Mercier, J.; Aubertin, K.; Desroches, J.; Urmey, K.; Karamchandiani, J.; Marple, E.; Guiot, M.C.; Leblond, F.; Petrecca, K. Highly Accurate Detection of Cancer In Situ with Intraoperative, Label-Free, Multimodal Optical Spectroscopy. Cancer Res. 2017, 77, 3942–3950. [Google Scholar] [CrossRef] [Green Version]
- McGregor, H.C.; Short, M.A.; McWilliams, A.; Shaipanich, T.; Ionescu, D.N.; Zhao, J.; Wang, W.; Chen, G.; Lam, S.; Zeng, H. Real-time endoscopic Raman spectroscopy for in vivo early lung cancer detection. J. Biophotonics 2017, 10, 98–110. [Google Scholar] [CrossRef]
- Shipp, D.W.; Rakha, E.A.; Koloydenko, A.A.; Macmillan, R.D.; Ellis, I.O.; Notingher, I. Intra-operative spectroscopic assessment of surgical margins during breast conserving surgery. Breast Cancer Res. 2018, 20, 69. [Google Scholar] [CrossRef] [Green Version]
- Jermyn, M.; Desroches, J.; Mercier, J.; St-Arnaud, K.; Guiot, M.C.; Leblond, F.; Petrecca, K. Raman spectroscopy detects distant invasive brain cancer cells centimeters beyond MRI capability in humans. Biomed. Opt. Express 2016, 7, 5129–5137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanai, N.; Eschbacher, J.; Hattendorf, G.; Coons, S.W.; Preul, M.C.; Smith, K.A.; Nakaji, P.; Spetzler, R.F. Intraoperative Confocal Microscopy for Brain Tumors: A Feasibility Analysis in Humans. Oper. Neurosurg. 2011, 68, ons282–ons290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, L.; Chen, Y.; Yin, C.; Borwege, S.; Sanai, N.; Liu, J.T.C. Optical-sectioning microscopy of protoporphyrin IX fluorescence in human gliomas: Standardization and quantitative comparison with histology. J. Biomed. Opt. 2017, 22, 46005. [Google Scholar] [CrossRef] [PubMed]
- Haj-Hosseini, N.; Richter, J.; Andersson-Engels, S.; Wårdell, K. Optical touch pointer for fluorescence guided glioblastoma resection using 5-aminolevulinic acid. Lasers Surg. Med. 2010, 42, 9–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valdés, P.A.; Leblond, F.; Kim, A.; Harris, B.T.; Wilson, B.C.; Fan, X.; Tosteson, T.D.; Hartov, A.; Ji, S.; Erkmen, K.; et al. Quantitative fluorescence in intracranial tumor: Implications for ALA-induced PpIX as an intraoperative biomarker. J. Neurosurg. 2011, 115, 11–17. [Google Scholar] [CrossRef] [Green Version]
- Kairdolf, B.A.; Bouras, A.; Kaluzova, M.; Sharma, A.K.; Wang, M.D.; Hadjipanayis, C.G.; Nie, S. Intraoperative Spectroscopy with Ultrahigh Sensitivity for Image-Guided Surgery of Malignant Brain Tumors. Anal. Chem. 2016, 88, 858–867. [Google Scholar] [CrossRef]
- Bravo, J.J.; Olson, J.D.; Davis, S.C.; Roberts, D.W.; Paulsen, K.D.; Kanick, S.C. Hyperspectral data processing improves PpIX contrast during fluorescence guided surgery of human brain tumors. Sci. Rep. 2017, 7, 9455. [Google Scholar] [CrossRef]
- Tamura, Y.; Kuroiwa, T.; Kajimoto, Y.; Miki, Y.; Miyatake, S.; Tsuji, M. Endoscopic identification and biopsy sampling of an intraventricular malignant glioma using a 5-aminolevulinic acid-induced protoporphyrin IX fluorescence imaging system. Technical note. J. Neurosurg. 2007, 106, 507–510. [Google Scholar] [CrossRef] [Green Version]
- Belykh, E.; Miller, E.J.; Hu, D.; Martirosyan, N.L.; Woolf, E.C.; Scheck, A.C.; Byvaltsev, V.A.; Nakaji, P.; Nelson, L.Y.; Seibel, E.J.; et al. Scanning Fiber Endoscope Improves Detection of 5-Aminolevulinic Acid-Induced Protoporphyrin IX Fluorescence at the Boundary of Infiltrative Glioma. World Neurosurg. 2018, 113, e51–e69. [Google Scholar] [CrossRef]
- Potaov, A.; Usachey, D.; Loshakov, V.; Cherekaev, V.A.; Kornienko, V.N.; Pronin, I.N.; Kobiakov, G.L.; Kalinin, P.L.; Gavrilov, A.G.; Stummer, W.; et al. First experience in 5-ALA fluorescence-guided and endoscopically assisted microsurgery of brain tumors. Med. Laser Appl. 2008, 23, 202–208. [Google Scholar] [CrossRef]
- Belykh, E.; Miller, E.J.; Carotenuto, A.; Patel, A.A.; Cavallo, C.; Martirosyan, N.L.; Healey, D.R.; Byvaltsev, V.A.; Scheck, A.C.; Lawton, M.T.; et al. Progress in Confocal Laser Endomicroscopy for Neurosurgery and Technical Nuances for Brain Tumor Imaging With Fluorescein. Front. Oncol. 2019, 9, 554. [Google Scholar] [CrossRef] [PubMed]
- Höhne, J.; Schebesch, K.M.; Zoubaa, S.; Proescholdt, M.; Riemenschneider, M.J.; Schmidt, N.O. Intraoperative imaging of brain tumors with fluorescein: Confocal laser endomicroscopy in neurosurgery. Clin. User Exp. Neurosurg. Focus 2021, 50, E19. [Google Scholar] [CrossRef] [PubMed]
| Author (Year) | Fluorophore | Study Design | Evidence Class | Description | Conclusions |
|---|---|---|---|---|---|
| Stummer et al. (2000) [6] | 5-ALA (PpIX) | Case series | II | Prospective study of 52 patients receiving 5-ALA for GBM resection. Rates of complete resection and predictors of survival were assessed. | Complete resection of contrast-enhanced tumor was achieved in 63% patients. Age, residual fluorescence and absence of contrast-enhancement on postoperative MRI were predictors of survival. |
| Stummer et al. (2006) [7] | 5-ALA (PpIX) | RCT | I | Randomized, controlled multicenter phase III trial of 322 patients who received either 5-ALA or conventional surgery. EOR and PFS were analyzed. | There was a significant improvement in complete resection of contrast-enhancing tumor in the 5-ALA group (36% vs. 27%), and improved six-month PFS (41.0% vs. 21.1%). |
| Eljamel et al. (2008) [8] | 5-ALA (PpIX) | RCT | I | Randomized, prospective phase III single center trial evaluating the use of 5-ALA and repetitive photodynamic therapy (PDT) for the treatment of GBM. Survival, Karnofsky performance score (KPS) and time to tumor progression were analyzed. | Patients who received 5-ALA and PDT had a significantly prolonged survival (53 vs. 25 weeks), improved KPS and prolonged time to tumor progression (8.6 vs. 4.8 months) compared to controls. |
| Nabavi et al. (2009) [9] | 5-ALA (PpIX) | Case series | II | Multicenter, prospective study of 36 patients with HGG undergoing surgery with 5-ALA. Positive predictive value (PPV) and survival data was analyzed. | 5-ALA had over 90% PPV in both areas of strong (96.9%) and weak (90.3%) fluorescence. No adverse events were found using the drug. |
| Diez Valle et al. (2011) [10] | 5-ALA (PpIX) | Case series | II | Prospective, single-center study of 36 patients with GBM who received 5-ALA prior to surgery. EOR, complete resection of contrast-enhanced tumor and survival analysis was conducted. | Strong fluorescence yielded 100% PPV, while vague fluorescence beyond the tumor core yielded 97% PPV and 66% negative predictive value (NPV). Complete resection of the contrast-enhanced tumor was removed in 83.3% patients. Patients had a 8.2% morbidity rate one month after surgery. |
| Stummer et al. (2011) [11] | 5-ALA (PpIX) | Case series | II | Prospective, multicenter phase II safety trial assessing adverse events in 219 patients undergoing HGG resection with 5-ALA who were also receiving concomitant radiochemotherapy with adjuvant temozolomide (Stupp protocol). Adverse events (AE) and survival analysis were conducted. | Three patients experienced four AEs possibly related to 5-ALA. GBM patients experienced a survival advantage if they received radiochemotherapy (16.3 vs. 11.9 months). Elderly patients additionally saw a benefit from concomitant therapies. |
| Diez Valle et al. (2014) [12] | 5-ALA (PpIX) | Cohort | II | Retrospective, multicenter study of 251 patients with malignant glioma who received 5-ALA with intended chemoradiotherapy with temozolomide. Complete resection rates and survival analyses were conducted. | Rates of complete resection (67% vs. 45%) and six-month progression-free survival for GBM patients (69% vs. 48%) were significantly higher in the 5-ALA group. |
| Eljamel (2015) [13] | 5-ALA (PpIX) | Meta-analysis | II | Meta-analysis of 20 studies on the use of 5-ALA for GBM surgery. Outcomes parameters included GTR rates, time to tumor progression, overall survival, and sensitivity and specificity data. | Mean GTR rate was 75.4%, and mean time to tumor progression was 8.1 months. Mean overall survival gain was 6.2 months. Mean specificity was 88.9% and sensitivity of 82.6%. 5-ALA is highly sensitive and specific, and improves GTR and time to tumor progression. |
| Teixidor et al. (2016) [14] | 5-ALA (PpIX) | Cohort | II | Prospective, multicenter cohort study of 85 patients with HGG receiving 5-ALA prior to surgery. Safety data, EOR and survival analyses were conducted. | Complete resection was achieved in 54% of patients. Six-month PFS was 58% and median overall survival was 14.2 months. No serious adverse events were reported. One-month postoperative morbidity was 6.5%. |
| Koc et al. (2008) [15] | Fluorescein | Cohort | II | Prospective study of 80 patients with GBM, 47 who received fluorescein during surgery and 33 who did not. EOR and survival analyses were conducted. | Patients who received fluorescein were more likely to receive a GTR, however, there were no differences in median survival between groups. |
| Acerbi et al. (2014) [16] | Fluorescein | Case series | II | Prospective study of 20 patients with HGG who received fluorescein during surgery. Safety data, EOR and survival analyses were conducted. | No adverse events related to fluorescein were observed. Complete removal of the contrast-enhanced tumor was found in 80% patients. Six-month PFS was found in 71.4% of patients, and median overall survival was 11 months. |
| Martirosyan et al. (2016) [17] | Fluorescein | Case series | II | Prospective, single-center study of 74 patients with gliomas and meningiomas who received fluorescein during surgery and resection with confocal laser endomicroscopy. Sensitivity and specificity data were analyzed. | Sensitivity and specificity for glioma tissue was 91% and 94%, respectively. |
| Acerbi et al. (2018) [18] | Fluorescein | Case series | II | Prospective, multicenter phase II trial of 46 patients with HGG who underwent resection. EOR, PFS and overall survival was recorded. | 82.6% gross total resection, PFS-6 and PFS-12 were 56.6% and 15.2%. Median survival was 12 months. No adverse reaction related to SF administration was recorded. The sensitivity and specificity of fluorescein in identifying tumor tissue were respectively 80.8% and 79.1%. |
| Cho et al. (2020) [19] | ICG | Case series | II | Retrospective study of 36 patients with HGG who received ICG prior to surgery. Accuracy of fluorescence was analyzed. | Near-infrared (NIR) imaging showed higher sensitivity and accuracy in diagnosing HGG tissue intraoperatively compared to white light. NIR imaging predicted postoperativce MRI gadolinium contrast with 91% accuracy, and patients with no residual NIR signal following resection were more likely to have complete resection on postoperative MRI. |
| Author (Year) | Modality | Study Design | Evidence Class | Description | Conclusions |
|---|---|---|---|---|---|
| Willems et al. (2006) [67] | Neuronavigation | RCT | I | 45 patients randomized to surgery with or without neuronavigation. Residual contrast-enhancing tumor and survival data was analyzed. | There were no differences in residual contrast-enhancing. Median survival was shorter in patients who received neuronavigation. |
| Senft et al. (2011) [68] | iMRI | RCT | I | 58 patients randomly selected to iMRI or control for glioma surgery. Extent of resection and postoperative neurological deficits were analyzed. | Patients in the iMRI group had higher rates of complete tumor resection, and no increased postoperative neurological deficits. |
| Roder et al. (2014) [69] | iMRI + 5-ALA | Case series | II | Retrospective comparative study of 117 patients undergoing GBM surgery with iMRI compared to conventional surgery with and without 5-ALA. | iMRI patients had a lower residual tumor volume and higher proportion of complete resection. Improved six-month PFS was seen in cases of complete resection. |
| Kubben et al. (2014) [70] | iMRI | RCT | I | Randomization of 14 patients with supratentorial GBM received iMRI or conventional neuronavigation. Residual tumor volume and postoperative outcomes were calculated. | There were no differences found in residual tumor volume or median survival. iMRI did not appear to be cost-effective, but limited by a small patient sample. |
| Wu et al. (2014) [71] | iMRI | RCT | I | 114 patients were randomized to iMRI or conventional neuronavigation. EOR was the primary endpoint, with secondary endpoints survival and morbidity data. | No differences in rates of GTR were detected in HGG patients. Six-month PFS trended toward the iMRI group in HGG patients. |
| Coburger et al. (2015) [72] | iMRI + 5-ALA | Case series | II | Prospective trial of 33 patients undergoing GBM surgery with iMRI and 5-ALA, compared to retrospective controls, EOR and survival data was analyzed. | EOR was higher in the iMRI+5-ALA group compared to iMRI alone. There were no differences in postoperative neurological deficits or survival data between groups. |
| Schatlo et al. (2015) [73] | iMRI + 5-ALA | Case series | II | Retrospective series of 200 HGG patients undergoing surgery with iMRI and 5-ALA or conventional surgery. EOR and survival data was analyzed. | Patients in the iMRI + 5-ALA group experienced prolonged overall survival upon univariate analysis, but no differences were detected upon multivariate analyses. |
| Neidert et al. (2016) [74] | Ultrasound | Case series | II | Retrospective analysis of 76 patients who underwent glioblastoma resection with intraoperative ultrasound or conventional surgery. Only patients who had a GTR achieved were included. Survival data was analyzed. | Median overall survival was longer in GTR patients were ultrasound was used, and ultrasound was associated with prolonged overall and progression-free survival. |
| Golub et al. (2020) [75] | iMRI + 5-ALA, Neuronavigation | Meta-analysis | II | Meta-analysis of 11 studies assessing neuronavigation, iMRI and 5-ALA for HGG resection. Rates of GTR and survival comparisons were analyzed. | iMRI and 5-ALA were superior to neuronavigation in achieving GTR, and both modalities were shown to improve patient survival. However, no differences were found between iMRI and 5-ALA. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schupper, A.J.; Yong, R.L.; Hadjipanayis, C.G. The Neurosurgeon’s Armamentarium for Gliomas: An Update on Intraoperative Technologies to Improve Extent of Resection. J. Clin. Med. 2021, 10, 236. https://doi.org/10.3390/jcm10020236
Schupper AJ, Yong RL, Hadjipanayis CG. The Neurosurgeon’s Armamentarium for Gliomas: An Update on Intraoperative Technologies to Improve Extent of Resection. Journal of Clinical Medicine. 2021; 10(2):236. https://doi.org/10.3390/jcm10020236
Chicago/Turabian StyleSchupper, Alexander J., Raymund L. Yong, and Constantinos G. Hadjipanayis. 2021. "The Neurosurgeon’s Armamentarium for Gliomas: An Update on Intraoperative Technologies to Improve Extent of Resection" Journal of Clinical Medicine 10, no. 2: 236. https://doi.org/10.3390/jcm10020236





