Gadolinium-Based Contrast Media Nephrotoxicity in Kidney Impairment: The Physio-Pathological Conditions for the Perfect Murder
Abstract
:1. Introduction
2. The Pathological Mechanism of Gadolinium Toxicity
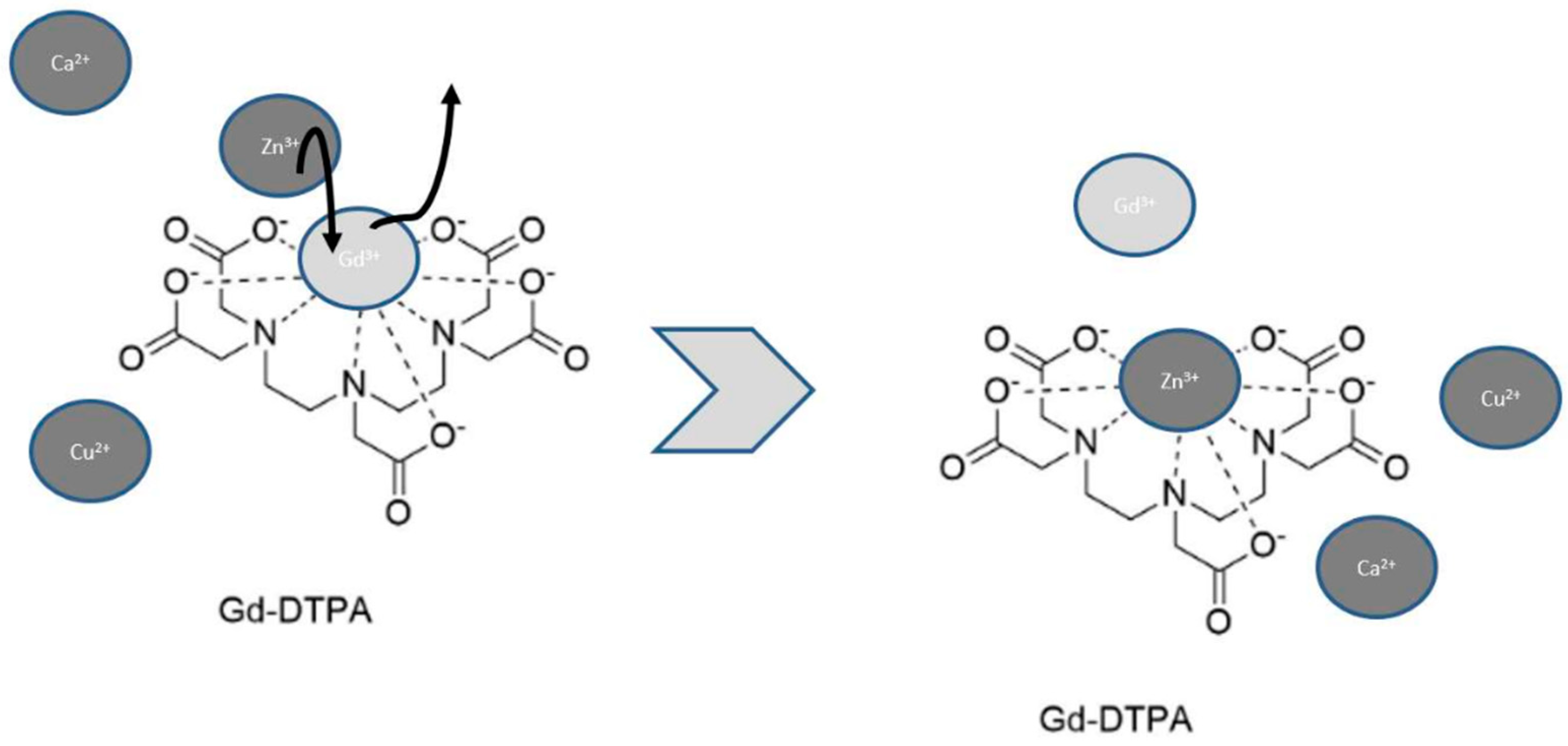
2.1. The Influence of Carrier Molecules on Complex Stability
2.2. The Influence of External Conditions on Complex Stability
3. Pathological Mechanism of Gadolinium Nephrotoxicity
3.1. Physical Features: GBCM Viscosity and Osmolality
3.2. Direct Toxicity of Gd3+
3.3. Comparison with I-CM
4. The Role of Chronic Kidney Disease in Gd3+ Toxicity
Clinical Evidence
5. Recommendations for GBCM Use
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Perazella, M.A.; Rodby, R.A. Gadolinium Use in Patients with Kidney Disease: A Cause for Concern. Semin. Dial. 2007, 20, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Wagner, B.; Drel, V.; Gorin, Y. Pathophysiology of Gadolinium-Associated Systemic Fibrosis. Am. J. Physiol. Ren. Physiol. 2016, 311, F1–F11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lmståhl, B.; Nyman, U.; Leander, P.; Chai, C.M.; Golman, K.; Björk, J.; Almén, T. Gadolinium Contrast Media Are More Nephrotoxic Than Iodine Media. The Importance of Osmolality in Direct Renal Artery Injections. Eur. Radiol. 2006, 16, 2712–2720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deray, G.; Rouviere, O.; Bacigalupo, L.; Maes, B.; Hannedouche, T.; Vrtovsnik, F.; Rigothier, C.; Billiouw, J.M.; Campioni, P.; Ferreiros, J.; et al. Safety of Meglumine Gadoterate (Gd-DOTA)-enhanced MRI Compared to Unenhanced MRI in Patients with Chronic Kidney Disease (RESCUE Study). Eur. Radiol. 2013, 23, 1250–1259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ergün, I.; Keven, K.; Uruc, I.; Ekmekci, Y.; Canbakan, B.; Erden, I.; Karatan, O. The Safety of Gadolinium in Patients with Stage 3 and 4 Renal Failure. Nephrol. Dial. Transplant. 2006, 21, 697–700. [Google Scholar] [CrossRef] [Green Version]
- Haley, T.J.; Raymond, K.; Komesu, N.; Upham, H.C. Toxicological and Pharmacological Effects of Gadolinium and Samarium Chlorides. Br. J. Pharmacol. Chemother. 1961, 17, 526–532. [Google Scholar] [CrossRef] [Green Version]
- Rogosnitzky, M.; Branch, S. Gadolinium-based Contrast Agent Toxicity: A Review of Known and Proposed Mechanisms. Biometals 2016, 29, 365–376. [Google Scholar] [CrossRef] [Green Version]
- Sherry, A.D.; Caravan, P.; Lenkinski, R.E. Primer on Gadolinium Chemistry. J. Magn. Reason. Imaging 2009, 30, 1240–1248. [Google Scholar] [CrossRef] [Green Version]
- Murata, N.; Gonzalez-Cuyar, L.F.; Murata, K.; Fligner, C.; Dills, R.; Hippe, D.; Maravilla, K.R. Macrocyclic and Other Non-Group 1 Gadolinium Contrast Agents Deposit Low Levels of Gadolinium in Brain and Bone Tissue: Preliminary Results From 9 Patients with Normal Renal Function. Investig. Radiol. 2016, 51, 447–453. [Google Scholar] [CrossRef]
- Wáng, Y.X.J.; Schroeder, J.; Siegmund, H.; Idée, J.M.; Fretellier, N.; Jestin-Mayer, G.; Factor, C.; Deng, M.; Kang, W.; Morcos, S.K. Total Gadolinium Tissue Deposition and Skin Structural Findings Following the Administration of Structurally Different Gadolinium Chelates in Healthy and Ovariectomized Female Rats. Quant. Imaging Med. Surg. 2015, 5, 534–545. [Google Scholar]
- Darrah, T.H.; Prutsman-Pfeiffer, J.J.; Poreda, R.J.; Campbell, M.E.; Hauschka, V.E.; Hannigan, R.E. Incorporation of Excess Gadolinium Into Human Bone From Medical Contrast Agents. Metallomics 2009, 1, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Idée, J.M.; Port, M.; Raynal, I.; Schaefer, M.; Le Greneu Sr Corot, C. Clinical and Biological Consequences of Transmetallation Induced by Contrast Agents for Magnetic Resonance Imaging: A Review. Fundam. Clin. Pharmacol. 2006, 20, 563–576. [Google Scholar] [CrossRef]
- Baranyai, Z.; Brücher, E.; Uggeri, F.; Maiocchi, A.; Tóth, I.; Andrási, M.; Gáspár, A.; Zékány, L.; Aime, S. The Role of Equilibrium and Kinetic Properties in the Dissociation of Gd [DTPA-bis (methylamide)] (Omniscan) at near to Physiological Conditions. Chemistry 2015, 21, 4789–4799. [Google Scholar] [CrossRef] [PubMed]
- Prybylski, J.P.; Jay, M. The Impact of Excess Ligand on the Retention of Nonionic, Linear Gadolinium-Based Contrast Agents in Patients with Various Levels of Renal Dysfunction: A Review and Simulation Analysis. Adv. Chronic Kidney Dis. 2017, 24, 176–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cacheris, W.P.; Quay, S.C.; Rocklage, S.M. The Relationship between Thermodynamics and the Toxicity of Gadolinium Complexes. Magn. Reason. Imaging 1990, 8, 467–481. [Google Scholar] [CrossRef]
- Takahashi, E.A.; Kallmes, D.F.; Mara, K.C.; Harmsen, W.S.; Misra, S. Nephrotoxicity of Gadolinium-Based Contrast in the Setting of Renal Artery Intervention: Retrospective Analysis With 10-year Follow-Up. Diagn. Interv. Radiol. 2018, 24, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Sambol, E.B.; Van der Meer, J.G.; Graham, A.; Goldstein, L.J.; Karwowski, J.K.; Dayal, R.; DeRubertis, B.; Kent, K.C. The Use of Gadolinium for Arterial Interventions. Ann. Vasc. Surg. 2011, 25, 366–376. [Google Scholar] [CrossRef]
- Hogstrom, B.; Ikei, N. Physicochemical Properties of Radiographic Contrast Media, Potential Nephrotoxicity, and Prophylaxis. Clin. Exp. Pharmacol. Physiol. 2015, 42, 1251–1257. [Google Scholar] [CrossRef] [Green Version]
- Bucher, A.M.; De Cecco, C.N.; Schoepf, U.J.; Meinel, F.G.; Krazinski, A.W.; Spearman, J.V.; McQuiston, A.D.; Wang, R.; Bucher, J.; Vogl, T.J.; et al. Is Contrast Medium Osmolality a Causal Factor for Contrast-Induced Nephropathy? Biomed. Res. Int. 2014, 2014. [Google Scholar] [CrossRef]
- Elmståhl, B.; Leander, P.; Grant, D.; Doughty, R.W.; Chai, C.M.; Björk, J.; Almén, T.; Nyman, U. Histomorphological Changes After Renal X-ray Arteriography Using Iodine and Gadolinium Contrast Media in an Ischemic Porcine Model. Acta Radiol. 2007, 48, 1109–1119. [Google Scholar] [CrossRef]
- Seeliger, E.; Lenhard, D.C.; Persson, P.B. Contrast Media Viscosity Versus Osmolality in Kidney Injury: Lessons from Animal Studies. Biomed. Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasouli, M. Basic Concepts and Practical Equations on Osmolality: Biochemical Approach. Clin. Biochem. 2016, 49, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Késmárky, G.; Kenyeres, P.; Rábai, M.; Tóth, K. Plasma Viscosity: A Forgotten Variable. Clin. Hemorheol. Microcirc. 2008, 39, 243–246. [Google Scholar]
- Persson, P.B.; Hansell, P.; Liss, P. Pathophysiology of Contrast Medium-Induced Nephropathy. Kidney Int. 2005, 68, 14–22. [Google Scholar] [CrossRef] [Green Version]
- Persson, P.B.; Patzak, A. Renal Haemodynamic Alterations in Contrast Medium-Induced Nephropathy and the Benefit of Hydration. Nephrol. Dial. Transplant. 2005, 20 (Suppl. 1), 2–5. [Google Scholar] [CrossRef] [Green Version]
- Bartolini, M.E.; Pekar, J.; Chettle, D.R.; McNeill, F.; Scott, A.; Sykes, J.; Prato, F.S.; Moran, G.R. An Investigation of the Toxicity of Gadolinium Based MRI Contrast Agents Using Neutron Activation Analysis. Magn. Reson. Imaging 2003, 21, 541–544. [Google Scholar] [CrossRef]
- Kay, J.; Bazari, H.; Avery, L.L.; Koreishi, A.F. Case Records of the Massachusetts General Hospital. Case 6–2008. A 46-year-old Woman with Renal Failure and Stiffness of the Joints and Skin. N. Engl. J. Med. 2008, 358, 827–838. [Google Scholar] [CrossRef]
- Ward, D.B.; Valentovic, M.A. Contrast Induced Acute Kidney Injury and Direct Cytotoxicity of Iodinated Rediocontrast Media on Renal Proximal Tubule Cells. J. Pharmacol. Exp. Ther. 2019, 370, 160–171. [Google Scholar] [CrossRef]
- Leander, P.; Allard, M.; Caille, J.M.; Golman, K. Early Effect of Gadopentetate and Iodinated Contrast Media on Rabbit Kidneys. Investig. Radiol. 1992, 27, 922–926. [Google Scholar] [CrossRef]
- Chien, C.C.; Sheu, M.J.; Chu, C.C.; Sun, Y.M.; Kan, W.C.; Wang, H.Y.; Hwang, J.C.; Wang, J.J. Prophylactic 0.9% Saline Hydration Inhibited High-Dose Gadodiamide-Induced Nephropathy in Rats. Hum. Exp. Toxicol. 2012, 31, 1170–1178. [Google Scholar] [CrossRef]
- Brillet, G.; Dubois, M.; Beaufils, H.; Bourbouze, R.; Deray, G. Renal Tolerance of gadolinium-DOTA and gadolinium-DTPA in Rats. Investig. Radiol. 1994, 29, 352–354. [Google Scholar] [CrossRef]
- Barbosa Pereira, L.V.; Massola Shimizu, M.H.; Ruiz Rodrigues, L.P.M.; Costa Leite, C.; Andrade, L.; Seguro, A.C. N-acetylcysteine Protects Rats With Chronic Renal Failure From Gadolinium-Chelate Nephrotoxicity. PLoS ONE 2012, 7, e39528. [Google Scholar]
- Elmståhl, B.; Nyman, U.; Leander, P.; Chai, C.-M.; Frennby, B.; Almén, T. Gadolinium Contrast Media Are More Nephrotoxic Than a Low Osmolar Iodine Medium Employing Doses with Equal X-ray Attenuation in Renal Arteriography: An Experimental Study in Pigs. Acad. Radiol. 2004, 11, 1219–1228. [Google Scholar] [CrossRef] [Green Version]
- Elmståhl, B.; Leander, P.; Nyman, U.; Chai, C.M.; Almén, T.; Frennby, B. Nephrotoxicity after renal angiography using iodine and gadolinium contrast media in pigs with renal damage. Acad. Radiol. 2002, 9 (Suppl. 2), S531–S534. [Google Scholar] [CrossRef]
- Kwak, H.S.; Lee, Y.H.; Han, Y.M.; Jin, G.Y.; Kim, W.; Chung, G.H. Comparison of renal damage by iodinated contrast or gadolinium in an acute renal failure rat model based on serum creatinine levels and apoptosis degree. J. Korean Med. Sci. 2005, 20, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Safi, W.; Rauscher, I.; Umgelter, A. Contrast-induced acute kidney injury in cirrhotic patients. A retrospective analysis. Ann. Hepatol. 2015, 14, 895–901. [Google Scholar] [CrossRef]
- Briguori, C.; Colombo, A.; Airoldi, F.; Melzi, G.; Michev, I.; Carlino, M.; Montorfano, M.; Chieffo, A.; Bellanca, R.; Ricciardelli, B. Gadolinium-based contrast agents and nephrotoxicity in patients undergoing coronary artery procedures. Catheter. Cardiovasc. Interv. 2006, 67, 175–180. [Google Scholar] [CrossRef]
- Sam, A.D.; Morasch, M.D.; Collins, J.; Song, G.; Chen, R.; Pereles, F.S. Safety of gadolinium contrast angiography in patients with chronic renal insufficiency. J. Vasc. Surg. 2003, 38, 313–318. [Google Scholar] [CrossRef] [Green Version]
- Rieger, J.; Sitter, T.; Toepfer, M.; Linsenmaier, U.; Pfeifer, K.J.; Schiffl, H. Gadolinium as an alternative contrast agent for diagnostic and interventional angiographic procedures in patients with impaired renal function. Nephrol. Dial. Transplant. 2002, 17, 824–828. [Google Scholar] [CrossRef]
- Naito, S.; Tazaki, H.; Okamoto, T.; Takeuchi, K.; Kan, S.; Takeuchi, Y.; Kamata, K. Comparison of nephrotoxicity between two gadolinium-contrasts, gadodiamide and gadopentetate in patients with mildly diminished renal failure. J. Toxicol. Sci. 2017, 42, 379–384. [Google Scholar] [CrossRef] [Green Version]
- Spasojević-Dimitrijeva, B.; Kotur-Stevuljević, J.; Đukić, M.; Paripović, D.; Miloševski-Lomić, G.; Spasojević-Kalimanovska, V.; Pavićević, P.; Mitrović, J.; Kostić, M. Serum Neutrophil Gelatinase-Associated Lipocalin and Urinary Kidney Injury Molecule-1 as Potential Biomarkers of Subclinical Nephrotoxicity After Gadolinium-Based and Iodinated-Based Contrast Media Exposure in Pediatric Patients With Normal Kidney Function. Med. Sci. Monit. 2017, 23, 4299–4305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mawad, H.; Laurin, L.P.; Naud, J.F.; Leblond, F.A.; Henley, N.; Vallée, M.; Pichette, V.; Leblanc, M. Changes in Urinary and Serum Levels of Novel Biomarkers after Administration of Gadolinium-based Contrast Agents. Biomark. Insights 2016, 11, 91–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erley, C.M.; Bader, B.D.; Berger, E.D.; Tuncel, N.; Winkler, S.; Tepe, G.; Risler, T.; Duda, S. Gadolinium-based contrast media compared with iodinated media for digital subtraction angiography in azotaemic patients. Nephrol. Dial. Transplant. 2004, 19, 2526–2531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jürgensen, T.; Brossmann, J.; Herrlinger, J.D. Acute renal failure after gadolinium-containing contrast medium in preexisting chronic renal failure stage. Med. Klin. 2007, 102, 480–482. [Google Scholar] [CrossRef] [PubMed]
- Giozzet, M.; Cavagna, E.; De, M.D.; Tarroni, G.; Casol, D.; De, L.S.; Tessarin, C.; De, E.P.V. Gadolinium for DSA in two patients with azotemia: Images of suitable quality and risk of acute renal failure. G. Ital. Nefrol. 2003, 20, 298–301. [Google Scholar] [PubMed]
- Thomsen, H.S. Gadolinium-based contrast media may be nephrotoxic even at approved doses. Eur. Radiol. 2004, 14, 1654–1656. [Google Scholar] [CrossRef] [PubMed]
- Schenker, M.P.; Solomon, J.A.; Roberts, D.A. Gadolinium Arteriography Complicated by Acute Pancreatitis and Acute Renal Failure. J. Vasc. Interv. Radiol. 2001, 12, 393. [Google Scholar] [CrossRef]
- Gemery, J.; Idelson, B.; Reid, S.; Yucel, E.K.; Pagan-Marin, H.; Ali, S.; Casserly, L. Acute renal failure after arteriography with a gadolinium-based contrast agent. AJR Am. J. Roentgenol. 1998, 171, 1277–1278. [Google Scholar] [CrossRef]
- Akgun, H.; Gonlusen, G.; Cartwright, J., Jr.; Suki, W.N.; Truong, L.D. Are Gadolinium-Based Contrast Media Nephrotoxic? A Renal Biopsy Study. Arch. Pathol. Lab. Med. 2006, 130, 1354–1357. [Google Scholar]
- Fujisaki, K.; Ono-Fujisaki, A.; Kura-Nakamura, N.; Komune, N.; Hirakawa, N.; Tsuruya, K.; Komune, S.; Iida, M. Rapid deterioration of renal insufficiency after magnetic resonance imaging with gadolinium-based contrast agent. Clin. Nephrol. 2011, 75, 251–254. [Google Scholar] [CrossRef]
- Badero, O.J.; Schlanger, L.; Rizk, D. Gadolinium nephrotoxicity: Case report of a rare entity and review of the literature. Clin. Nephrol. 2008, 70, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Khairallah, P.; Isakova, T.; Asplin, J.; Hamm, L.; Dobre, M.; Rahman, M.; Sharma, K.; Leonard, M.; Miller, E., III; Jaar, B.; et al. Acid Load and Phosphorus Homeostasis in CKD. Am. J. Kidney Dis. 2017, 70, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Razzaque, M.S. The FGF23-Klotho Axis: Endocrine Regulation of Phosphate Homeostasis. Nat. Rev. Endocrinol. 2009, 5, 611–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirano, S.; Suzuki, K.T. Exposure, metabolism, and toxicity of rare earths and related compounds. Environ. Health Perspect. 1996, 104 (Suppl. 1), 85–95. [Google Scholar]
- Li, N.; Wang, S.; Liu, J.; Ma, L.; Duan, Y.; Hong, F.L. The oxidative damage in lung of mice caused by lanthanoide. Biol. Trace Elem. Res. 2010, 134, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Lauber, C.; Bossaller, L.; Abujudeh, H.H.; Vladimer, G.I.; Christ, A.; Fitzgerald, K.A.; Latz, E.; Gravallese, E.M.; Marshak-Rothstein, A.; Kay, J. Gadolinium-based Compounds Induce NLRP3-dependent IL-1β Production and Peritoneal Inflammation. Ann. Rheum. Dis. 2015, 74, 2062–2069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreucci, M.; Faga, T.; Pisani, A.; Perticone, M.; Michael, A. The ischemic/nephrotoxic Acute Kidney Injury and the Use of Renal Biomarkers in Clinical Practice. Eur. J. Intern. Med. 2017, 39, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanmassenhove, J.; Van Biesen, W.; Vanholder, R.; Lameire, N. Subclinical AKI: Ready for Primetime in Clinical Practice? J. Nephrol. 2019, 32, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Gadolinium-Based Constrast Agents in Kidney Disease. A Comprehensive Review and Clinical Practice Guideline. Canadian Association of Radiology and Supported by the Canadian Society of Nephrology. Available online: https://car.ca/wp-content/uploads/CAR-NSF-Guidelines-2017-EN.pdf (accessed on 17 December 2020).
- Guidance on Gadolinium-Based Contrast Agent Administration to Adult Patients. The Royal College of Radiologist April 2019. Available online: https://www.rcr.ac.uk/system/files/publication/field_publication_files/bfcr193-gadolinium-based-contrast-agent-adult-patients.pdf (accessed on 17 December 2020).
- Joffe, P.; Thomsen, H.S.; Meusel, M. Pharmacokinetics of Gadodiamide Injection in Patients with Severe Renal Insufficiency and Patients Undergoing Hemodialysis or Continuous Ambulatory Peritoneal Dialysis. Acad. Radiol. 1998, 5, 491–502. [Google Scholar] [CrossRef]
- Gheuens, E.; Daelemans, R.; Mesens, S. Dialysability of Gadoteric Acid in Patients with End-Stage Renal Disease Undergoing Hemodialysis. Investig. Radiol. 2014, 49, 505–508. [Google Scholar] [CrossRef]
- Murashima, M.; Drott, H.R.; Carlow, D.; Shaw, L.M.; Milone, M.; Bachman, M.; Tsai, D.E.; Yang, S.L.; Bloom, R.D. Removal of Gadolinium by Peritoneal Dialysis. Clin. Nephrol. 2008, 69, 368–372. [Google Scholar] [CrossRef] [PubMed]


| GBCM | Chelant Structure | Charge | Viscosity (mPa’s) at 37 °C | Osmolality (mOsm/kg) at 37 °C | Conditional Stability (logKcond) | Excess Ligand (mmol/L) | Renal Excretion (T1/2 in Hours) | EMA Recommendation |
|---|---|---|---|---|---|---|---|---|
| Gadopentetate (Magnevist) | linear | ionic | 2.9 | 1960 | 18.4 | 1 | 1.6 | Suspended use in EU |
| Gadopentetate (Magnevision) | linear | ionic | 2.9 | 1960 | not available | not available | not available | Suspended use in EU |
| Gadodiamide (Omniscan) | linear | non-ionic | 1.4 | 789 | 14.9 | 25 | 1.3 | Suspended use in EU |
| Gadoxetate (primovist) | linear | ionic | 1.4 | 688 | 18.7 | 1.3 | 1.6 | Maintained use in EU |
| Gadoteridol (Prohance) | macrocyclic | non-ionic | 1.3 | 630 | 17.1 | 0.5 | 1.6 | Maintained use in EU |
| Gadobenate dimeglumine (Multihance) | linear | ionic | 5.3 | 1970 | 18.4 | 0 | 1.2–2 | Restrict use in liver scan |
| Gadoversetamide (OptiMark) | linear | non-ionic | 2.0 | 1110 | 15.0 | 50 | 1.7 | Suspended use in EU |
| Gadoterate meglumine (Doratem) | macrocyclic | ionic | 2.0 | 1350 | 19 | 0 | 1.6 | Maintained use in EU |
| Gadobytrol (Gadovist) | macrocyclic | non-ionic | 4.9 | 1603 | 14.8 | 1 | 1.5 | Maintained use in EU |
| Gadoterate (Clariscan) | macrocyclic | ionic | 2.1 | 1350 | not available | not available | 1.6 | Maintained use in EU |
| Gadoterate (Dotagraf) | macrocyclic | ionic | 1.8 | 1350 | not available | not available | 1.6 | Maintained use in EU |
| Gadobutrol (Gadovist) | macrocyclic | non-ionic | 4.9 | 1603 | 14.8 | 1 | 1.5 | Maintained use in EU |
| Report | Study Design | Aim | Number of Subjects | GFR (mL/min) | GBCM | Dose of Gd (mmol/kg) | Results |
|---|---|---|---|---|---|---|---|
| Preclinical studies | |||||||
| Leader et al. [29] | Experimental animal model | Evaluation of nephrotoxicity in a rabbit model | 31 | Not reported | Gadopentetate (L, I) | Not reported | Brushborder enzyme (LAP, ALP, and GGT) and lysosomal enzyme of tubular cell increase after GBCM intravenous administration. |
| Chien et al. [30] | Experimental animal model | Evaluation con 0.9% saline hydration to prevent kidney failure in a rat model | 12 | Cr-Cl 2.5 | Gadodiamide (L, non-I) | 5 | High doses of GBCM impact kidney function (reduction in Cr-Cl 40%) and lead to vacuolization of proximal tubules. Hydration limits the nephrotoxicity. |
| Brillet et al. [31] | Experimental animal model | Comparison changing in kidney function between GBMC (M and I) and GBCM (L and I) | GBMC (M, I): 10 GBCM (L, I): 10 | Cr-Cl 1.6 | Gadoterate (M, I) Gadopentetate (L, I) | Not reported | There is no change in S-Cr with GBCM (M and L); conversely, there is a significant change in S-Cr with GBCM (L and I). |
| Barbosa Pereira et al. [32] | Experimental animal model | Evaluation of nephrotoxicity in a rat model and acetylcysteine nephron protection | 31 | 16 normal kidney function and 13 with kidney impairment | Gadoterate meglumine (M, I) | Not applicable | In kidney impairment rats, GBCM shows a reduction of GFR. Acetylcysteine seems to reduce nephrotoxicity. |
| Elmstahl et al. [33] | Experimental animal model | Comparison between GBCM and I-CM group versus control group, intraarterial route | Case group 40 Control group 24 | Decutered by nephrectomy | Gadopentetate, (L, I) gadodiamide (L, nonI) | Not applicable | GBCMs are more nephrotoxic than I-CM. |
| Elmstahl et al. [34] | Experimental animal model | Comparison between GBCM and I-CM, intraarterial route | 64 | Kidney impaired (Partial nephrectomy) | Gadopentetate (L, I) Gadodiamide (L, non-I) | 3 mL/kg | GBCMs induce more kidney damage than I-CM. |
| Elmstahl et al. [20] | Experimental animal model | Kidney biopsy description | 152 | Gadopentetate (L, I), Gadobutrol (M, non-I) Gadodiamide (L, non-I) | Necrosis of proximal tubules and glomerulus Hemorrhage and congestion of the cortex, medulla, and glomerulus Vacuolation of proximal tubules Protein-filled tubules in the cortex and medulla | ||
| Kwak et al. [35] | Experimental animal model | Comparison of apoptosis in medulla and cortex between the control, GBCM group, and I-CM. | Control:3 GBCM: 9 I-CM: 9 | Not reported | Gadopentetate (L, I) | Not reported | No difference in S-Cr between GBCM and I-CM and increase in apoptosis between the control and GBCM |
| Clinical studies | |||||||
| Safi et al. [36] | Retrospective series | Comparison in the AKI rate between GBCM and I-CM in cirrhotic patients | GBCM: 68 I-CM:84 | S-Cr 0.88 | Gadobytrol (M, non-I) | Not reported | The rate of AKI (defined as an increase of S-Cr of 0.5 mg/dL) is 17.9% in I-CM and 5.9% in GBCM. |
| Sambol et al. [17] | Retrospettive series | Comparison between the GBCM group and GBCM + I-CM group | 153 GBCM group 59 | 43.3 | Gadodiamide (L, non-I) | Non reported | Rate of AKI (defined as an increase of S-Cr >0.5 mg/dL within 48 h) is 25% in the GBCM group. |
| Takahashi et al. [16] | Retrospective series | Incidence of AKI after endovascular intervention with GBCM | 68 | With AKI 18.2 No Aki 25 | Gadodiamide (L, non-I) Gadoteridol (M, non-I) | Not reported | The rate of AKI within 48 h is 14.78%. Pre-hydration limits AKI incidence. |
| Ergun e al. [5] | Retrospective series | Evaluation of CIN after GBCM | 91 | 33 | Gadopentetate (L, I), Gadodiamide (L, non-I), or Gadoterate (M, I) | 0.2 | 12% of patients had S-Cr increase (≥0.5 mg/dL) |
| Briguori et al. [37] | Retrospective series | Comparison after coronary arterial procedure between GBCM and I-CM | GBCM:32 I-CM: 32 | Cr-Cl GBCM 33 I-CM 30 | Gadobutrol (M, non-I) or Gadodiamide (L-non-I) | <0.4 | In the GBCM group, 28% of patients had S-Cr increase (≥0.5 mg/dL), while in the I-CM group, only 6.5% had S-Cr increase (≥0.5 mg/dL). |
| Sam et al. [38] | Retrospective series | Evaluation of CIN after GBCM | 195 | 38 | Gadopentetate (L, I) | 0.28 | 3.5% of patients had S-Cr increase (>1 mg/dL). |
| Rieger et al. [39] | Prospective series | Evaluation of CIN after GBCM | 29 | 23 | Gadopentetate (L, I) | 0.34 | 6.7% of patients had S-Cr increase (≥0.5 mg/dL). |
| Naito et al. [40] | Randomized trial | Comparison CIN between non-I and I GBCM | 102 | I: 94.1 Non-I: 90.5 | Gadopentetate (L, I) Gadodiamide (L, non-I) | Not reported | Significant S-Cystatin C increase in non-I GBCM |
| Spasojevic-Dimitrijeva et al. [41] | Prospective series | Comparison kidney damage between I-CM and GBCM | 123 | 133 | Gadopentetate (L, I) | 0.20 | Significant S-Cr and u-KIM1 increase after 24 h |
| Mawad et al. [42] | prospective series | Evaluation of urinary marker of kidney damage (IL-18, NAG, and NGAL) | 28 | >60 mL/min | Not reported | Not reported | Significant IL-18 and NAG increase, and no increase in NGAL |
| Erley et al. [43] | Randomized trial | Comparison of CIN between I-CM and GBCM | 21 | 31 | Gadobutrol | 0.57 | 50% of patients had over a 1.5-fold increase in basal S-Cr |
| Jurgensen et al. [44] | Case report | Description | 1 | 55 | Gadoteridol | Not reported | Cr-Cl descreased (<20 mL/min) within 10 days. |
| Giozzet et al. [45] | Case report | Description | 2 | 80 years: 40 84 years: 23 | 0.6 0.9 | The need for dialysis, and partial recovery of kidney function | |
| Thomsen et al. [46] | Case report | Description | 1 | 20 | Gadodiamide (L, non-I) | 0.14 | Need for dialysis |
| Schenker et al. [47] | Case report | Description | 1 | 15 | Gadodiamide (L, non-I) | Not reported | AKI |
| Gemery et al. [48] | Case report | Description | 1 | 13 | Gadoteridol (M, non-I) | 0.44 | S-Cr incresed to 9.3 mg/dL. |
| Akgun et al. [49] | Case report | Biopsy on human | 1 | 1 | Gadopentetate (L, I) + Gadodiamide | 0.1 + 0.19 | S-Cr increased to 3.4 mg/dL. Tubular cell necrosis, tubular cell degeneration, and marked proliferation of tubular cells together with mild interstitial edema and interstitial inflammation |
| Fujisaki et al. [50] | Case report | Description | 1 | 20 | Gadopentetate (L, I) | 0.2 | Need for dialysis |
| Badero et al. [51] | Case report | Description | 1 | 38 | 2 times Gadobenate (L, I) in 24 ore | Not reported. Total volume of 98 cc | S-Cr increased to 7.4 mg/dL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martino, F.; Amici, G.; Rosner, M.; Ronco, C.; Novara, G. Gadolinium-Based Contrast Media Nephrotoxicity in Kidney Impairment: The Physio-Pathological Conditions for the Perfect Murder. J. Clin. Med. 2021, 10, 271. https://doi.org/10.3390/jcm10020271
Martino F, Amici G, Rosner M, Ronco C, Novara G. Gadolinium-Based Contrast Media Nephrotoxicity in Kidney Impairment: The Physio-Pathological Conditions for the Perfect Murder. Journal of Clinical Medicine. 2021; 10(2):271. https://doi.org/10.3390/jcm10020271
Chicago/Turabian StyleMartino, Francesca, Gianpaolo Amici, Mitchell Rosner, Claudio Ronco, and Giacomo Novara. 2021. "Gadolinium-Based Contrast Media Nephrotoxicity in Kidney Impairment: The Physio-Pathological Conditions for the Perfect Murder" Journal of Clinical Medicine 10, no. 2: 271. https://doi.org/10.3390/jcm10020271





