Prolonged Carriage of Carbapenemase-Producing Enterobacteriaceae: Clinical Risk Factors and the Influence of Carbapenemase and Organism Types
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Definitions and Clinical Data
2.3. Microbiologic Methods
2.4. Statistical Analysis
3. Results
3.1. Study Patients and Identified Isolates
3.2. Probability of Prolonged Carriage by Different Types of Carbapenemase and Organism
3.3. Risk Factor Analysis for Prolonged Carriage of CPE
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van Duin, D.; Doi, Y. The global epidemiology of carbapenemase-producing Enterobacteriaceae. Virulence 2017, 8, 460–469. [Google Scholar] [CrossRef]
- French, C.E.; Coope, C.; Conway, L.; Higgins, J.P.; McCulloch, J.; Okoli, G.; Patel, B.C.; Oliver, I. Control of carbapenemase-producing Enterobacteriaceae outbreaks in acute settings: An evidence review. J. Hosp. Infect. 2017, 95, 3–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, A.M.; Mathema, B.; Larson, E.L. Carbapenem-resistant Enterobacteriaceae in the community: A scoping review. Int. J. Antimicrob. Agents 2017, 50, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Tischendorf, J.; de Avila, R.A.; Safdar, N. Risk of infection following colonization with carbapenem-resistant Enterobacteriaceae. A systemic review. Am. J. Infect. Control 2016, 44, 539–543. [Google Scholar] [CrossRef] [Green Version]
- Soontaros, S.; Leelakanok, N. Association between carbapenem-resistant Enterobacteriaceae and death: A systemic review. Am. J. Infect. Control 2019, 47, 1200–1212. [Google Scholar] [CrossRef]
- Gagliotti, C.; Ciccarese, V.; Sarti, M.; Giordani, S.; Barozzi, A.; Braglia, C.; Gallerani, C.; Gargiulo, R.; Lenzotti, G.; Manzi, O.; et al. Active surveillance for asymptomatic carriers of carbapenemase-producing Klebsiella pneumoniae in a hospital setting. J. Hosp. Infect. 2013, 83, 330–332. [Google Scholar] [CrossRef]
- Snitkin, E.S.; Zelazny, A.M.; Thomas, P.J.; Stock, F.; NISC Comparative Sequencing Program Group; Henderson, D.K.; Palmore, T.N.; Segre, J.A. Tracking a hospital outbreak of carbapenemase-resistant Klebsiella pneumoniae with whole-genome sequencing. Sci. Transl. Med. 2012, 4, 148ra116. [Google Scholar] [CrossRef] [Green Version]
- Tamma, P.D.; Goodman, K.E.; Harris, A.D.; Tekle, T.; Roberts, A.; Taiwo, A.; Simner, P.J. Comparing the outcomes of patients with carbapenemase-producing and non-carbapenemase- producing carbapenem-resistant Enterobacteriaceae bacteremia. Clin. Infect. Dis. 2017, 64, 257–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamma, P.D.; Kazmi, A.; Bergman, Y.; Goodman, K.E.; Ekunseitan, E.; Amoah, J.; Simner, P.J. The likelihood of developing a carbapenem-resistant Enterobacteriaceae infection during a hospital stay. Antimicrob. Agents Chemother. 2019, 63, e00757-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munoz-Price, L.S.; Quinn, J.P. Deconstructing the infection control bundles for the containment of carbapenem-resistant Enterobacteriaceae. Curr. Opin. Infect. Dis. 2013, 26, 378–387. [Google Scholar] [CrossRef]
- Friedman, N.D.; Carmeli, Y.; Walton, A.L.; Schwaber, M.J. Carbapenem-resistant Enterobacteriaceae: A strategic roadmap for infection control. Infect. Control Hosp. Epidemiol. 2017, 38, 580–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiotos, K.; Rock, C.; Schweizer, M.L.; Deloney, V.M.; Morgan, D.J.; Milstone, A.M.; Henderson, D.K.; Harris, A.D.; Han, J.H. Current infection prevention and antibiotic stewardship program practices: A survey of the Society for Healthcare Epidemiology of America (SHEA) Research Network (SRN). Infect. Control Hosp. Epidemiol. 2019, 40, 1046–1049. [Google Scholar] [CrossRef] [PubMed]
- Martischang, R.; Buetti, N.; Balmeli, C.; Saam, M.; Widmer, A.; Harbarth, S. Nation-wide survey of screening practices to detect carriers of multi-drug resistant organisms upon admission to Swiss healthcare institutions. Antimicrob. Resist. Infect. Control 2019, 8, 37. [Google Scholar] [CrossRef] [PubMed]
- Goodman, K.E.; Simner, P.J.; Klein, E.Y.; Kazmi, A.Q.; Gadala, A.; Toerper, M.F.; Levin, S.; Tamma, P.D.; Rock, C.; Cosgrove, S.E.; et al. Predicting probability of perirectal colonization with carbapenem-resistant Enterobacteriaceae (CRE) and other carbapenem-resistant organisms (CROs) at hospital admission. Infect. Control Hosp. Epidemiol. 2019, 40, 541–550. [Google Scholar] [CrossRef]
- Otter, J.A.; Burgess, P.; Davies, F.; Mookerjee, S.; Singleton, J.; Gilchrist, M.; Parsons, D.; Brannigan, E.T.; Robotham, J.; Holmes, A.H. Counting the cost of an outbreak of carbapenemase-producing Enterobacteriaceae: An economic evaluation from a hospital perspective. Clin. Microbiol. Infect. 2017, 3, 188–196. [Google Scholar] [CrossRef] [Green Version]
- Mouloudi, E.; Protonotariou, E.; Zagorianou, A.; Iosifidis, E.; Karapanagiotou, A.; Giasnetsova, T.; Tsioka, A.; Roilides, E.; Sofianou, D.; Gritsi-Gerogianni, N. Bloodstream infections caused by metallo-β-lactamase/Klebsiella pneumoniae carbapenemase-producing K. pneumoniae among intensive care unit patients in Greece: Risk factors for infection and impact of type of resistance on outcomes. Infect. Control Hosp. Epidemiol. 2010, 12, 1250–1256. [Google Scholar] [CrossRef]
- Park, J.W.; Kwak, S.H.; Jung, J.; Lee, J.Y.; Lim, Y.J.; Choi, H.S.; Hong, M.J.; Choi, S.H.; Kim, M.N.; Kim, S.H. The rate of acquisition of carbapenemase-producing Enterobacteriaceae among close contact patients depending on carbapenemase enzymes. Infect. Chemother. 2020, 52, 39–47. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.K.; Song, S.A.; Lee, J.N.; Oh, M.; Jo, K.M.; Kim, H.J.; Lee, J.H.; Park, J.; Jang, H.J.; Kim, H.K.; et al. Clinical factors predicting persistent carriage of Klebsiella pneumoniae carbapenemase-producing carbapenem-resistant Enterobacteriaceae among patients with known carriage. J. Hosp. Infect. 2018, 99, 405–412. [Google Scholar] [CrossRef]
- Clinical & Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, 26th ed.; CLSI Document M100S; Clinical Laboratory Standard Institute: Wayne, PA, USA, 2016. [Google Scholar]
- Song, W.; Hong, S.G.; Yong, D.; Jeong, S.H.; Kim, H.S.; Kim, H.S.; Kim, J.S.; Bae, I.K. Combined use of the modified Hodge test and carbapenemase inhibition test for detection of carbapenemase-producing Enterobacteriaceae and metallo-β-lactamase-producing Pseudomonas spp. Ann. Lab. Med. 2015, 35, 212–219. [Google Scholar] [CrossRef] [Green Version]
- Jeong, S.H.; Kim, H.S.; Kim, J.S.; Shin, D.H.; Kim, H.S.; Park, M.J.; Shin, S.; Hong, J.S.; Lee, S.S.; Song, W. Prevalence and molecular characteristics of carbapenemase-producing Enterobacteriaceae from five hospitals in Korea. Ann. Lab. Med. 2016, 36, 529–535. [Google Scholar] [CrossRef] [Green Version]
- Bar-Yoseph, H.; Hussein, K.; Braun, E.; Paul, M. Natural history and decolonization strategies for ESBL/carbapenem-resistant Enterobacteriaceae carriage: Systematic review and meta-analysis. J. Antimicrob. Chemother. 2016, 71, 2729–2739. [Google Scholar] [CrossRef]
- Lübbert, C.; Lippmann, N.; Busch, T.; Kaisers, U.X.; Ducomble, T.; Eckmanns, T.; Rodloff, A.C. Long-term carriage of Klebsiella pneumoniae carbapenemase-2 producing K. pneumoniae after a large single-center outbreak in Germany. Am. J. Infect. Control 2014, 42, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, F.S.; Assous, M.V.; Bdolah-Abram, T.; Lachish, T.; Yinnon, A.M.; Wiener-Well, Y. Duration of carriage of carbapenem-resistant Enterobacteriaceae (CRE) following hospital discharge. Am. J. Infect. Control 2013, 41, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Schechner, V.; Kotlovsky, T.; Tarabeia, J.; Kazma, M.; Schwartz, D.; Navon-Venezia, S.; Carmeli, Y. Predictors of rectal carriage of carbapenem-resistant Enterobacteriaceae (CRE) among patients with known CRE carriage at their next hospital encounter. Infect. Control Hosp. Epidemiol. 2011, 32, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Feldman, N.; Adler, A.; Molshatzki, N.; Navon-Venezia, S.; Khabra, E.; Cohen, D.; Carmeli, Y. Gastrointestinal colonization by KPC-producing Klebsiella pneumoniae following hospital discharge: Duration of carriage and risk factors for persistent carriage. Clin. Microbiol. Infect. 2013, 19, E190–E196. [Google Scholar] [CrossRef] [Green Version]
- Lim, Y.J.; Park, H.Y.; Lee, J.Y.; Kwak, S.H.; Kim, M.N.; Sung, H.; Kim, S.H.; Choi, S.H. Clearance of carbapenemase-producing Enterobacteriaceae (CPE) carriage: A comparative study of NDM-1 and KPC CPE. Clin. Microbiol. Infect. 2018, 24, 1104.e5–1104.e8. [Google Scholar] [CrossRef] [Green Version]
- Davido, B.; Moussiegt, A.; Dinh, A.; Bouchand, F.; Matt, M.; Senard, O.; Deconinck, L.; Espinasse, F.; Lawrence, C.; Fortineau, N.; et al. Germs of thrones-spontaneous decolonization of carbapenem-resistant Enterobacteriaceae (CRE) and vancomycin-resistant Enterococci (VRE) in western Europe: Is this myth or reality? Antimicrob. Resist. Infect. Control 2018, 7, 100. [Google Scholar] [CrossRef]
- Cheng, V.C.; Chen, J.H.; So, S.Y.; Wong, S.C.; Chau, P.H.; Wong, L.M.; Ching, R.H.; Ng, M.L.; Lee, W.M.; Hung, I.F.; et al. A novel risk factors associated with colonization by carbapenemase-producing Enterobacteriaceae: Use of proton pump inhibitors in addition to antimicrobial treatment. Infect. Control Hosp. Epidemiol. 2016, 37, 1418–1425. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Diseases, Division of Healthcare Quality Promotion. Facility Guidance for Control of Carbapenem-resistant Enterobacteriaceae (CRE). Update—CRE Toolkit; CDC: Atlanta, GA, USA, 2015.
- Liang, Q.; Yan, C.; Xu, Z.; Huang, M. Preemptive isolation and active surveillance in the prevention and control of nosocomial infection reduce the incidence of carbapenem-resistant Enterobacteriaceae. Infect. Dis. 2019, 51, 377–379. [Google Scholar] [CrossRef]
- Rooney, C.M.; Sheppard, A.E.; Clark, E.; Davies, K.; Hubbard, A.T.; Sebra, R.; Crook, D.W.; Walker, A.S.; Wilcox, M.H.; Chilton, C.H. Dissemination of multiple carbapenem resistance genes in an in vitro gut model simulating the human colon. J. Antimicrob. Chemother. 2019, 74, 1876–1883. [Google Scholar] [CrossRef]
- Kunishima, H.; Ishibashi, N.; Wada, K.; Oka, K.; Takahashi, M.; Yamasaki, Y.; Aoyagi, T.; Takemura, H.; Kitagawa, M.; Kaku, M. The effect of gut microbiota and probiotic organisms on the properties of extended spectrum beta-lactamase producing and carbapenem resistant Enterobacteriaceae including growth, beta-lactamase activity and gene transmissibility. J. Infect. Chemother. 2019, 25, 894–900. [Google Scholar] [CrossRef] [PubMed]
- Millan, B.; Park, H.; Hotte, N.; Mathieu, O.; Burguiere, P.; Tompkins, T.A.; Kao, D.; Madsen, K.L. Fecal microbial transplants reduce antibiotic-resistant genes in patients with recurrent Clostridium difficile infection. Clin. Infect. Dis. 2016, 62, 1479–1486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seekatz, A.M.; Bassis, C.M.; Fogg, L.; Moore, N.M.; Rhee, Y.; Lolans, K.; Weinstein, R.A.; Lin, M.Y.; Young, V.B.; Hayden, M.K.; et al. Gut microbiota and clinical features distinguish colonization with Klebsiella pneumoniae carbapenemase-producing Klebsiella pneumoniae at the time of admission to a long-term acute care hospital. Open Forum Infect. Dis. 2018, 5, ofy190. [Google Scholar] [CrossRef] [PubMed]
- Tavoukjian, V. Fecal microbiota transplantation for the decolonization of antibiotic-resistant bacteria in the gut: A systemic review and meta-analysis. J. Hosp. Infect. 2019, 102, 174–188. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Tariq, R.; Tosh, P.K.; Pardi, D.S.; Khanna, S. Fecal microbiota transplantation for eradicating carriage of multidrug-resistant organisms: A systemic review. Clin. Microbiol. Infect. 2019, 25, 958–963. [Google Scholar] [CrossRef]
- Ruppé, E.; Burdet, C.; Grall, N.; de Lastours, V.; Lescure, F.X.; Andremont, A.; Armand-Lefèvre, L. Impact of antibiotics on the intestinal microbiota needs to be re-defined to optimize antibiotic usage. Clin. Microbiol. Infect. 2018, 24, 3–5. [Google Scholar] [CrossRef] [Green Version]
- Woerther, P.L.; Lepeule, R.; Burdet, C.; Decousser, J.W.; Ruppé, É.; Barbier, F. Carbapenems and alternative β-lactams for the treatment of infections due to extended-spectrum β-lactamase-producing Enterobacteriaceae: What impact on intestinal colonisation resistance? Int. J. Antimicrob. Agents 2018, 52, 762–770. [Google Scholar] [CrossRef]
- Shimasaki, T.; Seekatz, A.; Bassis, C.; Rhee, Y.; Yelin, R.D.; Fogg, L.; Dangana, T.; Cisneros, E.C.; Weinstein, R.A.; Okamoto, K.; et al. Increased relative abundance of Klebsiella pneumoniae carbapenemase-producing Klebsiella pneumoniae within the gut microbiota is associated with risk of bloodstream infection in long-term acute care hospital patients. Clin. Infect. Dis. 2019, 68, 2053–2059. [Google Scholar] [CrossRef]
- Chotiprasitsakul, D.; Srichatrapimuk, S.; Kirdlarp, S.; Pyden, A.D.; Santanirand, P. Epidemiology of carbapenem-resistant Enterobacteriaceae: A 5-year experience at a tertiary care hospital. Infect. Drug. Resist. 2019, 12, 461–468. [Google Scholar] [CrossRef] [Green Version]
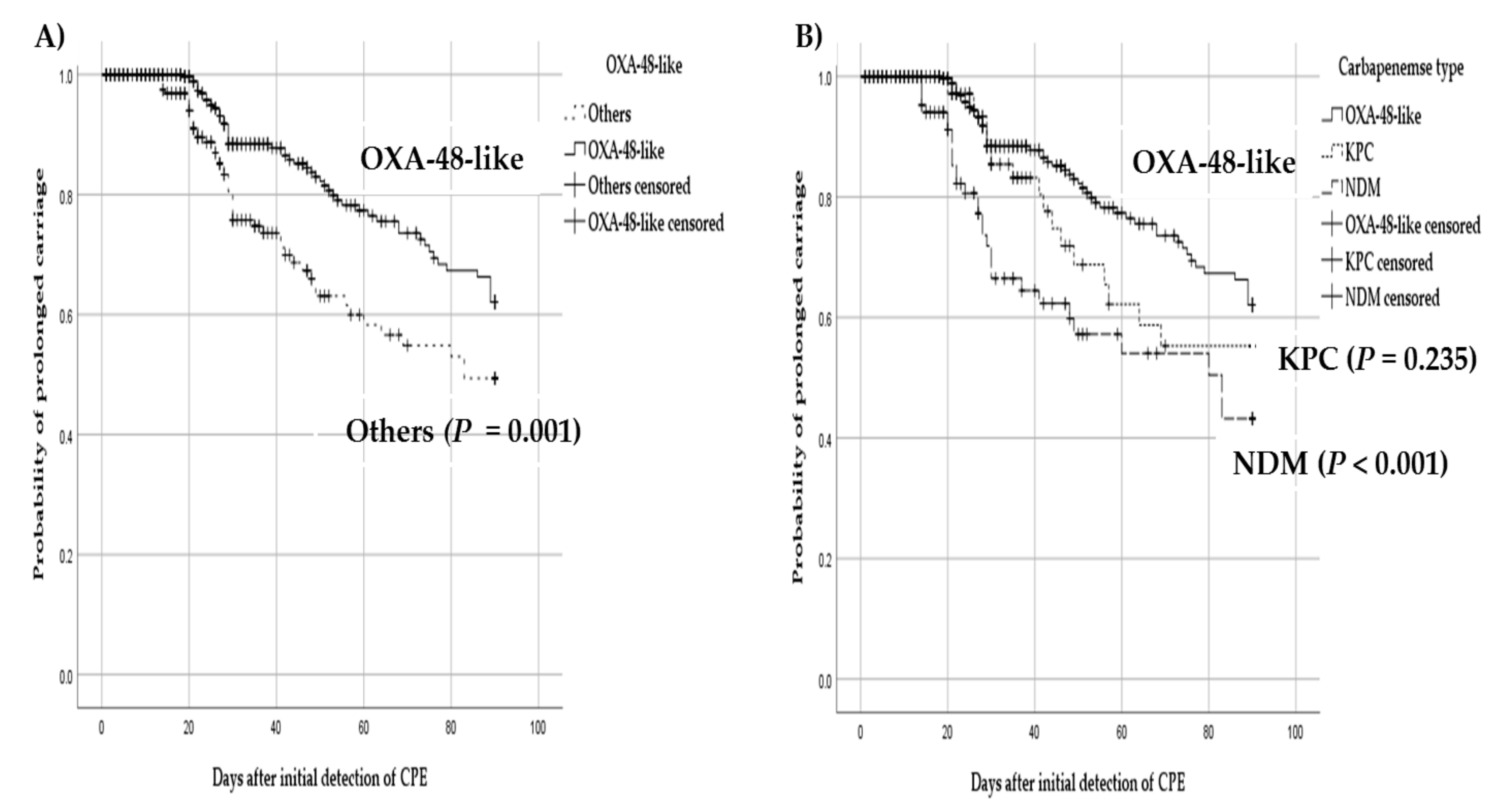

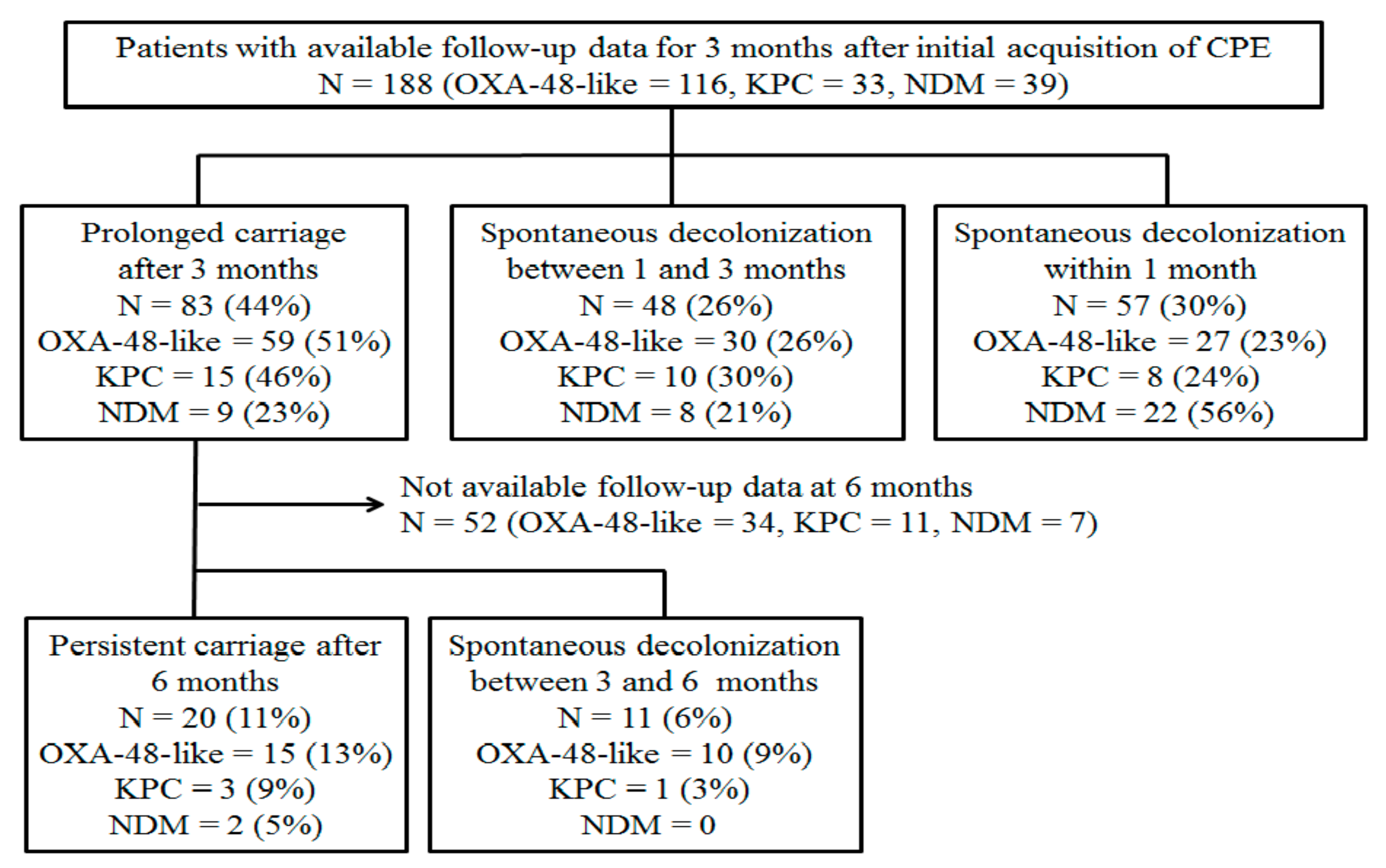
| Variables | Decolonization within 3 Months a (n = 105) | Prolonged Carriage after 3 Months (n = 83) | p-Value |
|---|---|---|---|
| Age in years, median (IQR) | 69 (53–78) | 68 (54–79) | 0.61 |
| Male | 60 (57) | 43 (52) | 0.47 |
| Type of carbapenemase | |||
| OXA-48-like | 57 (54) | 59 (71) | 0.019 |
| NDM | 30 (29) | 9 (11) | 0.003 |
| KPC | 18 (17) | 15 (18) | 0.87 |
| Type of organism | |||
| Polymicrobial carriage b | 10 (10) | 20 (24) | 0.007 |
| K. pneumoniae | 83 (79) | 81 (98) | <0.001 |
| E. coli | 1 (1) | 13 (16) | <0.001 |
| Enterobacter spp. | 21 (20) | 5 (6) | 0.006 |
| C. freundii | 10 (10) | 7 (8) | 0.80 |
| Underlying comorbidity | |||
| Charlson’s score, median (IQR) | 5 (3–7) | 5 (3–7) | 0.69 |
| Immunosuppressive therapy c | 10 (10) | 11 (13) | 0.42 |
| Surgery in the last 3 months | 59 (56) | 39 (47) | 0.21 |
| Catheter present d | 96 (91) | 79 (95) | 0.39 |
| ICU admission e | 66 (63) | 63 (76) | 0.056 |
| VRE colonization | 25 (24) | 35 (42) | 0.007 |
| Clostridioides difficile infection f | 12 (11) | 24 (29) | 0.002 |
| Duration of index hospitalization, median days (IQR) g | 41 (24–60) | 85 (56–119) | <0.001 |
| Re-admission | 34 (32) | 20 (24) | 0.21 |
| Clinical positive culture | 16 (15) | 66 (81) | <0.001 |
| Antibiotics use h | |||
| antipseudomonal penicillin | 52 (50) | 43 (52) | 0.76 |
| cephalosporin | 54 (51) | 48 (58) | 0.38 |
| carbapenem | 39 (37) | 63 (76) | <0.001 |
| fluoroquinolones | 37 (35) | 44 (53) | 0.015 |
| amikacin | 6 (6) | 10 (12) | 0.12 |
| colistin | 20 (19) | 26 (31) | 0.052 |
| tigecycline | 4 (4) | 15 (18) | 0.003 |
| glycopeptide | 52 (50) | 59 (71) | 0.003 |
| In-hospital mortality | 6 (6) | 10 (12) | 0.12 |
| Variables a | Univariable Analysis | Multivariable Analysis | |
|---|---|---|---|
| OR (95% CI) | aOR (95% CI) | p Value | |
| OXA-48-like | 2.07 (1.12–3.81) | 1.14 (0.18–7.18) | 0.69 |
| NDM | 0.30 (0.14–0.68) | ||
| Polymicrobial carriage | 3.02 (1.32–6.87) | ||
| K. pneumoniae | 10.74 (2.45–47.14) | 6.58 (1.05–41.27) | 0.044 |
| E. coli | 19.31 (2.47–150.99) | 13.48 (1.18–153.48) | 0.036 |
| Enterobacter spp. | 0.26 (0.09–0.71) | ||
| ICU admission | 1.86 (0.98–3.53) | ||
| VRE colonization | 2.33 (1.25–4.36) | ||
| Clostridioides difficile infection | 3.15 (1.47–6.78) | 3.98 (1.29–12.26) | 0.016 |
| Duration of index hospitalization, median days (IQR) | 1.02 (1.01–1.03) | 1.01 (1.004–1.03) | 0.008 |
| Clinical positive culture | 21.60 (10.17–45.87) | 11.14 (4.73–26.25) | <0.001 |
| Carbapenem | 5.33 (2.81–10.11) | 2.32 (0.97–5.55) | 0.06 |
| Fluoroquinolones | 2.07 (1.15–3.73) | ||
| Colistin | 1.94 (0.99–3.80) | ||
| Tigecycline | 5.57 (1.77–17.50) | ||
| Glycopeptide | 2.51 (1.36–4.61) | ||
| Variables a | Univariable Analysis | Multivariable Analysis | |
|---|---|---|---|
| OR (95% CI) | aOR (95% CI) | p-Value | |
| E. coli | 15.83 (1.99–125.55) | ||
| Intensive care unit admission | 2.02 (0.91–4.51) | ||
| VRE colonization | 2.32 (1.07–5.01) | ||
| Clostridioides difficile infection | 4.57 (1.56–13.34) | 10.49 (2.01–54.88) | 0.005 |
| Duration of index hospitalization, median days (IQR) | 1.02 (1.01–1.03) | ||
| Clinical positive culture | 18.25 (7.21–46.18) | 13.93 (4.53–42.81) | <0.001 |
| Carbapenem | 10.27 (4.32–24.43) | 8.96 (2.24–35.83) | 0.002 |
| Fluoroquinolones | 2.35 (1.11–4.96) | ||
| Colistin | 2.69 (1.06–6.82) | ||
| Tigecycline | 4.13 (1.09–15.67) | ||
| Glycopeptide | 3.26 (1.49–7.13) | ||
| Variables a | Univariable Analysis | Multivariable Analysis | |
|---|---|---|---|
| OR (95% CI) | aOR (95% CI) | p-Value | |
| Intensive care unit admission | 2.01 (1.03–3.93) | ||
| VRE colonization | 2.61 (1.32–5.18) | ||
| Clostridioides difficile infection | 4.57 (1.84–11.35) | 4.38 (1.37–13.94) | 0.013 |
| Polymicrobial carriage | 4.21 (1.59–11.12) | ||
| Duration of index hospitalization, median days (IQR) | 1.02 (1.01–1.03) | 1.01 (1.00–1.03) | 0.009 |
| Clinical positive culture | 17.01 (7.86–36.83) | 10.40 (4.45–23.34) | <0.001 |
| Carbapenem | 6.08 (3.07–12.04) | 2.95 (1.23–7.06) | 0.015 |
| Fluoroquinolones | 2.00 (1.07–3.74) | ||
| Colistin | 2.40 (1.13–5.12) | ||
| Tigecycline | 6.06 (1.68–21.84) | ||
| Glycopeptide | 2.62 (1.36–5.02) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.K.; Chang, I.B.; Kim, H.S.; Song, W.; Lee, S.S. Prolonged Carriage of Carbapenemase-Producing Enterobacteriaceae: Clinical Risk Factors and the Influence of Carbapenemase and Organism Types. J. Clin. Med. 2021, 10, 310. https://doi.org/10.3390/jcm10020310
Kim YK, Chang IB, Kim HS, Song W, Lee SS. Prolonged Carriage of Carbapenemase-Producing Enterobacteriaceae: Clinical Risk Factors and the Influence of Carbapenemase and Organism Types. Journal of Clinical Medicine. 2021; 10(2):310. https://doi.org/10.3390/jcm10020310
Chicago/Turabian StyleKim, Yong Kyun, In Bok Chang, Han Sung Kim, Wonkeun Song, and Seung Soon Lee. 2021. "Prolonged Carriage of Carbapenemase-Producing Enterobacteriaceae: Clinical Risk Factors and the Influence of Carbapenemase and Organism Types" Journal of Clinical Medicine 10, no. 2: 310. https://doi.org/10.3390/jcm10020310





