Correlation between Speech Perception Outcomes after Cochlear Implantation and Postoperative Acoustic and Electric Hearing Thresholds
Abstract
1. Introduction
2. Experimental Section
2.1. Study Participants
2.2. Audiometric Evaluation
2.3. Electric CI Hearing Thresholds
2.4. Electrocochleography
2.5. Statistical Analysis
3. Results
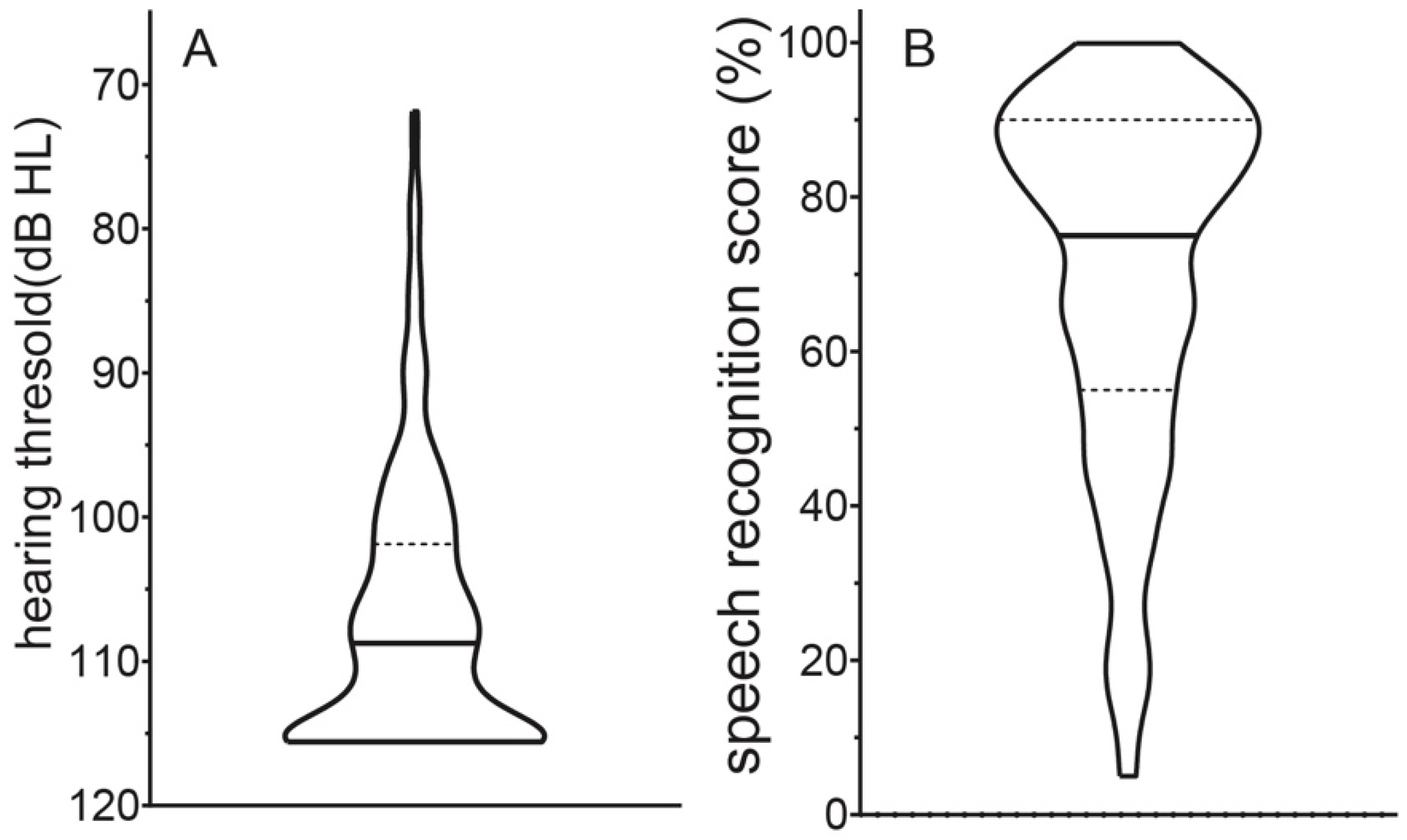
3.1. Audiometric Evaluation
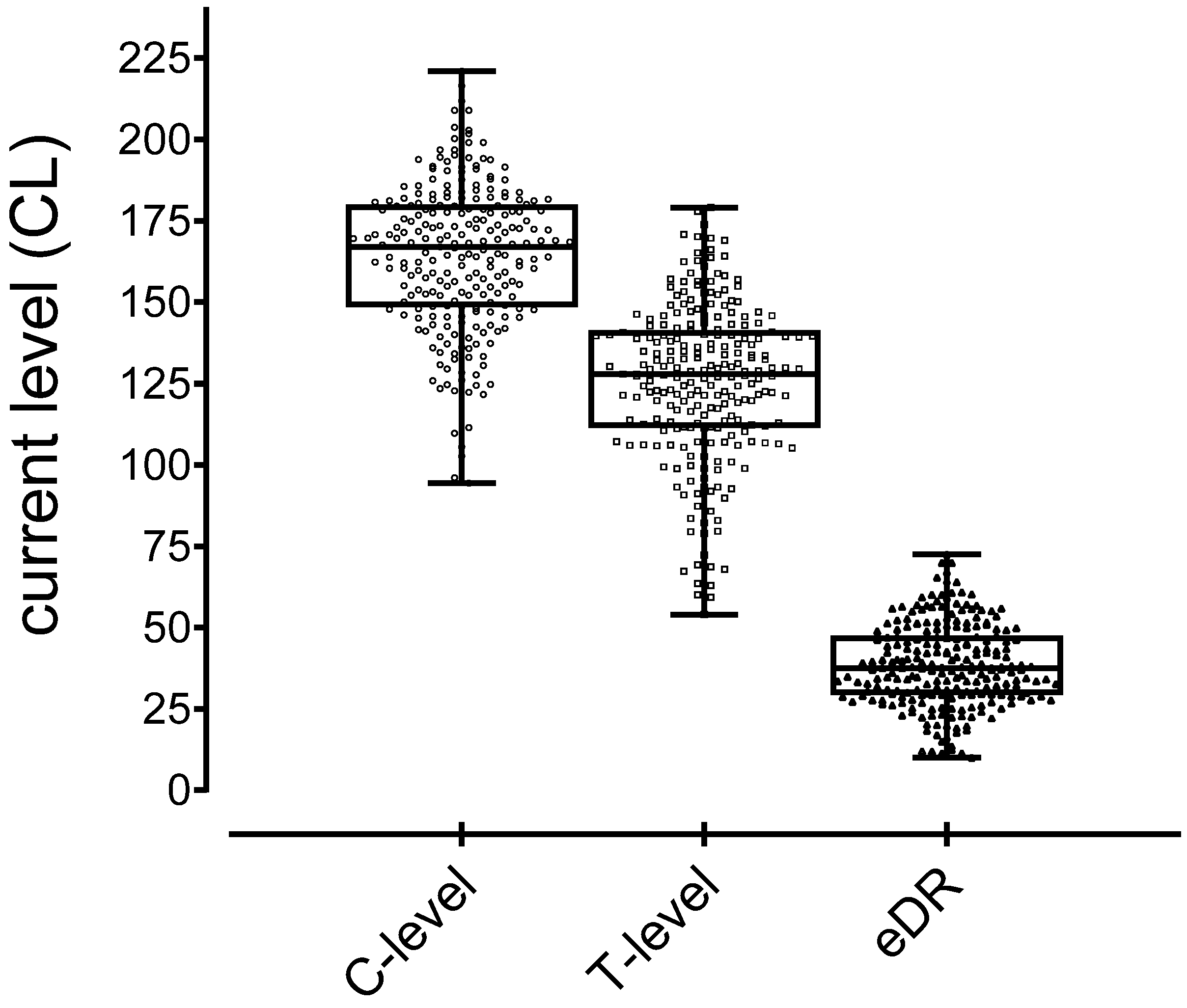
3.2. Electric CI Hearing Thresholds and Electric Dynamic Range
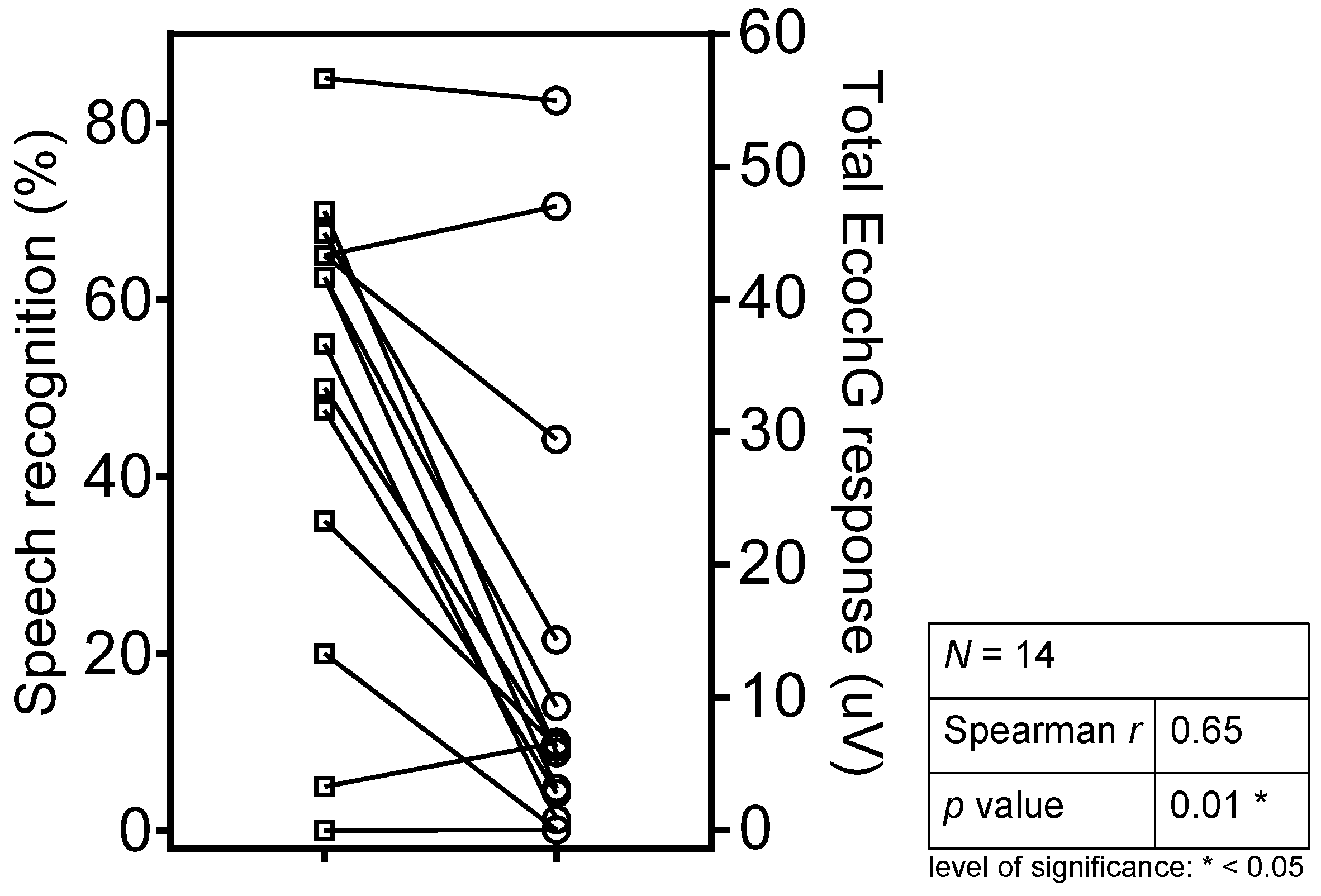
3.3. ECochG Responses and Speech Understanding
3.4. Correlations
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Blamey, P.; Artieres, F.; Baskent, D.; Bergeron, F.; Beynon, A.; Burke, E.; Dillier, N.; Dowell, R.; Fraysse, B.; Gallégo, S.; et al. Factors affecting auditory performance of postlinguistically deaf adults using cochlear implants: An update with 2251 patients. Audiol. Neurootol. 2013, 18, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Holden, L.K.; Finley, C.C.; Firszt, J.B.; Holden, T.A.; Brenner, C.; Potts, L.G.; Gotter, B.D.; Vanderhood, S.S.; Mispagel, K.; Heydebrand, G.; et al. Factors affecting open-set word recognition in adults with cochlear implants. Ear Hear. 2013, 34, 342–360. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, U.; Hocke, T.; Hast, A.; Iro, H. Maximum preimplantation monosyllabic score as predictor of cochlear implant outcome. HNO 2019, 67, 62–68. [Google Scholar] [CrossRef] [PubMed]
- James, C.J.; Karoui, C.; Laborde, M.L.; Lepage, B.; Molinier, C.E.; Tartayre, M.; Bernard, E.; Deguine, O.; Marx, M.; Fraysse, B.; et al. Early Sentence Recognition in Adult Cochlear Implant Users. Ear Hear. 2019, 40, 905–917. [Google Scholar] [CrossRef] [PubMed]
- Lazard, D.S.; Vincent, C.; Venail, F.; Heyning, P.; Truy, E.; Sterkers, O.; Skarzynski, P.; Skarzynski, H.; Schauwers, K.; Leary, S.; et al. Pre-, per- and postoperative factors affecting performance of postlinguistically deaf adults using cochlear implants: A new conceptual model over time. PLoS ONE 2012, 7, e048739. [Google Scholar] [CrossRef]
- Rubinstein, J.T.; Parkinson, W.S.; Tyler, R.S.; Gantz, B.J. Residual speech recognition and cochlear implant performance: Effects of implantation criteria. Am. J. Otol. 1999, 20, 445–452. [Google Scholar]
- Kaandorp, M.W.; Smits, C.; Merkus, P.; Festen, J.M.; Goverts, S.T. Lexical-Access Ability and Cognitive Predictors of Speech Recognition in Noise in Adult Cochlear Implant Users. Trends Hear. 2017, 21. [Google Scholar] [CrossRef]
- Zhan, K.Y.; Lewis, J.H.; Vasil, K.J.; Tamati, T.N.; Harris, M.S.; Pisoni, D.B.; William, K.G.; Ray, C.; Moberly, A.C. Cognitive Functions in Adults Receiving Cochlear Implants: Predictors of Speech Recognition and Changes After Implantation. Otol. Neurotol. 2020, 41, e322–e329. [Google Scholar] [CrossRef]
- Jolink, C.; Helleman, H.W.; Spronsen, E.; Ebbens, F.A.; Ravesloot, M.J.; Dreschler, W.A. The long-term results of speech perception in elderly cochlear implant users. Cochlear Implant. Int. 2016, 17, 146–150. [Google Scholar] [CrossRef]
- Leung, J.; Wang, N.Y.; Yeagle, J.D.; Chinnici, J.; Bowditch, S.; Francis, H.W.; Niparko, J.K. Predictive models for cochlear implantation in elderly candidates. Arch. Otolaryngol. Head Neck Surg. 2005, 131, 1049–1054. [Google Scholar] [CrossRef]
- Fontenot, T.E.; Giardina, C.K.; Dillon, M.; Rooth, M.A.; Teagle, H.F.; Park, L.R.; Brown, K.D.; Adunka, O.F.; Buchman, C.A.; Pillsbury, H.C.; et al. Residual Cochlear Function in Adults and Children Receiving Cochlear Implants: Correlations with Speech Perception Outcomes. Ear Hear. 2019, 40, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Kamakura, T.; Nadol, J.B., Jr. Correlation between word recognition score and intracochlear new bone and fibrous tissue after cochlear implantation in the human. Hear. Res. 2016, 339, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Pfingst, B.E.; Zhou, N.; Colesa, D.J.; Watts, M.M.; Strahl, S.B.; Garadat, S.N.; Schvartz, L.K.C.; Budenz, C.L.; Raphael, Y.; Zwolan, T.A.; et al. Importance of cochlear health for implant function. Hear. Res. 2015, 322, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Balkany, T.J.; Connell, S.S.; Hodges, A.V.; Payne, S.L.; Telischi, F.F.; Eshraghi, A.A.; Angeli, S.I.; Germani, R.; Messiah, S.A.; Kristopher, L.; et al. Conservation of residual acoustic hearing after cochlear implantation. Otol. Neurotol. 2006, 27, 1083–1088. [Google Scholar] [CrossRef] [PubMed]
- Carlson, M.L.; Driscoll, C.L.; Gifford, R.H.; Service, G.J.; Tombers, N.M.; Hughes, B.B.J.; Neff, B.A.; Beatty, C.W. Implications of minimizing trauma during conventional cochlear implantation. Otol. Neurotol. 2011, 32, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Dalbert, A.; Huber, A.; Baumann, N.; Veraguth, D.; Roosli, C.; Pfiffner, F. Hearing Preservation After Cochlear Implantation May Improve Long-term Word Perception in the Electric-only Condition. Otol. Neurotol. 2016, 37, 1314–1319. [Google Scholar] [CrossRef]
- Cosetti, M.K.; Friedmann, D.R.; Zhu, B.Z.; Heman, A.S.E.; Fang, Y.; Keller, R.G.; Shapiro, W.H.; Roland, J.T., Jr.; Waltzman, S.B. The effects of residual hearing in traditional cochlear implant candidates after implantation with a conventional electrode. Otol. Neurotol. 2013, 34, 516–521. [Google Scholar] [CrossRef]
- Graaff, F.; Lissenberg, W.B.I.; Kaandorp, M.W.; Merkus, P.; Goverts, S.T.; Kramer, S.E. Relationship Between Speech Recognition in Quiet and Noise and Fitting Parameters, Impedances and ECAP Thresholds in Adult Cochlear Implant Users. Ear Hear. 2020, 41, 935–947. [Google Scholar] [CrossRef]
- Beek, F.B.; Briaire, J.J.; Frijns, J.H. Population-based prediction of fitting levels for individual cochlear implant recipients. Audiol. Neurootol. 2015, 20, 1–16. [Google Scholar] [CrossRef]
- Kim, S.Y.; Jeon, S.K.; Oh, S.H.; Lee, J.H.; Suh, M.W.; Lee, S.Y.; Lim, Y.J.; Park, M.K. Electrical dynamic range is only weakly associated with auditory performance and speech recognition in long-term users of cochlear implants. Int. J. Pediatric Otorhinolaryngol. 2018, 111, 170–173. [Google Scholar] [CrossRef]
- Elia, A.; Bartoli, R.; Giagnotti, F.; Quaranta, N. The role of hearing preservation on electrical thresholds and speech performances in cochlear implantation. Otol. Neurotol. 2012, 33, 343–347. [Google Scholar] [PubMed]
- Clellan, J.H.; Formeister, E.J.; Merwin, W.H., 3rd; Dillon, M.T.; Calloway, N.; Iseli, C.; Buchman, C.A.; Fitzpatrick, D.C.; Adunka, O.F. Round window electrocochleography and speech perception outcomes in adult cochlear implant subjects: Comparison with audiometric and biographical information. Otol. Neurotol. 2014, 35, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, D.C.; Campbell, A.P.; Choudhury, B.; Dillon, M.T.; Forgues, M.; Buchman, C.A. Round window electrocochleography just before cochlear implantation: Relationship to word recognition outcomes in adults. Otol. Neurotol. 2014, 35, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Dalbert, A.; Pfiffner, F.; Hoesli, M.; Koka, K.; Veraguth, D.; Roosli, C. Assessment of Cochlear Function during Cochlear Implantation by Extra- and Intracochlear Electrocochleography. Front. Neurosci. 2018, 12, 18. [Google Scholar] [CrossRef] [PubMed]
- Campbell, L.; Kaicer, A.; Briggs, R.; Leary, S. Cochlear response telemetry: Intracochlear electrocochleography via cochlear implant neural response telemetry pilot study results. Otol. Neurotol. 2015, 36, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Kompis, M.; Krebs, M.; Hausler, R. Verification of normative values for the Swiss version of the Freiburg speech intelligibility test. HNO 2006, 54, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Plesch, J.; Ernst, B.P.; Strieth, S.; Rader, T. A psychoacoustic application for the adjustment of electrical hearing thresholds in cochlear implant patients. PLoS ONE. 2019, 14, e0223625. [Google Scholar] [CrossRef]
- Wesarg, T.; Battmer, R.D.; Garrido, L.C.; Dillier, N.; Garcia, I.L.; Hey, M. Effect of changing pulse rate on profile parameters of perceptual thresholds and loudness comfort levels and relation to ECAP thresholds in recipients of the Nucleus CI24RE device. Int. J. Audiol. 2010, 49, 775–787. [Google Scholar] [CrossRef]
- Dalbert, A.; Pfiffner, F.; Roosli, C.; Thoele, K.; Sim, J.H.; Gerig, R. Extra- and Intracochlear Electrocochleography in Cochlear Implant Recipients. Audiol. Neurootol. 2015, 20, 339–348. [Google Scholar] [CrossRef]
- Blamey, P.J.; Pyman, B.C.; Gordon, M.; Clark, G.M.; Brown, A.M.; Dowell, R.C. Factors predicting postoperative sentence scores in postlinguistically deaf adult cochlear implant patients. Ann. Otol. Rhinol. Laryngol. 1992, 101, 342–348. [Google Scholar] [CrossRef]
- Bento, R.F.; Brito, N.R.V.; Castilho, A.M.; Gomez, M.V.; Sant, A.S.B.; Guedes, M.C. Psychoacoustic dynamic range and cochlear implant speech-perception performance in nucleus 22 users. Cochlear Implant. Int. 2005, 6, 31–34. [Google Scholar] [CrossRef]
- Spahr, A.J.; Dorman, M.F. Effects of minimum stimulation settings for the Med El Tempo+ speech processor on speech understanding. Ear Hear. 2005, 26, 2S–6S. [Google Scholar] [CrossRef] [PubMed]
- Busby, P.A.; Arora, K. Effects of Threshold Adjustment on Speech Perception in Nucleus Cochlear Implant Recipients. Ear Hear. 2016, 37, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.J.; Shannon, R.V. Effects of dynamic range and amplitude mapping on phoneme recognition in Nucleus-22 cochlear implant users. Ear Hear. 2000, 21, 227–235. [Google Scholar] [CrossRef]
- Loizou, P.C.; Dorman, M.; Fitzke, J. The effect of reduced dynamic range on speech understanding: Implications for patients with cochlear implants. Ear Hear. 2000, 21, 25–31. [Google Scholar] [CrossRef]
- Kawano, A.; Seldon, H.L.; Clark, G.M.; Ramsden, R.T.; Raine, C.H. Intracochlear factors contributing to psychophysical percepts following cochlear implantation. Acta Otolaryngol. 1998, 118, 313–326. [Google Scholar]
- Trecca, E.M.C.; Riggs, W.J.; Mattingly, J.K.; Hiss, M.M.; Cassano, M.; Adunka, O.F. Electrocochleography and Cochlear Implantation: A Systematic Review. Otol. Neurotol. 2020, 41, 864–878. [Google Scholar] [CrossRef]
- Dalbert, A.; Sim, J.H.; Gerig, R.; Pfiffner, F.; Roosli, C.; Huber, A. Correlation of Electrophysiological Properties and Hearing Preservation in Cochlear Implant Patients. Otol. Neurotol. 2015, 36, 1172–1180. [Google Scholar] [CrossRef]
- Haumann, S.; Imsiecke, M.; Bauernfeind, G.; Buchner, A.; Helmstaedter, V.; Lenarz, T.; Salcher, R.B. Monitoring of the Inner Ear Function During and After Cochlear Implant Insertion Using Electrocochleography. Trends Hear. 2019, 23. [Google Scholar] [CrossRef]
- Connell, B.P.; Holder, J.T.; Dwyer, R.T.; Gifford, R.H.; Noble, J.H.; Bennett, M.L.; Labadie, R.F. Intra- and Postoperative Electrocochleography May Be Predictive of Final Electrode Position and Postoperative Hearing Preservation. Front. Neurosci. 2017, 11, 291. [Google Scholar] [CrossRef]
| Group I | Group II | |
|---|---|---|
| Total (n) | 237 | 14 |
| Age at implantation (years: mean, (SD)) | 47 (21) | 56 (16) |
| Sex (% female) | 57 | 36 |
| Implant side (% right) | 52 | 57 |
| Bilateral implanted participants (n) | 25 | 0 |
| Cochlear implant model | ||
| Cochlear™ Nucleus® 512 | 71 | |
| Cochlear™ Nucleus® 422 | 49 | |
| Cochlear™ Nucleus® 522 | 17 | |
| Cochlear™ Nucleus® 24RE(CA) | 100 | |
| Advanced Bionics HiRes 90KTM Advantage | 11 | |
| Advanced Bionics HiResTM Ultra | 3 |
| Speech Recognition (%) vs. pPTA (dB HL) | Speech Recognition (%) vs. T-Level (CL) | Speech Recognition (%) vs. C-Level (CL) | Speech Recognition (%) vs. eDR (nC) | |
|---|---|---|---|---|
| Spearman r | −0.06 | 0.09 | 0.15 | 0.10 |
| p-value | 0.32 | 0.16 | 0.02 * | 0.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rüegg, U.; Dalbert, A.; Veraguth, D.; Röösli, C.; Huber, A.; Pfiffner, F. Correlation between Speech Perception Outcomes after Cochlear Implantation and Postoperative Acoustic and Electric Hearing Thresholds. J. Clin. Med. 2021, 10, 324. https://doi.org/10.3390/jcm10020324
Rüegg U, Dalbert A, Veraguth D, Röösli C, Huber A, Pfiffner F. Correlation between Speech Perception Outcomes after Cochlear Implantation and Postoperative Acoustic and Electric Hearing Thresholds. Journal of Clinical Medicine. 2021; 10(2):324. https://doi.org/10.3390/jcm10020324
Chicago/Turabian StyleRüegg, Ursina, Adrian Dalbert, Dorothe Veraguth, Christof Röösli, Alexander Huber, and Flurin Pfiffner. 2021. "Correlation between Speech Perception Outcomes after Cochlear Implantation and Postoperative Acoustic and Electric Hearing Thresholds" Journal of Clinical Medicine 10, no. 2: 324. https://doi.org/10.3390/jcm10020324
APA StyleRüegg, U., Dalbert, A., Veraguth, D., Röösli, C., Huber, A., & Pfiffner, F. (2021). Correlation between Speech Perception Outcomes after Cochlear Implantation and Postoperative Acoustic and Electric Hearing Thresholds. Journal of Clinical Medicine, 10(2), 324. https://doi.org/10.3390/jcm10020324





