Immune Tolerance of the Human Decidua
Abstract
:1. Introduction
2. Decidualization: Morphological Differentiation in the Human Endometrium
3. Functional Differentiation in Human Endometrium: IGFBP-1 and PRL as Decidual Markers
4. Spontaneous Decidualization of Human ESCs
5. Uterine Natural Killer (uNK) Cells in Human Endometrium
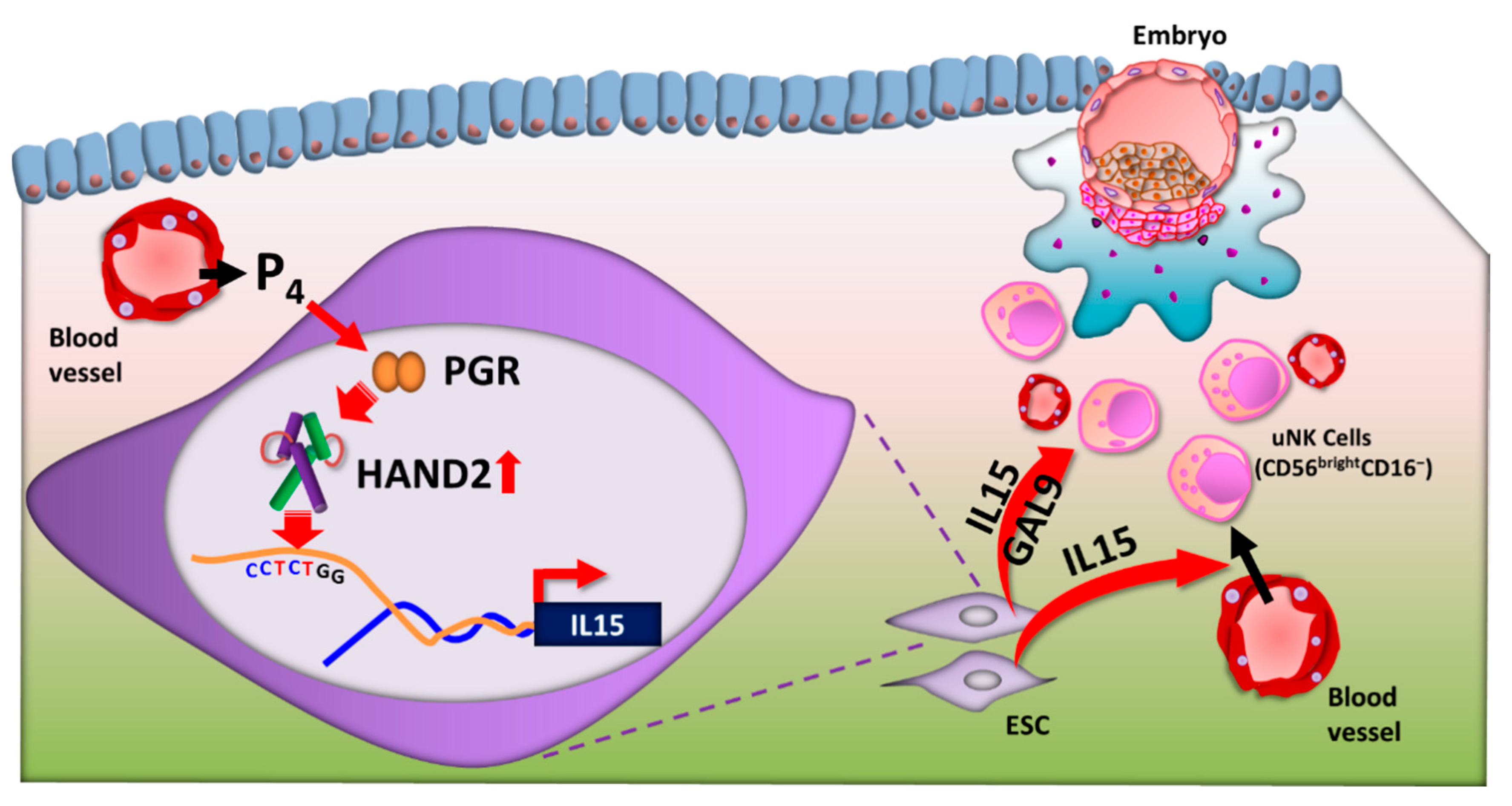
6. IL-15
7. Heart- and Neural Crest Derivatives-Expressed Protein 2 (HAND2): A key Decidua Transcription Factor for IL15 Transcription
8. T Cell
9. Other Immune Cells
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fraser, I.S.; Critchley, H.O.; Broder, M.; Munro, M.G. The FIGO recommendations on terminologies and definitions for normal and abnormal uterine bleeding. Semin. Reprod. Med. 2011, 29, 383–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okada, H.; Tsuzuki, T.; Murata, H. Decidualization of the human endometrium. Reprod. Med. Biol. 2018, 17, 220–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henriet, P.; Gaide Chevronnay, H.P.; Marbaix, E. The endocrine and paracrine control of menstruation. Mol. Cell. Endocrinol. 2012, 358, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.; Sun, X.; Dey, S.K. Mechanisms of implantation: Strategies for successful pregnancy. Nat. Med. 2012, 18, 1754–1767. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.; Morin, S.; Jeong, J.W.; Scott, R.T., Jr.; Lessey, B.A. Local and systemic factors and implantation: What is the evidence? Fertil. Steril. 2016, 105, 873–884. [Google Scholar] [CrossRef] [Green Version]
- Hoshina, M.; Boothby, M.; Hussa, R.; Pattillo, R.; Camel, H.M.; Boime, I. Linkage of human chorionic gonadotrophin and placental lactogen biosynthesis to trophoblast differentiation and tumorigenesis. Placenta 1985, 6, 163–172. [Google Scholar] [CrossRef]
- Hawkins, S.M.; Matzuk, M.M. The menstrual cycle: Basic biology. Ann. N. Y. Acad. Sci. 2008, 1135, 10–18. [Google Scholar] [CrossRef]
- Gellersen, B.; Brosens, J.J. Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr. Rev. 2014, 35, 851–905. [Google Scholar] [CrossRef]
- Brar, A.K.; Frank, G.R.; Kessler, C.A.; Cedars, M.I.; Handwerger, S. Progesterone-dependent decidualization of the human endometrium is mediated by cAMP. Endocrine 1997, 6, 301–307. [Google Scholar] [CrossRef]
- Murata, H.; Tanaka, S.; Tsuzuki-Nakao, T.; Kido, T.; Kakita-Kobayashi, M.; Kida, N.; Hisamatsu, Y.; Tsubokura, H.; Hashimoto, Y.; Kitada, M.; et al. The transcription factor HAND2 up-regulates transcription of the. J. Biol. Chem. 2020, 295, 9596–9605. [Google Scholar] [CrossRef]
- Dunn, C.L.; Kelly, R.W.; Critchley, H.O. Decidualization of the human endometrial stromal cell: An enigmatic transformation. Reprod. Biomed. Online 2003, 7, 151–161. [Google Scholar] [CrossRef]
- Ihnatovych, I.; Hu, W.; Martin, J.L.; Fazleabas, A.T.; de Lanerolle, P.; Strakova, Z. Increased phosphorylation of myosin light chain prevents in vitro decidualization. Endocrinology 2007, 148, 3176–3184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ihnatovych, I.; Livak, M.; Reed, J.; de Lanerolle, P.; Strakova, Z. Manipulating actin dynamics affects human in vitro decidualization. Biol. Reprod. 2009, 81, 222–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ronnov-Jessen, L.; Petersen, O.W. A function for filamentous alpha-smooth muscle actin: Retardation of motility in fibroblasts. J. Cell Biol. 1996, 134, 67–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okada, H.; Nakajima, T.; Yoshimura, T.; Yasuda, K.; Kanzaki, H. The inhibitory effect of dienogest, a synthetic steroid, on the growth of human endometrial stromal cells in vitro. Mol. Hum. Reprod. 2001, 7, 341–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garrido-Gomez, T.; Dominguez, F.; Quinonero, A.; Diaz-Gimeno, P.; Kapidzic, M.; Gormley, M.; Ona, K.; Padilla-Iserte, P.; McMaster, M.; Genbacev, O.; et al. Defective decidualization during and after severe preeclampsia reveals a possible maternal contribution to the etiology. Proc. Natl. Acad. Sci. USA 2017, 114, E8468–E8477. [Google Scholar] [CrossRef] [Green Version]
- Irwin, J.C.; Utian, W.H.; Eckert, R.L. Sex steroids and growth factors differentially regulate the growth and differentiation of cultured human endometrial stromal cells. Endocrinology 1991, 129, 2385–2392. [Google Scholar] [CrossRef]
- Tabanelli, S.; Tang, B.; Gurpide, E. In vitro decidualization of human endometrial stromal cells. J. Steroid Biochem. Mol. Biol. 1992, 42, 337–344. [Google Scholar] [CrossRef]
- Garrido-Gomez, T.; Dominguez, F.; Lopez, J.A.; Camafeita, E.; Quinonero, A.; Martinez-Conejero, J.A.; Pellicer, A.; Conesa, A.; Simon, C. Modeling human endometrial decidualization from the interaction between proteome and secretome. J. Clin. Endocrinol. Metab. 2011, 96, 706–716. [Google Scholar] [CrossRef]
- Schatz, F.; Lockwood, C.J. Progestin regulation of plasminogen activator inhibitor type 1 in primary cultures of endometrial stromal and decidual cells. J. Clin. Endocrinol. Metab. 1993, 77, 621–625. [Google Scholar] [CrossRef]
- Hatayama, H.; Kanzaki, H.; Iwai, M.; Kariya, M.; Fujimoto, M.; Higuchi, T.; Kojima, K.; Nakayama, H.; Mori, T.; Fujita, J. Progesterone enhances macrophage colony-stimulating factor production in human endometrial stromal cells in vitro. Endocrinology 1994, 135, 1921–1927. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T.; Kanzaki, H.; Nakayama, H.; Fujimoto, M.; Hatayama, H.; Kojima, K.; Iwai, M.; Mori, T.; Fujita, J. Induction of tissue inhibitor of metalloproteinase 3 gene expression during in vitro decidualization of human endometrial stromal cells. Endocrinology 1995, 136, 4973–4981. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Salamonsen, L.A. Tissue inhibitor of metalloproteinases (TIMP)-1, -2 and -3 in human endometrium during the menstrual cycle. Mol. Hum. Reprod. 1997, 3, 735–741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krikun, G.; Schatz, F.; Mackman, N.; Guller, S.; Lockwood, C.J. Transcriptional regulation of the tissue factor gene by progestins in human endometrial stromal cells. J. Clin. Endocrinol. Metab. 1998, 83, 926–930. [Google Scholar] [CrossRef] [PubMed]
- Okada, S.; Okada, H.; Sanezumi, M.; Nakajima, T.; Yasuda, K.; Kanzaki, H. Expression of interleukin-15 in human endometrium and decidua. Mol. Hum. Reprod. 2000, 6, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Akison, L.K.; Robker, R.L. The critical roles of progesterone receptor (PGR) in ovulation, oocyte developmental competence and oviductal transport in mammalian reproduction. Reprod. Domest. Anim. Zuchthyg. 2012, 47 (Suppl. 4), 288–296. [Google Scholar] [CrossRef]
- Patel, B.; Elguero, S.; Thakore, S.; Dahoud, W.; Bedaiwy, M.; Mesiano, S. Role of nuclear progesterone receptor isoforms in uterine pathophysiology. Hum. Reprod. Update 2015, 21, 155–173. [Google Scholar] [CrossRef] [Green Version]
- Vento-Tormo, R.; Efremova, M.; Botting, R.A.; Turco, M.Y.; Vento-Tormo, M.; Meyer, K.B.; Park, J.-E.; Stephenson, E.; Polański, K.; Goncalves, A.; et al. Single-cell reconstruction of the early maternal–fetal interface in humans. Nature 2018, 563, 347–353. [Google Scholar] [CrossRef] [Green Version]
- Stefanoska, I.; Jovanovic Krivokuca, M.; Vasilijic, S.; Cujic, D.; Vicovac, L. Prolactin stimulates cell migration and invasion by human trophoblast in vitro. Placenta 2013, 34, 775–783. [Google Scholar] [CrossRef]
- Matsumoto, H.; Sakai, K.; Iwashita, M. Insulin-like growth factor binding protein-1 induces decidualization of human endometrial stromal cells via alpha5beta1 integrin. Mol. Hum. Reprod. 2008, 14, 485–489. [Google Scholar] [CrossRef] [Green Version]
- Maaskant, R.A.; Bogic, L.V.; Gilger, S.; Kelly, P.A.; Bryant-Greenwood, G.D. The human prolactin receptor in the fetal membranes, decidua, and placenta. J. Clin. Endocrinol. Metab. 1996, 81, 396–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, R.L.; Critchley, H.O.; Brooks, J.; Jabbour, H.N.; McNeilly, A.S. Localization and temporal expression of prolactin receptor in human endometrium. J. Clin. Endocrinol. Metab. 1998, 83, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, I.; Lebrun, J.J.; Ali, S.; Kelly, P.A. Expression of prolactin and its receptor in human lymphoid cells. Mol. Endocrinol. 1992, 6, 1023–1031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, A.; Loke, Y.W. Uterine large granular lymphocytes: A possible role in embryonic implantation? Am. J. Obstet. Gynecol. 1990, 162, 308–310. [Google Scholar] [CrossRef]
- Jabbour, H.N.; Critchley, H.O.; Boddy, S.C. Expression of functional prolactin receptors in nonpregnant human endometrium: Janus kinase-2, signal transducer and activator of transcription-1 (STAT1), and STAT5 proteins are phosphorylated after stimulation with prolactin. J. Clin. Endocrinol. Metab. 1998, 83, 2545–2553. [Google Scholar] [CrossRef] [PubMed]
- Boutin, J.M.; Edery, M.; Shirota, M.; Jolicoeur, C.; Lesueur, L.; Ali, S.; Gould, D.; Djiane, J.; Kelly, P.A. Identification of a cDNA encoding a long form of prolactin receptor in human hepatoma and breast cancer cells. Mol. Endocrinol. 1989, 3, 1455–1461. [Google Scholar] [CrossRef] [Green Version]
- Irving, J.A.; Lala, P.K. Functional role of cell surface integrins on human trophoblast cell migration: Regulation by TGF-beta, IGF-II, and IGFBP-1. Exp. Cell Res. 1995, 217, 419–427. [Google Scholar] [CrossRef]
- Hamilton, G.S.; Lysiak, J.J.; Han, V.K.; Lala, P.K. Autocrine-paracrine regulation of human trophoblast invasiveness by insulin-like growth factor (IGF)-II and IGF-binding protein (IGFBP)-1. Exp. Cell Res. 1998, 244, 147–156. [Google Scholar] [CrossRef]
- Bellofiore, N.; Rana, S.; Dickinson, H.; Temple-Smith, P.; Evans, J. Characterization of human-like menstruation in the spiny mouse: Comparative studies with the human and induced mouse model. Hum. Reprod. 2018, 33, 1715–1726. [Google Scholar] [CrossRef]
- Van Der Horst, C.J.; Gillman, J. The menstrual cycle in Elephantulus. S. Afr. J. Med. Sci. 1941, 6, 27–42. [Google Scholar]
- Rasweiler, J.J.T. Spontaneous decidual reactions and menstruation in the black mastiff bat, Molossus ater. Am. J. Anat. 1991, 191, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhu, C.; Lin, H.; Yang, Q.; Ou, Q.; Li, Y.; Chen, Z.; Racey, P.; Zhang, S.; Wang, H. Wild fulvous fruit bats (Rousettus leschenaulti) exhibit human-like menstrual cycle. Biol. Reprod. 2007, 77, 358–364. [Google Scholar] [CrossRef]
- Rasweiler, J.J.T.; de Bonilla, H. Menstruation in short-tailed fruit bats (Carollia spp.). J. Reprod. Fertil. 1992, 95, 231–248. [Google Scholar] [CrossRef] [PubMed]
- Erkenbrack, E.M.; Maziarz, J.D.; Griffith, O.W.; Liang, C.; Chavan, A.R.; Nnamani, M.C.; Wagner, G.P. The mammalian decidual cell evolved from a cellular stress response. PLoS Biol. 2018, 16, e2005594. [Google Scholar] [CrossRef]
- Evers, J.L. Female subfertility. Lancet 2002, 360, 151–159. [Google Scholar] [CrossRef]
- Vanneste, E.; Voet, T.; Le Caignec, C.; Ampe, M.; Konings, P.; Melotte, C.; Debrock, S.; Amyere, M.; Vikkula, M.; Schuit, F.; et al. Chromosome instability is common in human cleavage-stage embryos. Nat. Med. 2009, 15, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Teklenburg, G.; Salker, M.; Molokhia, M.; Lavery, S.; Trew, G.; Aojanepong, T.; Mardon, H.J.; Lokugamage, A.U.; Rai, R.; Landles, C.; et al. Natural selection of human embryos: Decidualizing endometrial stromal cells serve as sensors of embryo quality upon implantation. PloS ONE 2010, 5, e10258. [Google Scholar] [CrossRef]
- Dimitriadis, E.; Menkhorst, E.; Saito, S.; Kutteh, W.H.; Brosens, J.J. Recurrent pregnancy loss. Nat. Rev. Dis. Primers 2020, 6, 98. [Google Scholar] [CrossRef]
- Salker, M.; Teklenburg, G.; Molokhia, M.; Lavery, S.; Trew, G.; Aojanepong, T.; Mardon, H.J.; Lokugamage, A.U.; Rai, R.; Landles, C.; et al. Natural selection of human embryos: Impaired decidualization of endometrium disables embryo-maternal interactions and causes recurrent pregnancy loss. PloS ONE 2010, 5, e10287. [Google Scholar] [CrossRef] [Green Version]
- Weimar, C.H.; Kavelaars, A.; Brosens, J.J.; Gellersen, B.; de Vreeden-Elbertse, J.M.; Heijnen, C.J.; Macklon, N.S. Endometrial stromal cells of women with recurrent miscarriage fail to discriminate between high- and low-quality human embryos. PLoS ONE 2012, 7, e41424. [Google Scholar] [CrossRef] [Green Version]
- Teklenburg, G.; Salker, M.; Heijnen, C.; Macklon, N.S.; Brosens, J.J. The molecular basis of recurrent pregnancy loss: Impaired natural embryo selection. Mol. Hum. Reprod. 2010, 16, 886–895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, S.W.; Norwitz, G.A.; Pavlicev, M.; Tilburgs, T.; Simón, C.; Norwitz, E.R. Endometrial Decidualization: The Primary Driver of Pregnancy Health. Int. J. Mol. Sci. 2020, 21, 4092. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Godbole, G.; Modi, D. Decidual Control of Trophoblast Invasion. Am. J. Reprod. Immunol. 2016, 75, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Sojka, D.K.; Yang, L.; Yokoyama, W.M. Uterine Natural Killer Cells. Front. Immunol. 2019, 10, 960. [Google Scholar] [CrossRef]
- Rätsep, M.T.; Felker, A.M.; Kay, V.R.; Tolusso, L.; Hofmann, A.P.; Croy, B.A. Uterine natural killer cells: Supervisors of vasculature construction in early decidua basalis. Reproduction 2015, 149, R91–R102. [Google Scholar] [CrossRef] [Green Version]
- Jabrane-Ferrat, N. Features of Human Decidual NK Cells in Healthy Pregnancy and During Viral Infection. Front. Immunol. 2019, 10, 1397. [Google Scholar] [CrossRef]
- Hanna, J.; Goldman-Wohl, D.; Hamani, Y.; Avraham, I.; Greenfield, C.; Natanson-Yaron, S.; Prus, D.; Cohen-Daniel, L.; Arnon, T.I.; Manaster, I.; et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat. Med. 2006, 12, 1065–1074. [Google Scholar] [CrossRef]
- Wallace, A.E.; Fraser, R.; Cartwright, J.E. Extravillous trophoblast and decidual natural killer cells: A remodelling partnership. Hum. Reprod. Update 2012, 18, 458–471. [Google Scholar] [CrossRef] [Green Version]
- Brighton, P.J.; Maruyama, Y.; Fishwick, K.; Vrljicak, P.; Tewary, S.; Fujihara, R.; Muter, J.; Lucas, E.S.; Yamada, T.; Woods, L.; et al. Clearance of senescent decidual cells by uterine natural killer cells in cycling human endometrium. Elife 2017, 6. [Google Scholar] [CrossRef]
- Lucas, E.S.; Vrljicak, P.; Muter, J.; Diniz-da-Costa, M.M.; Brighton, P.J.; Kong, C.S.; Lipecki, J.; Fishwick, K.J.; Odendaal, J.; Ewington, L.J.; et al. Recurrent pregnancy loss is associated with a pro-senescent decidual response during the peri-implantation window. Commun. Biol. 2020, 3, 37. [Google Scholar] [CrossRef] [Green Version]
- Moretta, L.; Pietra, G.; Montaldo, E.; Vacca, P.; Pende, D.; Falco, M.; Del Zotto, G.; Locatelli, F.; Moretta, A.; Mingari, M.C. Human NK cells: From surface receptors to the therapy of leukemias and solid tumors. Front. Immunol. 2014, 5, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burton, G.J.; Woods, A.W.; Jauniaux, E.; Kingdom, J.C. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta 2009, 30, 473–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, S.J. Why is placentation abnormal in preeclampsia? Am. J. Obstet. Gynecol. 2015, 213, S115–S122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jauniaux, E.; Burton, G.J. Placenta accreta spectrum: A need for more research on its aetiopathogenesis. Bjog. Int. J. Obstet. Gynaecol. 2018, 125, 1449–1450. [Google Scholar] [CrossRef] [PubMed]
- Apps, R.; Murphy, S.P.; Fernando, R.; Gardner, L.; Ahad, T.; Moffett, A. Human leucocyte antigen (HLA) expression of primary trophoblast cells and placental cell lines, determined using single antigen beads to characterize allotype specificities of anti-HLA antibodies. Immunology 2009, 127, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Apps, R.; Gardner, L.; Moffett, A. A critical look at HLA-G. Trends Immunol. 2008, 29, 313–321. [Google Scholar] [CrossRef]
- Robert, R.; Robert, C.; Jay, I.; Charles, L.; Thomas, M.G. Creasy and Resnik’s Maternal-Fetal Medicine: Principles and Practice, 7th ed.; Saunders: Philadelphia, PA, USA, 2013. [Google Scholar]
- Chazara, O.; Xiong, S.; Moffett, A. Maternal KIR and fetal HLA-C: A fine balance. J. Leukoc. Biol. 2011, 90, 703–716. [Google Scholar] [CrossRef]
- Parham, P.; Moffett, A. Variable NK cell receptors and their MHC class I ligands in immunity, reproduction and human evolution. Nat. Rev. Immunol. 2013, 13, 133–144. [Google Scholar] [CrossRef] [Green Version]
- Sharkey, A.M.; Gardner, L.; Hiby, S.; Farrell, L.; Apps, R.; Masters, L.; Goodridge, J.; Lathbury, L.; Stewart, C.A.; Verma, S.; et al. Killer Ig-like receptor expression in uterine NK cells is biased toward recognition of HLA-C and alters with gestational age. J. Immunol. 2008, 181, 39–46. [Google Scholar] [CrossRef]
- Moffett, A.; Colucci, F. Co-evolution of NK receptors and HLA ligands in humans is driven by reproduction. Immunol. Rev. 2015, 267, 283–297. [Google Scholar] [CrossRef] [Green Version]
- Moffett, A.; Chazara, O.; Colucci, F.; Johnson, M.H. Variation of maternal KIR and fetal HLA-C genes in reproductive failure: Too early for clinical intervention. Reprod. Biomed. Online 2016, 33, 763–769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alecsandru, D.; Garrido, N.; Vicario, J.L.; Barrio, A.; Aparicio, P.; Requena, A.; García-Velasco, J.A. Maternal KIR haplotype influences live birth rate after double embryo transfer in IVF cycles in patients with recurrent miscarriages and implantation failure. Hum. Reprod. 2014, 29, 2637–2643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanier, L.L. Up on the tightrope: Natural killer cell activation and inhibition. Nat. Immunol. 2008, 9, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Hammer, Q.; Rückert, T.; Borst, E.M.; Dunst, J.; Haubner, A.; Durek, P.; Heinrich, F.; Gasparoni, G.; Babic, M.; Tomic, A.; et al. Peptide-specific recognition of human cytomegalovirus strains controls adaptive natural killer cells. Nat. Immunol. 2018, 19, 453–463. [Google Scholar] [CrossRef] [PubMed]
- King, A.; Allan, D.S.; Bowen, M.; Powis, S.J.; Joseph, S.; Verma, S.; Hiby, S.E.; McMichael, A.J.; Loke, Y.W.; Braud, V.M. HLA-E is expressed on trophoblast and interacts with CD94/NKG2 receptors on decidual NK cells. Eur. J. Immunol. 2000, 30, 1623–1631. [Google Scholar] [CrossRef]
- Kamishikiryo, J.; Maenaka, K. HLA-G molecule. Curr. Pharm. Des. 2009, 15, 3318–3324. [Google Scholar] [CrossRef]
- Li, C.; Houser, B.L.; Nicotra, M.L.; Strominger, J.L. HLA-G homodimer-induced cytokine secretion through HLA-G receptors on human decidual macrophages and natural killer cells. Proc. Natl. Acad. Sci. USA 2009, 106, 5767–5772. [Google Scholar] [CrossRef] [Green Version]
- Apps, R.; Gardner, L.; Sharkey, A.M.; Holmes, N.; Moffett, A. A homodimeric complex of HLA-G on normal trophoblast cells modulates antigen-presenting cells via LILRB1. Eur. J. Immunol. 2007, 37, 1924–1937. [Google Scholar] [CrossRef] [Green Version]
- Genbacev, O.; Schubach, S.A.; Miller, R.K. Villous culture of first trimester human placenta—Model to study extravillous trophoblast (EVT) differentiation. Placenta 1992, 13, 439–461. [Google Scholar] [CrossRef]
- Menkhorst, E.M.; Van Sinderen, M.L.; Rainczuk, K.; Cuman, C.; Winship, A.; Dimitriadis, E. Invasive trophoblast promote stromal fibroblast decidualization via Profilin 1 and ALOX5. Sci. Rep. 2017, 7, 8690. [Google Scholar] [CrossRef] [Green Version]
- Kitaya, K.; Yamaguchi, T.; Honjo, H. Central role of interleukin-15 in postovulatory recruitment of peripheral blood CD16(-) natural killer cells into human endometrium. J. Clin. Endocrinol. Metab. 2005, 90, 2932–2940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barber, E.M.; Pollard, J.W. The uterine NK cell population requires IL-15 but these cells are not required for pregnancy nor the resolution of a Listeria monocytogenes infection. J. Immunol. 2003, 171, 37–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keskin, D.B.; Allan, D.S.; Rybalov, B.; Andzelm, M.M.; Stern, J.N.; Kopcow, H.D.; Koopman, L.A.; Strominger, J.L. TGFbeta promotes conversion of CD16+ peripheral blood NK cells into CD16- NK cells with similarities to decidual NK cells. Proc. Natl. Acad. Sci. USA 2007, 104, 3378–3383. [Google Scholar] [CrossRef] [Green Version]
- Henderson, T.A.; Saunders, P.T.; Moffett-King, A.; Groome, N.P.; Critchley, H.O. Steroid receptor expression in uterine natural killer cells. J. Clin. Endocrinol. Metab. 2003, 88, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Waldmann, T.A.; Tagaya, Y. The multifaceted regulation of interleukin-15 expression and the role of this cytokine in NK cell differentiation and host response to intracellular pathogens. Annu. Rev. Immunol. 1999, 17, 19–49. [Google Scholar] [CrossRef] [Green Version]
- Steel, J.C.; Waldmann, T.A.; Morris, J.C. Interleukin-15 biology and its therapeutic implications in cancer. Trends Pharm. Sci. 2012, 33, 35–41. [Google Scholar] [CrossRef] [Green Version]
- Meazza, R.; Azzarone, B.; Orengo, A.M.; Ferrini, S. Role of common-gamma chain cytokines in NK cell development and function: Perspectives for immunotherapy. J. Biomed. Biotechnol. 2011, 2011, 861920. [Google Scholar] [CrossRef]
- Okada, H.; Nakajima, T.; Yasuda, K.; Kanzaki, H. Interleukin-1 inhibits interleukin-15 production by progesterone during in vitro decidualization in human. J. Reprod. Immunol. 2004, 61, 3–12. [Google Scholar] [CrossRef]
- Díaz-Gimeno, P.; Horcajadas, J.A.; Martínez-Conejero, J.A.; Esteban, F.J.; Alamá, P.; Pellicer, A.; Simón, C. A genomic diagnostic tool for human endometrial receptivity based on the transcriptomic signature. Fertil. Steril. 2011, 95, 50–60.e15. [Google Scholar] [CrossRef]
- Lynch, V.J.; Leclerc, R.D.; May, G.; Wagner, G.P. Transposon-mediated rewiring of gene regulatory networks contributed to the evolution of pregnancy in mammals. Nat. Genet. 2011, 43, 1154–1159. [Google Scholar] [CrossRef]
- Christian, M.; Pohnke, Y.; Kempf, R.; Gellersen, B.; Brosens, J.J. Functional association of PR and CCAAT/enhancer-binding protein beta isoforms: Promoter-dependent cooperation between PR-B and liver-enriched inhibitory protein, or liver-enriched activatory protein and PR-A in human endometrial stromal cells. Mol. Endocrinol. 2002, 16, 141–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godbole, G.; Modi, D. Regulation of decidualization, interleukin-11 and interleukin-15 by homeobox A 10 in endometrial stromal cells. J. Reprod. Immunol. 2010, 85, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Ma, L.; Ma, W.G.; Maas, R.L.; Dey, S.K. Hoxa-10 regulates uterine stromal cell responsiveness to progesterone during implantation and decidualization in the mouse. Mol. Endocrinol. 1999, 13, 1005–1017. [Google Scholar] [CrossRef] [PubMed]
- Buzzio, O.L.; Lu, Z.; Miller, C.D.; Unterman, T.G.; Kim, J.J. FOXO1A differentially regulates genes of decidualization. Endocrinology 2006, 147, 3870–3876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takano, M.; Lu, Z.; Goto, T.; Fusi, L.; Higham, J.; Francis, J.; Withey, A.; Hardt, J.; Cloke, B.; Stavropoulou, A.V.; et al. Transcriptional cross talk between the forkhead transcription factor forkhead box O1A and the progesterone receptor coordinates cell cycle regulation and differentiation in human endometrial stromal cells. Mol. Endocrinol. 2007, 21, 2334–2349. [Google Scholar] [CrossRef]
- Li, Q.; Kannan, A.; DeMayo, F.J.; Lydon, J.P.; Cooke, P.S.; Yamagishi, H.; Srivastava, D.; Bagchi, M.K.; Bagchi, I.C. The antiproliferative action of progesterone in uterine epithelium is mediated by Hand2. Science 2011, 331, 912–916. [Google Scholar] [CrossRef] [Green Version]
- Shindoh, H.; Okada, H.; Tsuzuki, T.; Nishigaki, A.; Kanzaki, H. Requirement of heart and neural crest derivatives-expressed transcript 2 during decidualization of human endometrial stromal cells in vitro. Fertil. Steril. 2014, 101, 1781–1790.e5. [Google Scholar] [CrossRef]
- Yamagishi, H.; Olson, E.N.; Srivastava, D. The basic helix-loop-helix transcription factor, dHAND, is required for vascular development. J. Clin. Investig. 2000, 105, 261–270. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, D.; Thomas, T.; Lin, Q.; Kirby, M.L.; Brown, D.; Olson, E.N. Regulation of cardiac mesodermal and neural crest development by the bHLH transcription factor, dHAND. Nat. Genet. 1997, 16, 154–160. [Google Scholar] [CrossRef]
- Huyen, D.V.; Bany, B.M. Evidence for a conserved function of heart and neural crest derivatives expressed transcript 2 in mouse and human decidualization. Reproduction 2011, 142, 353–368. [Google Scholar] [CrossRef]
- Cho, H.; Okada, H.; Tsuzuki, T.; Nishigaki, A.; Yasuda, K.; Kanzaki, H. Progestin-induced heart and neural crest derivatives expressed transcript 2 is associated with fibulin-1 expression in human endometrial stromal cells. Fertil. Steril. 2013, 99, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Mazur, E.C.; Vasquez, Y.M.; Li, X.; Kommagani, R.; Jiang, L.; Chen, R.; Lanz, R.B.; Kovanci, E.; Gibbons, W.E.; DeMayo, F.J. Progesterone receptor transcriptome and cistrome in decidualized human endometrial stromal cells. Endocrinology 2015, 156, 2239–2253. [Google Scholar] [CrossRef] [PubMed]
- Murata, H.; Tsuzuki, T.; Kido, T.; Kakita-Kobayashi, M.; Kida, N.; Hisamatsu, Y.; Okada, H. Progestin-induced heart and neural crest derivatives-expressed transcript 2 inhibits angiopoietin 2 via fibroblast growth factor 9 in human endometrial stromal cells. Reprod. Biol. 2019, 19, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Ticconi, C.; Pietropolli, A.; Di Simone, N.; Piccione, E.; Fazleabas, A. Endometrial Immune Dysfunction in Recurrent Pregnancy Loss. Int. J. Mol. Sci. 2019, 20, 5332. [Google Scholar] [CrossRef] [Green Version]
- Bettelli, E.; Carrier, Y.; Gao, W.; Korn, T.; Strom, T.B.; Oukka, M.; Weiner, H.L.; Kuchroo, V.K. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 2006, 441, 235–238. [Google Scholar] [CrossRef]
- Wilczynski, J.R.; Kalinka, J.; Radwan, M. The role of T-regulatory cells in pregnancy and cancer. Front. Biosci. A J. Virtual Libr. 2008, 13, 2275–2289. [Google Scholar] [CrossRef] [Green Version]
- Samstein, R.M.; Josefowicz, S.Z.; Arvey, A.; Treuting, P.M.; Rudensky, A.Y. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal-fetal conflict. Cell 2012, 150, 29–38. [Google Scholar] [CrossRef] [Green Version]
- Mold, J.E.; Venkatasubrahmanyam, S.; Burt, T.D.; Michaelsson, J.; Rivera, J.M.; Galkina, S.A.; Weinberg, K.; Stoddart, C.A.; McCune, J.M. Fetal and adult hematopoietic stem cells give rise to distinct T cell lineages in humans. Science 2010, 330, 1695–1699. [Google Scholar] [CrossRef] [Green Version]
- Shima, T.; Sasaki, Y.; Itoh, M.; Nakashima, A.; Ishii, N.; Sugamura, K.; Saito, S. Regulatory T cells are necessary for implantation and maintenance of early pregnancy but not late pregnancy in allogeneic mice. J. Reprod. Immunol. 2010, 85, 121–129. [Google Scholar] [CrossRef]
- La Rocca, C.; Carbone, F.; Longobardi, S.; Matarese, G. The immunology of pregnancy: Regulatory T cells control maternal immune tolerance toward the fetus. Immunol. Lett. 2014, 162, 41–48. [Google Scholar] [CrossRef]
- Guerin, L.R.; Prins, J.R.; Robertson, S.A. Regulatory T-cells and immune tolerance in pregnancy: A new target for infertility treatment? Hum. Reprod. Update 2009, 15, 517–535. [Google Scholar] [CrossRef] [PubMed]
- Darrasse-Jèze, G.; Darasse-Jèze, G.; Klatzmann, D.; Charlotte, F.; Salomon, B.L.; Cohen, J.L. CD4+CD25+ regulatory/suppressor T cells prevent allogeneic fetus rejection in mice. Immunol. Lett. 2006, 102, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.X.; Kang, X.M.; Zhao, A.M. Regulation of CD4⁺FOXP3⁺ T cells by CCL20/CCR6 axis in early unexplained recurrent miscarriage patients. Genet. Mol. Res. 2015, 14, 9145–9154. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.J.; Hao, C.F.; Yi, L.; Yin, G.J.; Bao, S.H.; Qiu, L.H.; Lin, Q.D. Increased prevalence of T helper 17 (Th17) cells in peripheral blood and decidua in unexplained recurrent spontaneous abortion patients. J. Reprod. Immunol. 2010, 84, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Zhang, N.; Lin, J.; Wang, C.; Pan, X.; Chen, L.; Li, D.; Wang, L. Distinct pattern of Th17/Treg cells in pregnant women with a history of unexplained recurrent spontaneous abortion. Biosci. Trends 2018, 12, 157–167. [Google Scholar] [CrossRef] [Green Version]
- Wira, C.R.; Fahey, J.V.; Rodriguez-Garcia, M.; Shen, Z.; Patel, M.V. Regulation of mucosal immunity in the female reproductive tract: The role of sex hormones in immune protection against sexually transmitted pathogens. Am. J. Reprod. Immunol. 2014, 72, 236–258. [Google Scholar] [CrossRef] [Green Version]
- Salamonsen, L.A.; Lathbury, L.J. Endometrial leukocytes and menstruation. Hum. Reprod. Update 2000, 6, 16–27. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, K.; Kwak-Kim, J.; Ota, K.; Kuroda, K.; Hisano, M.; Sugiyama, R.; Yamaguchi, K. Immunosuppression with tacrolimus improved reproductive outcome of women with repeated implantation failure and elevated peripheral blood TH1/TH2 cell ratios. Am. J. Reprod. Immunol. 2015, 73, 353–361. [Google Scholar] [CrossRef]
- Kwak-Kim, J.Y.; Chung-Bang, H.S.; Ng, S.C.; Ntrivalas, E.I.; Mangubat, C.P.; Beaman, K.D.; Beer, A.E.; Gilman-Sachs, A. Increased T helper 1 cytokine responses by circulating T cells are present in women with recurrent pregnancy losses and in infertile women with multiple implantation failures after IVF. Hum. Reprod. 2003, 18, 767–773. [Google Scholar] [CrossRef] [Green Version]
- Zhu, C.; Anderson, A.C.; Schubart, A.; Xiong, H.; Imitola, J.; Khoury, S.J.; Zheng, X.X.; Strom, T.B.; Kuchroo, V.K. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat. Immunol. 2005, 6, 1245–1252. [Google Scholar] [CrossRef]
- Sanchez-Fueyo, A.; Tian, J.; Picarella, D.; Domenig, C.; Zheng, X.X.; Sabatos, C.A.; Manlongat, N.; Bender, O.; Kamradt, T.; Kuchroo, V.K.; et al. Tim-3 inhibits T helper type 1-mediated auto- and alloimmune responses and promotes immunological tolerance. Nat. Immunol. 2003, 4, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Zhou, W.H.; Tao, Y.; Wang, S.C.; Jiang, Y.L.; Zhang, D.; Piao, H.L.; Fu, Q.; Li, D.J.; Du, M.R. The Galectin-9/Tim-3 pathway is involved in the regulation of NK cell function at the maternal-fetal interface in early pregnancy. Cell. Mol. Immunol. 2016, 13, 73–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayaraman, P.; Sada-Ovalle, I.; Beladi, S.; Anderson, A.C.; Dardalhon, V.; Hotta, C.; Kuchroo, V.K.; Behar, S.M. Tim3 binding to galectin-9 stimulates antimicrobial immunity. J. Exp. Med. 2010, 207, 2343–2354. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Kim, C.J.; Kim, D.J.; Kang, J.H. Immune cells in the female reproductive tract. Immune Netw. 2015, 15, 16–26. [Google Scholar] [CrossRef] [Green Version]
- Faas, M.M.; de Vos, P. Uterine NK cells and macrophages in pregnancy. Placenta 2017, 56, 44–52. [Google Scholar] [CrossRef]
- Bulmer, J.N.; Williams, P.J.; Lash, G.E. Immune cells in the placental bed. Int. J. Dev. Biol. 2010, 54, 281–294. [Google Scholar] [CrossRef]
- Bonatz, G.; Hansmann, M.L.; Buchholz, F.; Mettler, L.; Radzun, H.J.; Semm, K. Macrophage- and lymphocyte-subtypes in the endometrium during different phases of the ovarian cycle. Int. J. Gynaecol. Obstet. 1992, 37, 29–36. [Google Scholar] [CrossRef]
- Song, J.Y.; Fraser, I.S. Effects of progestogens on human endometrium. Obstet. Gynecol. Surv. 1995, 50, 385–394. [Google Scholar] [CrossRef]
- King, A. Uterine leukocytes and decidualization. Hum. Reprod. Update 2000, 6, 28–36. [Google Scholar] [CrossRef]
- Salamonsen, L.A.; Zhang, J.; Brasted, M. Leukocyte networks and human endometrial remodelling. J. Reprod. Immunol. 2002, 57, 95–108. [Google Scholar] [CrossRef]
- Pollard, J.W. Uterine DCs are essential for pregnancy. J. Clin. Investig. 2008, 118, 3832–3835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plaks, V.; Birnberg, T.; Berkutzki, T.; Sela, S.; BenYashar, A.; Kalchenko, V.; Mor, G.; Keshet, E.; Dekel, N.; Neeman, M.; et al. Uterine DCs are crucial for decidua formation during embryo implantation in mice. J. Clin. Investig. 2008, 118, 3954–3965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Travis, M.A.; Reizis, B.; Melton, A.C.; Masteller, E.; Tang, Q.; Proctor, J.M.; Wang, Y.; Bernstein, X.; Huang, X.; Reichardt, L.F.; et al. Loss of integrin alpha(v)beta8 on dendritic cells causes autoimmunity and colitis in mice. Nature 2007, 449, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Tseng, S.Y.; Otsuji, M.; Gorski, K.; Huang, X.; Slansky, J.E.; Pai, S.I.; Shalabi, A.; Shin, T.; Pardoll, D.M.; Tsuchiya, H. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J. Exp. Med. 2001, 193, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Latchman, Y.; Wood, C.R.; Chernova, T.; Chaudhary, D.; Borde, M.; Chernova, I.; Iwai, Y.; Long, A.J.; Brown, J.A.; Nunes, R.; et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol. 2001, 2, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Freeman, G.J.; Long, A.J.; Iwai, Y.; Bourque, K.; Chernova, T.; Nishimura, H.; Fitz, L.J.; Malenkovich, N.; Okazaki, T.; Byrne, M.C.; et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 2000, 192, 1027–1034. [Google Scholar] [CrossRef] [Green Version]
- Dong, H.; Zhu, G.; Tamada, K.; Chen, L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat. Med. 1999, 5, 1365–1369. [Google Scholar] [CrossRef]
- Steinman, R.M.; Hawiger, D.; Nussenzweig, M.C. Tolerogenic dendritic cells. Annu. Rev. Immunol. 2003, 21, 685–711. [Google Scholar] [CrossRef] [Green Version]
- Ghaebi, M.; Nouri, M.; Ghasemzadeh, A.; Farzadi, L.; Jadidi-Niaragh, F.; Ahmadi, M.; Yousefi, M. Immune regulatory network in successful pregnancy and reproductive failures. Biomed. Pharmacother. 2017, 88, 61–73. [Google Scholar] [CrossRef]
- Vendelova, E.; Ashour, D.; Blank, P.; Erhard, F.; Saliba, A.E.; Kalinke, U.; Lutz, M.B. Tolerogenic Transcriptional Signatures of Steady-State and Pathogen-Induced Dendritic Cells. Front. Immunol. 2018, 9, 333. [Google Scholar] [CrossRef]
- Jeziorska, M.; Salamonsen, L.A.; Woolley, D.E. Mast cell and eosinophil distribution and activation in human endometrium throughout the menstrual cycle. Biol. Reprod. 1995, 53, 312–320. [Google Scholar] [CrossRef] [Green Version]
- Salamonsen, L.A.; Woolley, D.E. Menstruation: Induction by matrix metalloproteinases and inflammatory cells. J. Reprod Immunol. 1999, 44, 1–27. [Google Scholar] [CrossRef]
- Yeaman, G.R.; Collins, J.E.; Currie, J.K.; Guyre, P.M.; Wira, C.R.; Fanger, M.W. IFN-gamma is produced by polymorphonuclear neutrophils in human uterine endometrium and by cultured peripheral blood polymorphonuclear neutrophils. J. Immunol. 1998, 160, 5145–5153. [Google Scholar] [PubMed]
- Song, J.Y.; Russell, P.; Markham, R.; Manconi, F.; Fraser, I.S. Effects of high dose progestogens on white cells and necrosis in human endometrium. Hum. Reprod. 1996, 11, 1713–1718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vincent, A.J.; Malakooti, N.; Zhang, J.; Rogers, P.A.; Affandi, B.; Salamonsen, L.A. Endometrial breakdown in women using Norplant is associated with migratory cells expressing matrix metalloproteinase-9 (gelatinase B). Hum. Reprod. 1999, 14, 807–815. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murata, H.; Tanaka, S.; Okada, H. Immune Tolerance of the Human Decidua. J. Clin. Med. 2021, 10, 351. https://doi.org/10.3390/jcm10020351
Murata H, Tanaka S, Okada H. Immune Tolerance of the Human Decidua. Journal of Clinical Medicine. 2021; 10(2):351. https://doi.org/10.3390/jcm10020351
Chicago/Turabian StyleMurata, Hiromi, Susumu Tanaka, and Hidetaka Okada. 2021. "Immune Tolerance of the Human Decidua" Journal of Clinical Medicine 10, no. 2: 351. https://doi.org/10.3390/jcm10020351




