Infantile Hemangiomas: An Update on Pathogenesis and Treatment
Abstract
:1. Introduction
1.1. Definition and Epidemiology
1.2. Risk Factors
1.3. ISSVA 2018 Classification
1.4. Differential Diagnosis
1.5. Syndromes Associated with IH
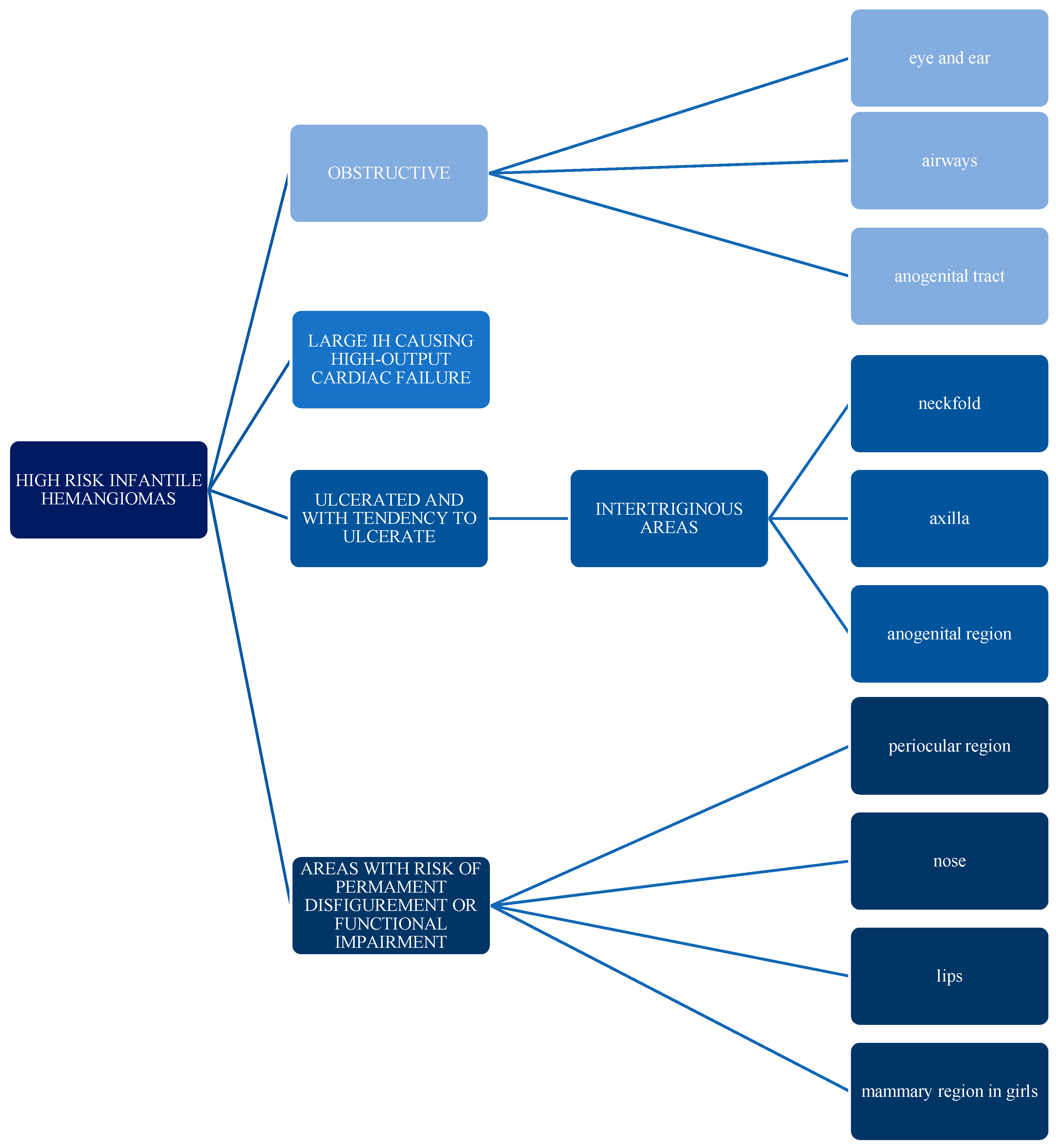
1.6. Complications
2. Pathogenesis
3. Treatment
3.1. Propranolol
3.1.1. Mechanism of Action
3.1.2. Recurrence and Residual Lesions after Propranolol Treatment
3.1.3. Visceral Hemangiomas
3.1.4. Side Effects
3.2. Other Beta-Blockers
3.2.1. Atenolol
3.2.2. Timolol
3.3. Other Drugs
3.4. Laser Treatment
3.4.1. Mechanism of Action
3.4.2. Laser versus Observation
3.4.3. Neodymium-Doped Yttrium Aluminum Garnet (Nd:YAG) Laser
3.5. Combined Treatment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Léauté-Labrèze, C.; Harper, J.I.; Hoeger, P.H. Infantile haemangioma. Lancet 2017, 390, 85–94. [Google Scholar] [CrossRef]
- Oksiuta, M.; Matuszczak, E.; Debek, W.; Dzienis-Koronkiewicz, E.; Hermanowicz, A. Treatment of rapidly proliferating haemangiomas in newborns with propranolol and review of the literature. J. Matern. Neonatal Med. 2014, 29, 64–68. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, J.-Z.; Yu, S.-R.; Xiang, F.; Kang, X.-J. Risk factors for infantile hemangioma: A meta-analysis. World J. Pediatr. 2019, 16, 377–384. [Google Scholar] [CrossRef]
- Drolet, B.A.; Swanson, E.A.; Frieden, I. Infantile Hemangiomas: An Emerging Health Issue Linked to an Increased Rate of Low Birth Weight Infants. J. Pediatr. 2008, 153, 712-715.e1. [Google Scholar] [CrossRef]
- ISSVA Classification of Vascular Anomalies ©2018 International Society for the Study of Vascular Anomalies. Available online: issva.org/classification (accessed on 31 October 2020).
- Yang, H.; Hu, D.-L.; Shu, Q.; Guo, X.-D. Efficacy and adverse effects of oral propranolol in infantile hemangioma: A meta-analysis of comparative studies. World J. Pediatr. 2019, 15, 546–558. [Google Scholar] [CrossRef]
- Harter, N.; Mancini, A.J. Diagnosis and Management of Infantile Hemangiomas in the Neonate. Pediatr. Clin. N. Am. 2019, 66, 437–459. [Google Scholar] [CrossRef]
- Bandera, A.I.R.; Sebaratnam, D.F.; Wargon, O.; Wong, L.-C.F. Infantile hemangioma. Part 1: Epidemiology, pathogenesis, clinical presentation and assessment. J. Am. Acad. Dermatol. 2021. [Google Scholar] [CrossRef]
- Haggstrom, A.N.; Drolet, B.A.; Baselga, E.; Chamlin, S.L.; Garzon, M.C.; Horii, K.A.; Lucky, A.W.; Mancini, A.J.; Metry, D.W.; Newell, B.; et al. Prospective Study of Infantile Hemangiomas: Clinical Characteristics Predicting Complications and Treatment. Pediatrics 2006, 118, 882–887. [Google Scholar] [CrossRef]
- Sebaratnam, D.; Bandera, A.I.R.; Wong, L.-C.F.; Wargon, O. Infantile hemangioma. Part 2: Management. J. Am. Acad. Dermatol. 2021. [Google Scholar] [CrossRef]
- Gomez-Acevedo, H.; Dai, Y.; Strub, G.; Shawber, C.; Wu, J.K.; Richter, G.T. Identification of putative biomarkers for Infantile Hemangiomas and Propranolol treatment via data integration. Sci. Rep. 2020, 10, 3261. [Google Scholar] [CrossRef]
- Mihm, M.C.; Nelson, J.S. Hypothesis: The metastatic niche theory can elucidate infantile hemangioma development. J. Cutan. Pathol. 2010, 37, 83–87. [Google Scholar] [CrossRef] [Green Version]
- Strub, G.M.; Kirsh, A.L.; Whipple, M.E.; Kuo, W.P.; Keller, R.B.; Kapur, R.P.; Majesky, M.W.; Perkins, J.A. Endothelial and circulating C19MC microRNAs are biomarkers of infantile hemangioma. JCI Insight 2016, 1, e88856. [Google Scholar] [CrossRef] [Green Version]
- Moisan, F.; Oucherif, S.; Kaulanjan-Checkmodine, P.; Prey, S.; Rousseau, B.; Bonneu, M.; Claverol, S.; Gontier, E.; Lacomme, S.; Dousset, L.; et al. Critical role of Aquaporin-1 and telocytes in infantile hemangioma response to propranolol beta blockade. Proc. Natl. Acad. Sci. USA 2021, 118, e2018690118. [Google Scholar] [CrossRef]
- Liu, H.; Zheng, Z.; Wintour, E. Aquaporins and Fetal Fluid Balance. Placenta 2008, 29, 840–847. [Google Scholar] [CrossRef]
- Chen, Z.-Y.; Wang, Q.-N.; Zhu, Y.-H.; Zhou, L.-Y.; Xu, T.; He, Z.-Y.; Yang, Y. Progress in the treatment of infantile hemangioma. Ann. Transl. Med. 2019, 7, 692. [Google Scholar] [CrossRef]
- Dornhoffer, J.R.; Wei, T.; Zhang, H.; Miller, E.; Cleves, M.A.; Richter, G.T. The expression of renin–angiotensin–aldosterone axis components in infantile hemangioma tissue and the impact of propranolol treatment. Pediatr. Res. 2017, 82, 155–163. [Google Scholar] [CrossRef]
- Pan, N.; Frome, W.L.; Dart, R.A.; Tewksbury, D.; Luo, J. Expression of the Renin-Angiotensin System in a Human Placental Cell Line. Clin. Med. Res. 2012, 11, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Léauté-Labrèze, C.; De La Roque, E.D.; Hubiche, T.; Boralevi, F.; Thambo, J.-B.; Taïeb, A. Propranolol for Severe Hemangiomas of Infancy. N. Engl. J. Med. 2008, 358, 2649–2651. [Google Scholar] [CrossRef]
- Hoeger, P.H.; Harper, J.I.; Baselga, E.; Bonnet, D.; Boon, L.M.; Degli Atti, M.C.; El Hachem, M.; Oranje, A.P.; Rubin, A.T.; Weibel, L.; et al. Treatment of infantile haemangiomas: Recommendations of a European expert group. Eur. J. Nucl. Med. Mol. Imaging 2015, 174, 855–865. [Google Scholar] [CrossRef]
- Léauté-Labrèze, C.; Hoeger, P.; Mazereeuw-Hautier, J.; Guibaud, L.; Baselga, E.; Posiunas, G.; Phillips, R.J.; Caceres, H.; Gutierrez, J.C.L.; Ballona, R.; et al. A Randomized, Controlled Trial of Oral Propranolol in Infantile Hemangioma. N. Engl. J. Med. 2015, 372, 735–746. [Google Scholar] [CrossRef] [Green Version]
- Baselga, E.; Dembowska-Baginska, B.; Przewratil, P.; González-Enseñat, M.A.; Wyrzykowski, D.; Torrelo, A.; Gutiérrez, J.-C.L.; Rychłowska-Pruszyńska, M.; De Lucas-Laguna, R.; Esteve-Martinez, A.; et al. Efficacy of Propranolol Between 6 and 12 Months of Age in High-Risk Infantile Hemangioma. Pediatrics 2018, 142, e20173866. [Google Scholar] [CrossRef] [Green Version]
- McGee, P.; Miller, S.; Black, C.; Hoey, S. Propranolol for infantile haemangioma: A Review of Current Dosing Regime in a Regional Paediatric Hospital. Ulst. Med. J. 2013, 82, 16–20. [Google Scholar]
- Der Sarkissian, S.A.; Wargon, O.; Sebaratnam, D.F. International heterogeneity in admission criteria and monitoring for the initiation of propranolol in infantile hemangioma. JAAD Int. 2020, 1, 111–113. [Google Scholar] [CrossRef]
- Prasad, A.; Sinha, A.; Kumar, B.; Prasad, A.; Kumari, M. Individualized dosing of oral propranolol for treatment of infantile hemangioma: A prospective study. Pan Afr. Med. J. 2019, 32, 155. [Google Scholar] [CrossRef]
- Guo, X.; Zhu, X.; Liu, D.; Gong, Y.; Sun, J.; Dong, C. Continuous delivery of propranolol from liposomes-in-microspheres significantly inhibits infantile hemangioma growth. Int. J. Nanomed. 2017, 12, 6923–6936. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Wang, X.; Zheng, J.; Zhang, L.; Li, X.; Yuan, W.-E.; Liu, X. Propranolol-Loaded Mesoporous Silica Nanoparticles for Treatment of Infantile Hemangiomas. Adv. Health Mater. 2019, 8, e1801261. [Google Scholar] [CrossRef]
- Storch, C.H.; Hoeger, P. Propranolol for infantile haemangiomas: Insights into the molecular mechanisms of action. Br. J. Dermatol. 2010, 163, 269–274. [Google Scholar] [CrossRef]
- Kleinman, M.E.; Greives, M.R.; Churgin, S.S.; Blechman, K.M.; Chang, E.I.; Ceradini, D.J.; Tepper, O.M.; Gurtner, G.C. Hypoxia-Induced Mediators of Stem/Progenitor Cell Trafficking Are Increased in Children with Hemangioma. Arter. Thromb. Vasc. Biol. 2007, 27, 2664–2670. [Google Scholar] [CrossRef] [Green Version]
- Greenberger, S.; Bischoff, J. Infantile Hemangioma—Mechanism(s) of Drug Action on a Vascular Tumor. Cold Spring Harb. Perspect. Med. 2011, 1, a006460. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Mai, H.-M.; Zheng, J.; Zheng, J.-W.; Wang, Y.-A.; Qin, Z.-P.; Li, K.-L. Propranolol inhibits angiogenesis via down-regulating the expression of vascular endothelial growth factor in hemangioma derived stem cell. Int. J. Clin. Exp. Pathol. 2013, 7, 48–55. [Google Scholar]
- Yuan, W.-L.; Jin, Z.-L.; Wei, J.-J.; Liu, Z.-Y.; Xue, L.; Wang, X.-K. Propranolol given orally for proliferating infantile haemangiomas: Analysis of efficacy and serological changes in vascular endothelial growth factor and endothelial nitric oxide synthase in 35 patients. Br. J. Oral Maxillofac. Surg. 2013, 51, 656–661. [Google Scholar] [CrossRef]
- Hickey, M.M.; Simon, M.C. Regulation of Angiogenesis by Hypoxia and Hypoxia-Inducible Factors. Curr. Top. Dev. Biol. 2006, 76, 217–257. [Google Scholar] [CrossRef]
- Rotter, A.; De Oliveira, Z.N.P. Infantile hemangioma: Pathogenesis and mechanisms of action of propranolol. J. Dtsch. Dermatol. Ges. 2017, 15, 1185–1190. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, M.; North, P.E.; Elsey, J.; Bubley, J.; Rao, S.; Jung, Y.; Wu, S.; Zou, M.-H.; Pollack, B.P.; Kumar, J.; et al. Propranolol exhibits activity against hemangiomas independent of beta blockade. NPJ Precis. Oncol. 2019, 3, 27. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Zeng, J.; Huang, Y.; Gong, M.; Ye, Y.; Zhao, H.; Chen, Z.; Zhang, H. Telocytes and their structural relationships with surrounding cell types in the skin of silky fowl by immunohistochemistrical, transmission electron microscopical and morphometric analysis. Poult. Sci. 2021, 100, 101367. [Google Scholar] [CrossRef]
- Shah, S.D.; Baselga, E.; McCuaig, C.; Pope, E.; Coulie, J.; Boon, L.M.; Garzon, M.C.; Haggstrom, A.N.; Adams, D.; Drolet, B.A.; et al. Rebound Growth of Infantile Hemangiomas after Propranolol Therapy. Pediatrics 2016, 137, e20151754. [Google Scholar] [CrossRef] [Green Version]
- Kagami, S.; Kaneko, M.; Kishi, A.; Katori, T. Prolonged growth of infantile hemangioma after pulsed dye laser and oral propranolol treatment. J. Dermatol. 2018, 45, 1109–1112. [Google Scholar] [CrossRef]
- Yu, Z.; Cai, R.; Chang, L.; Qiu, Y.; Chen, X.; Chen, Q.; Ma, G.; Jin, Y.; Lin, X. Clinical and radiological outcomes of infantile hemangioma treated with oral propranolol: A long-term follow-up study. J. Dermatol. 2018, 46, 376–382. [Google Scholar] [CrossRef]
- Chang, L.; Lv, D.; Yu, Z.; Ma, G.; Ying, H.; Qiu, Y.; Gu, Y.; Jin, Y.; Chen, H.; Lin, X. Infantile hemangioma: Factors causing recurrence after propranolol treatment. Pediatr. Res. 2017, 83, 175–182. [Google Scholar] [CrossRef]
- Buckmiller, L.M. Update on hemangiomas and vascular malformations. Curr. Opin. Otolaryngol. Head Neck Surg. 2004, 12, 476–487. [Google Scholar] [CrossRef]
- Galdeano, F.; Herón, A.; Moreno, S.; Aprea, G.; Meneses, M.; Torrelo, A. Multiple pulmonary infantile hemangiomas responsive to oral propranolol. Pediatr. Dermatol. 2020, 38, 226–228. [Google Scholar] [CrossRef]
- Nip, S.Y.A.; Hon, K.L.; Leung, W.K.A.; Leung, A.K.C.; Choi, P.C.L. Neonatal Abdominal Hemangiomatosis: Propranolol beyond Infantile Hemangioma. Case Rep. Pediatr. 2016, 2016, 9803975. [Google Scholar] [CrossRef] [Green Version]
- Laurens, C.; Abot, A.; Delarue, A.; Knauf, C. Central Effects of Beta-Blockers May Be Due to Nitric Oxide and Hydrogen Peroxide Release Independently of Their Ability to Cross the Blood-Brain Barrier. Front. Neurosci. 2019, 13, 33. [Google Scholar] [CrossRef] [Green Version]
- Calderón-Castrat, X.; Velásquez, F.; Castro, R.; Ballona, R. Atenolol oral en el manejo del hemangioma infantil: Serie de casos de 46 pacientes. Actas Dermo-Sifiliogr. 2019, 111, 59–62. [Google Scholar] [CrossRef]
- Gumina, M.E.; Yan, A.C. Atenolol as an alternative to propranolol for the management of sleep disturbances in the treatment of infantile hemangiomas. Pediatr. Dermatol. 2019, 36, 556–557. [Google Scholar] [CrossRef]
- Khan, M.; Boyce, A.; Prieto-Merino, D.; Wedgeworth, E.; Flohr, C. The Role of Topical Timolol in the Treatment of Infantile Hemangiomas: A Systematic Review and Meta-analysis. Acta Derm. Venereol. 2017, 97, 1167–1171. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.W.; Wang, X.; Zhang, L.; Zheng, J.W.; Liu, C.; Wang, Y.A. Topical Timolol Vs. Oral Propranolol for the Treatment of Superficial Infantile Hemangiomas. Front. Oncol. 2018, 8, 605. [Google Scholar] [CrossRef]
- Zheng, L.; Li, Y. Effect of topical timolol on response rate and adverse events in infantile hemangioma: A meta-analysis. Arch. Dermatol. Res. 2018, 310, 261–269. [Google Scholar] [CrossRef]
- Ying, H.; Zou, Y.; Yu, W.; Qiu, Y.; Ma, G.; Chang, L.; Gu, Y.; Lyu, D.; Lin, X. Prospective, open-label, rater-blinded and self-controlled pilot study of the treatment of proliferating superficial infantile hemangiomas with 0.5% topical timolol cream versus 595-nm pulsed dye laser. J. Dermatol. 2017, 23, 373–665. [Google Scholar] [CrossRef]
- Muñoz-Garza, F.Z.; Ríos, M.; Roé-Crespo, E.; Bernabeu-Wittel, J.; Montserrat-García, M.T.; Puig, L.; Gich, I.; Baselga, E. Efficacy and Safety of Topical Timolol for the Treatment of Infantile Hemangioma in the Early Proliferative Stage. JAMA Dermatol. 2021, 157, 583. [Google Scholar] [CrossRef]
- Gummi, R.; Stahl, E.D.; Marsh, J.D. Topical timolol for an iris hemangioma. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2018, 22, 156–158. [Google Scholar] [CrossRef]
- He, L.; Huang, G. Spectral Doppler ultrasound for predicting long-term response to topical timolol in children with infantile hemangioma. J. Clin. Ultrasound 2017, 45, 480–487. [Google Scholar] [CrossRef]
- Borok, J.; Gangar, P.; Admani, S.; Proudfoot, J.; Friedlander, S.F. Safety and efficacy of topical timolol treatment of infantile haemangioma: A prospective trial. Br. J. Dermatol. 2017, 178, e51–e52. [Google Scholar] [CrossRef]
- Almebayadh, M. Successful treatment of ulcerated infantile hemangioma with brimonidine-timolol cream: 2 cases report and review of the literature. J. Dermatol. Treat. 2019, 31, 433–434. [Google Scholar] [CrossRef]
- Gill, K.; Bayart, C.; Desai, R.; Golden, A.; Raimer, P.; Tamburro, J. Brimonidine Toxicity Secondary to Topical Use for an Ulcerated Hemangioma. Pediatr. Dermatol. 2016, 33, e232–e234. [Google Scholar] [CrossRef]
- Mannschreck, D.B.; Huang, A.H.; Lie, E.; Psoter, K.; Puttgen, K. Topical timolol as adjunct therapy to shorten oral propranolol therapy for infantile hemangiomas. Pediatr. Dermatol. 2019, 36, 283–289. [Google Scholar] [CrossRef]
- Qiao, J.; Lin, J.; Zhang, D.; Li, J.; Chen, C.; Yu, H.; Li, X.; Fang, B. Efficacy of Combined Topical Timolol and Oral Propranolol for Treating Infantile Hemangioma: A Meta-Analysis of Randomized Controlled Trials. Front. Pharmacol. 2020, 11, 554847. [Google Scholar] [CrossRef]
- Ge, J.; Zheng, J.; Zhang, L.; Yuan, W.; Zhao, H. Oral propranolol combined with topical timolol for compound infantile hemangiomas: A retrospective study. Sci. Rep. 2016, 6, 19765. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Yuan, W.-E.; Zheng, J.-W. Pharmacological therapies for infantile hemangiomas: A clinical study in 853 consecutive patients using a standard treatment algorithm. Sci. Rep. 2016, 6, 21670. [Google Scholar] [CrossRef] [Green Version]
- Tay, Y.-K.; Tan, S.-K. Treatment of infantile hemangiomas with the 595-nm pulsed dye laser using different pulse widths in an Asian population. Lasers Surg. Med. 2012, 44, 93–96. [Google Scholar] [CrossRef]
- Lin, M.-Y.; Lin, C.-S.; Hu, S.; Chang, J.-M.; Chung, W.-H.; Zhang, Z.-Y.; Chang, S.-C.; Huo, Y.-P. The application of 595-nm pulsed dye laser for vascular anomalies in a Chinese population: A 10-year experience. J. Cosmet. Laser Ther. 2018, 21, 171–178. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, F.; Jia, Q.; Xu, R.; Dang, W.; Chen, Q.; Lin, L.; Wang, Y. One Possible Mechanism of Pulsed Dye Laser Treatment on Infantile Hemangioma: Induction of Endothelial Apoptosis and Serum vascular endothelial growth factor (VEGF) Level Changes. J. Lasers Med Sci. 2014, 5, 75–81. [Google Scholar]
- Kessels, J.P.; Hamers, E.T.; Ostertag, J.U. Superficial Hemangioma: Pulsed Dye Laser Versus Wait-and-See. Dermatol. Surg. 2013, 39, 414–421. [Google Scholar] [CrossRef]
- Batta, K.; Goodyear, H.M.; Moss, C.; Williams, H.C.; Hiller, L.; Waters, R. Randomised controlled study of early pulsed dye laser treatment of uncomplicated childhood haemangiomas: Results of a 1-year analysis. Lancet 2002, 360, 521–527. [Google Scholar] [CrossRef]
- Witman, P.M.; Wagner, A.M.; Scherer, K.; Waner, M.; Frieden, I.J. Complications following pulsed dye laser treatment of superficial hemangiomas. Lasers Surg. Med. 2006, 38, 116–123. [Google Scholar] [CrossRef]
- Zhang, W.; Li, F.; Yang, Y.; Xue, L.; Cao, M.; Wang, L. Hemangioma treatment with pulsed dye laser—Distinct parameters used between neonatal and non-neonatal patients. J. Cosmet. Laser Ther. 2016, 18, 389–392. [Google Scholar] [CrossRef]
- Chelleri, C.; Monzani, N.A.; Gelmetti, C.; Milani, G.P.; Fossali, E.F.; Galeone, C.; Cavalli, R. Residual Lesions after Pharmacological and Dye-Laser Treatment of Infantile Hemangiomas: Critical Review of 432 Cases. Lasers Surg. Med. 2019, 52, 597–603. [Google Scholar] [CrossRef]
- Kaune, K.M.; Lauerer, P.; Kietz, S.; Eich, C.; Thoms, K.-M.; Schön, M.P.; Zutt, M. Combination therapy of infantile hemangiomas with pulsed dye laser and Nd:YAG laser is effective and safe. J. Dtsch. Dermatol. Ges. 2014, 12, 473–478. [Google Scholar] [CrossRef]
- Hartmann, F.; Lockmann, A.; Grönemeyer, L.-L.; Haenssle, H.; Zutt, M.; Von Fintel, H.; Kühnle, I.; Schön, M.; Thoms, K.-M. Nd:YAG and pulsed dye laser therapy in infantile haemangiomas: A retrospective analysis of 271 treated haemangiomas in 149 children. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1372–1379. [Google Scholar] [CrossRef]
- Alcántara-González, J.; Boixeda, P.; Truchuelo-Díez, M.; Pérez-García, B.; Alonso-Castro, L.; Olasolo, P.J. Hemangiomas infantiles tratados con aplicación secuencial de láser de colorante pulsado y Nd:YAG: Estudio retrospectivo. Actas Dermo-Sifiliográfica 2013, 104, 504–511. [Google Scholar] [CrossRef]
- Vlachakis, I.; Gardikis, S.; Michailoudi, E.; Charissis, G. Treatment of hemangiomas in children using a Nd:YAG laser in conjunction with ice cooling of the epidermis: Techniques and results. BMC Pediatr. 2003, 3, 2. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, F.; Lockmann, A.; Himpel, O.; Kühnle, I.; Hensen, J.; Schön, M.P.; Thoms, K. Combination therapy of oral propranolol and combined Nd:YAG/pulsed dye laser therapy in infantile hemangiomas: A retrospective analysis of 48 treated hemangiomas in 30 children. J. Dtsch. Dermatol. Ges. 2020, 18, 984–993. [Google Scholar] [CrossRef]
- Sugimoto, A.; Aoki, R.; Toyohara, E.; Ogawa, R. Infantile Hemangiomas Cleared by Combined Therapy with Pulsed Dye Laser and Propranolol. Dermatol. Surg. 2021, 47, 1052–1057. [Google Scholar] [CrossRef]
- Kamali, A.S.; Asilian, A.; Mokhtari, F.; Abtahi-Naeini, B.; Nilforoushzadeh, M.A.; Mostafaie, S. Pulsed dye laser and topical timolol gel versus Pulse dye laser in treatment of infantile hemangioma: A double-blind randomized controlled trial. Adv. Biomed. Res. 2015, 4, 257. [Google Scholar] [CrossRef]
- Chen, Q.; Chang, L.; Qiu, Y.; Ying, H.; Chang, S.; Zhang, Y.; Chen, Z.; Ma, G.; Lin, X. Comparison of the efficacy between topical timolol and pulsed dye laser in the treatment of ulcerated infantile haemangiomas: A randomized controlled study. J. Eur. Acad. Dermatol. Venereol. 2020, 35, e303–e305. [Google Scholar] [CrossRef]
- Lin, L.; Guo, P.; Cao, Y.; Li, Q.; Zhang, J.; Huo, R. Combination of Sclerotherapy and Dual-Wavelength Laser in the Management of Infantile Hemangiomas in Chinese Infants. Dermatol. Surg. 2019, 45, 1253–1259. [Google Scholar] [CrossRef]
- Grover, C.; Arora, P.; Kedar, A.; Pal, P.; Lal, B. Combination of Oral Corticosteroids and Polidocanol Sclerotherapy in the Management of Infantile Hemangiomas. Dermatol. Surg. 2010, 36, 2030–2036. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalska, M.; Dębek, W.; Matuszczak, E. Infantile Hemangiomas: An Update on Pathogenesis and Treatment. J. Clin. Med. 2021, 10, 4631. https://doi.org/10.3390/jcm10204631
Kowalska M, Dębek W, Matuszczak E. Infantile Hemangiomas: An Update on Pathogenesis and Treatment. Journal of Clinical Medicine. 2021; 10(20):4631. https://doi.org/10.3390/jcm10204631
Chicago/Turabian StyleKowalska, Małgorzata, Wojciech Dębek, and Ewa Matuszczak. 2021. "Infantile Hemangiomas: An Update on Pathogenesis and Treatment" Journal of Clinical Medicine 10, no. 20: 4631. https://doi.org/10.3390/jcm10204631
APA StyleKowalska, M., Dębek, W., & Matuszczak, E. (2021). Infantile Hemangiomas: An Update on Pathogenesis and Treatment. Journal of Clinical Medicine, 10(20), 4631. https://doi.org/10.3390/jcm10204631





