Lymphedema in Endometrial Cancer Survivor: A Nationwide Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Acquisition
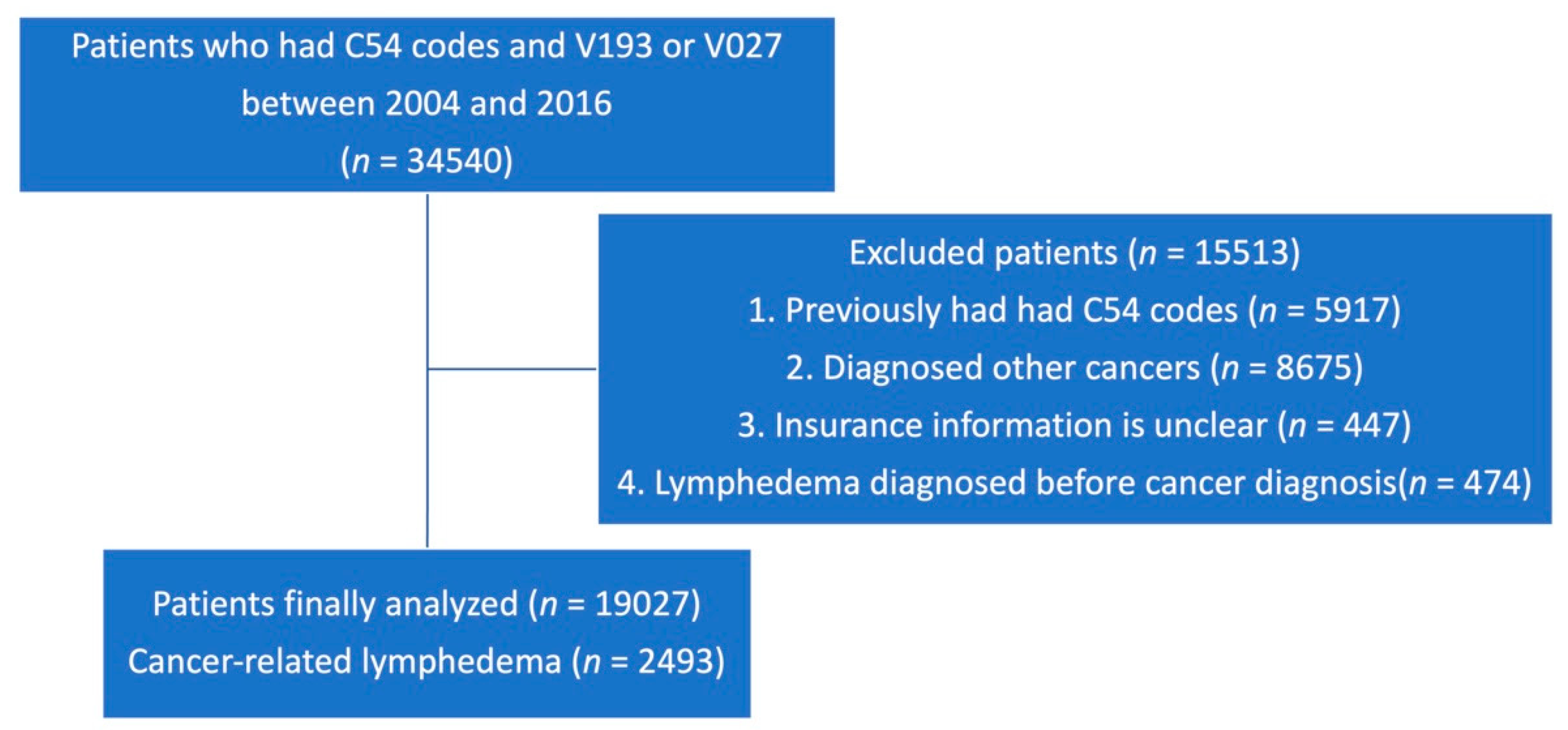
2.2. Study Population and Design
2.3. Statistical Analysis
3. Results
3.1. Basal Characteristics
3.2. Risk Factors for Developing Lymphedema
3.3. Treatment Cost for Lymphedema in Endometrial Cancer
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Loos, A.H.; Oostindier, M.; Weiderpass, E. Geographic and temporal variations in cancer of the corpus uteri: Incidence and mortality in pre- and postmenopausal women in Europe. Int. J. Cancer 2005, 117, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Korea Central Cancer Registry, National Cancer Center. Annual Report of Cancer Statistics in Korea in 2017. Available online: http://www.mohw.go.kr/react/gm/sgm0701vw.jsp?PAR_MENU_ID=13&MENU_ID=1304080401&CONT_SEQ=357199 (accessed on 20 July 2021).
- Hayes, S.C.; Janda, M.; Ward, L.C.; Reul-Hirche, H.; Steele, M.L.; Carter, J.; Quinn, M.; Cornish, B.; Obermair, A. Lymphedema following gynecological cancer: Results from a prospective, longitudinal cohort study on prevalence, incidence and risk factors. Gynecol. Oncol. 2017, 146, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Todo, Y.; Yamamoto, R.; Minobe, S.; Suzuki, Y.; Takeshi, U.; Nakatani, M.; Aoyagi, Y.; Ohba, Y.; Okamoto, K.; Kato, H. Risk factors for postoperative lower-extremity lymphedema in endometrial cancer survivors who had treatment including lymphadenectomy. Gynecol. Oncol. 2010, 119, 60–64. [Google Scholar] [CrossRef] [PubMed]
- de Vries, M.; Hoekstra, H.J.; Hoekstra-Weebers, J.E. Quality of life after axillary or groin sentinel lymph node biopsy, with or without completion lymph node dissection, in patients with cutaneous melanoma. Ann. Surg. Oncol. 2009, 16, 2840–2847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finnane, A.; Hayes, S.C.; Obermair, A.; Janda, M. Quality of life of women with lower-limb lymphedema following gynecological cancer. Expert Rev. Pharmacoecon. Outcomes Res. 2011, 11, 287–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McWayne, J.; Heiney, S.P. Psychologic and social sequelae of secondary lymphedema: A review. Cancer 2005, 104, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Abu-Rustum, N.R.; Alektiar, K.; Iasonos, A.; Lev, G.; Sonoda, Y.; Aghajanian, C.; Chi, D.S.; Barakat, R.R. The incidence of symptomatic lower-extremity lymphedema following treatment of uterine corpus malignancies: A 12-year experience at Memorial Sloan-Kettering Cancer Center. Gynecol. Oncol. 2006, 103, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Hareyama, H.; Hada, K.; Goto, K.; Watanabe, S.; Hakoyama, M.; Oku, K.; Hayakashi, Y.; Hirayama, E.; Okuyama, K. Prevalence, classification, and risk factors for postoperative lower extremity lymphedema in women with gynecologic malignancies: A retrospective study. Int. J. Gynecol. Cancer 2015, 25, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Yost, K.J.; Cheville, A.L.; Al-Hilli, M.M.; Mariani, A.; Barrette, B.A.; McGree, M.E.; Weaver, A.L.; Dowdy, S.C. Lymphedema after surgery for endometrial cancer: Prevalence, risk factors, and quality of life. Obstet. Gynecol. 2014, 124, 307–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armer, J.M.; Radina, M.E.; Porock, D.; Culbertson, S.D. Predicting breast cancer-related lymphedema using self-reported symptoms. Nurs. Res. 2003, 52, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Bakar, Y.; Tugral, A. Lower Extremity Lymphedema Management after Gynecologic Cancer Surgery: A Review of Current Management Strategies. Ann. Vasc. Surg. 2017, 44, 442–450. [Google Scholar] [CrossRef] [PubMed]
- De Vrieze, T.; Gebruers, N.; Nevelsteen, I.; Tjalma, W.A.A.; Thomis, S.; De Groef, A.; Dams, L.; Haenen, V.; Devoogdt, N. Breast cancer-related lymphedema and its treatment: How big is the financial impact? Support. Care Cancer 2021, 29, 3801–3813. [Google Scholar] [CrossRef] [PubMed]
- Dean, L.T.; Moss, S.L.; Ransome, Y.; Frasso-Jaramillo, L.; Zhang, Y.; Visvanathan, K.; Nicholas, L.H.; Schmitz, K.H. “It still affects our economic situation”: Long-term economic burden of breast cancer and lymphedema. Support. Care Cancer 2019, 27, 1697–1708. [Google Scholar] [CrossRef] [PubMed]
- Dean, L.T.; Moss, S.L.; Rollinson, S.I.; Frasso Jaramillo, L.; Paxton, R.J.; Owczarzak, J.T. Patient recommendations for reducing long-lasting economic burden after breast cancer. Cancer 2019, 125, 1929–1940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Health Insurance Service. H-Well NHIS. Available online: https://www.nhis.or.kr/static/html/wbd/g/a/wbdga0101.html (accessed on 20 July 2021).
- Kim, L.; Kim, J.A.; Kim, S. A guide for the utilization of Health Insurance Review and Assessment Service National Patient Samples. Epidemiol. Health 2014, 36, e2014008. [Google Scholar] [CrossRef] [PubMed]
- International Society of Lymphology. The diagnosis and treatment of peripheral lymphedema. Consensus document of the International Society of Lymphology. Lymphology 2003, 36, 84–91. [Google Scholar]
- Cragun, J.M.; Havrilesky, L.J.; Calingaert, B.; Synan, I.; Secord, A.A.; Soper, J.T.; Clarke-Pearson, D.L.; Berchuck, A. Retrospective analysis of selective lymphadenectomy in apparent early-stage endometrial cancer. J. Clin. Oncol. 2005, 23, 3668–3675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geppert, B.; Lonnerfors, C.; Bollino, M.; Persson, J. Sentinel lymph node biopsy in endometrial cancer-Feasibility, safety and lymphatic complications. Gynecol. Oncol. 2018, 148, 491–498. [Google Scholar] [CrossRef] [PubMed]



| Total (n = 19,027) | Lymphedema | ||||
|---|---|---|---|---|---|
| No (16,534, 86.9%) | Yes (2493, 13.1%) | HR (95% CI) | p-Value | ||
| Age | |||||
| <40 | 2276 (12.0%) | 2133 (93.7%) | 143 (6.3%) | 1.00 | |
| 40–59 | 11,823 (62.1%) | 10,273 (86.9%) | 1550 (13.1%) | 1.41 (1.20–1.66) | <0.0001 |
| >60 | 4928 (25.9%) | 4128 (83.8%) | 800 (16.23%) | 1.47 (1.24–1.75) | <0.0001 |
| Income | |||||
| 1~5 | 4720 (24.8%) | 4117 (87.2%) | 603 (12.8%) | 0.94 (0.85–1.05) | 0.2716 |
| 6~10 | 3653 (19.2%) | 3214 (88.0%) | 439 (12.0%) | 0.88 (0.79–0.99) | 0.0362 |
| 10~15 | 4533 (23.8%) | 3950 (87.1%) | 583 (12.9%) | 0.93 (0.34–1.03) | 0.1612 |
| 16~20 | 6121 (32.2%) | 5253 (85.8%) | 868 (14.2%) | 1.00 | |
| Residence | |||||
| Urban | 12,919 (67.9%) | 11,155 (86.4%) | 1764 (14.2%) | 1.00 | |
| Rural | 6108 (32.1%) | 5379 (88.1%) | 729 (11.9%) | 0.95 (0.88–1.04) | 0.2771 |
| Treatment | |||||
| Surgery | 8411 (44.2%) | 7460 (88.7%) | 951 (11.3%) | 1.00 | |
| Radiation | 447 (2.4%) | 384 (85.9%) | 63 (14.1%) | 1.42 (1.10–1.83) | 0.0078 |
| Chemotherapy | 346 (1.8%) | 293 (84.7%) | 53 (15.3%) | 1.81 (1.37–2.38) | <0.0001 |
| S + R | 2545 (13.4%) | 2015 (79.2%) | 530 (20.8%) | 1.87 (1.68–2.08) | <0.0001 |
| S + C | 1404 (7.4%) | 1171 (83.4%) | 233 (16.6%) | 1.61 (1.39–1.86) | <0.0001 |
| R + C | 319 (1.7%) | 259 (81.2%) | 60 (18.8%) | 2.15 (1.65–2.78) | <0.0001 |
| S + R + C | 1314 (6.9%) | 974 (74.1%) | 340 (25.9%) | 2.57 (2.27–2.91) | <0.0001 |
| other | 4241 (22.3%) | 3978 (93.8%) | 263 (6.2%) | 0.68 (0.59–0.78) | <0.0001 |
| Medication | Physiotherapy | Medication + Physiotherapy | Total | |
|---|---|---|---|---|
| 2004 | 20,696 | 4372 | 972 | 26,039 |
| 2005 | 16,313 | 2847 | 1495 | 20,654 |
| 2006 | 68,377 | 13,705 | 2890 | 84,972 |
| 2007 | 112,065 | 15,817 | 20,805 | 148,687 |
| 2008 | 144,772 | 34,270 | 63,320 | 242,362 |
| 2009 | 193,300 | 53,119 | 98,656 | 345,074 |
| 2010 | 172,975 | 50,082 | 74,014 | 297,070 |
| 2011 | 274,091 | 72,589 | 106,043 | 452,724 |
| 2012 | 293,853 | 75,010 | 104,135 | 472,998 |
| 2013 | 354,080 | 72,686 | 94,248 | 521,015 |
| 2014 | 560,701 | 142,837 | 305,295 | 1,008,833 |
| 2015 | 644,125 | 189,901 | 362,690 | 1,196,716 |
| 2016 | 943,558 | 173,691 | 326,195 | 1,443,444 |
| 2017 | 1,165,017 | 251,684 | 477,804 | 1,894,505 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-J.; Myong, J.-P.; Lee, Y.-H.; Cho, E.-J.; Lee, S.-J.; Kim, C.-J.; Kim, J.-H. Lymphedema in Endometrial Cancer Survivor: A Nationwide Cohort Study. J. Clin. Med. 2021, 10, 4647. https://doi.org/10.3390/jcm10204647
Lee S-J, Myong J-P, Lee Y-H, Cho E-J, Lee S-J, Kim C-J, Kim J-H. Lymphedema in Endometrial Cancer Survivor: A Nationwide Cohort Study. Journal of Clinical Medicine. 2021; 10(20):4647. https://doi.org/10.3390/jcm10204647
Chicago/Turabian StyleLee, Su-Jeong, Jun-Pyo Myong, Yun-Hee Lee, Eui-Jin Cho, Sung-Jong Lee, Chan-Joo Kim, and Jin-Hwi Kim. 2021. "Lymphedema in Endometrial Cancer Survivor: A Nationwide Cohort Study" Journal of Clinical Medicine 10, no. 20: 4647. https://doi.org/10.3390/jcm10204647
APA StyleLee, S.-J., Myong, J.-P., Lee, Y.-H., Cho, E.-J., Lee, S.-J., Kim, C.-J., & Kim, J.-H. (2021). Lymphedema in Endometrial Cancer Survivor: A Nationwide Cohort Study. Journal of Clinical Medicine, 10(20), 4647. https://doi.org/10.3390/jcm10204647







