Updates on Wound Infiltration Use for Postoperative Pain Management: A Narrative Review
Abstract
1. Introduction
2. Materials and Methods
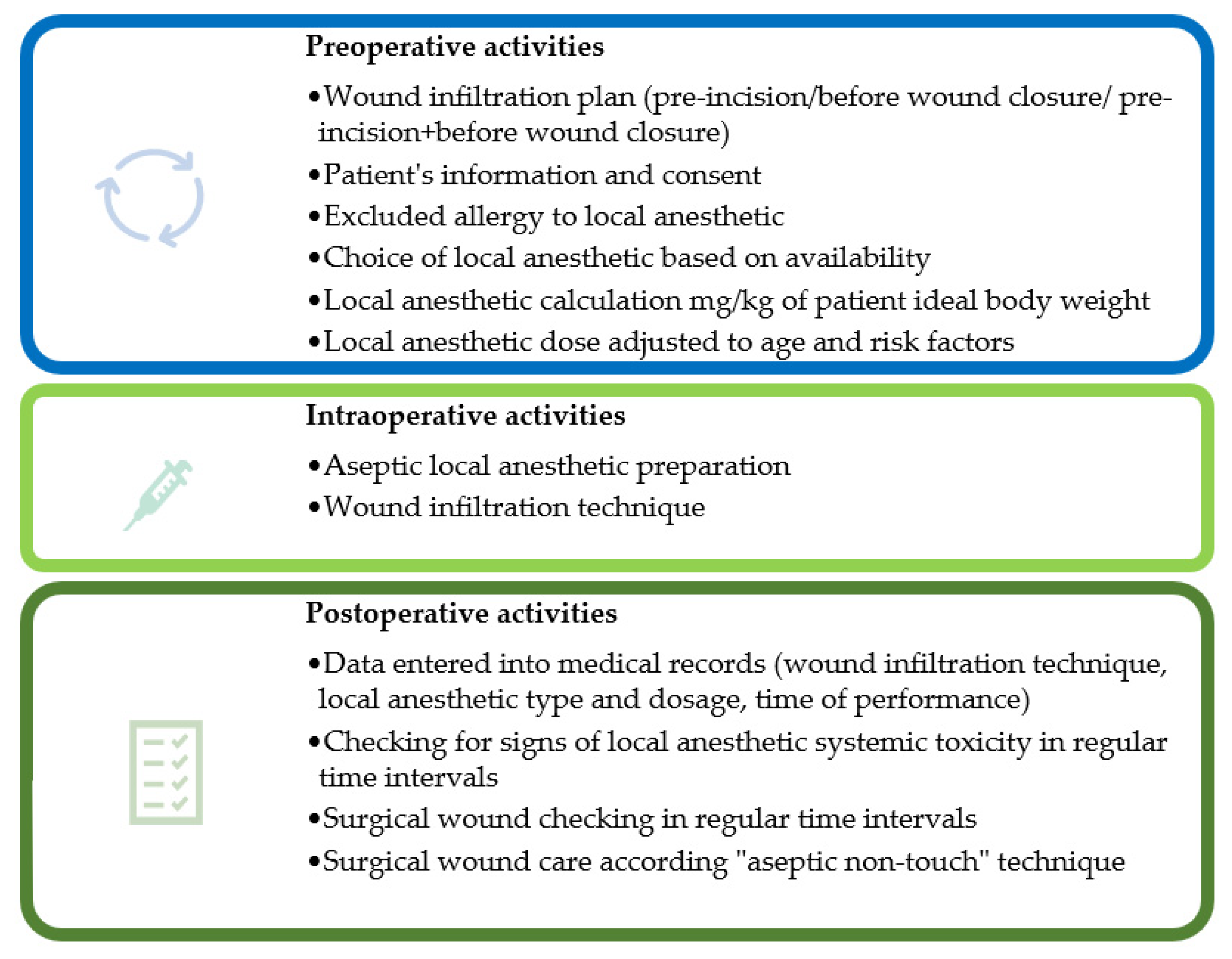
3. Wound Infiltration Technique
4. Local Anesthetics and Medications for Wound Infiltration
5. Complications of Wound Infiltration
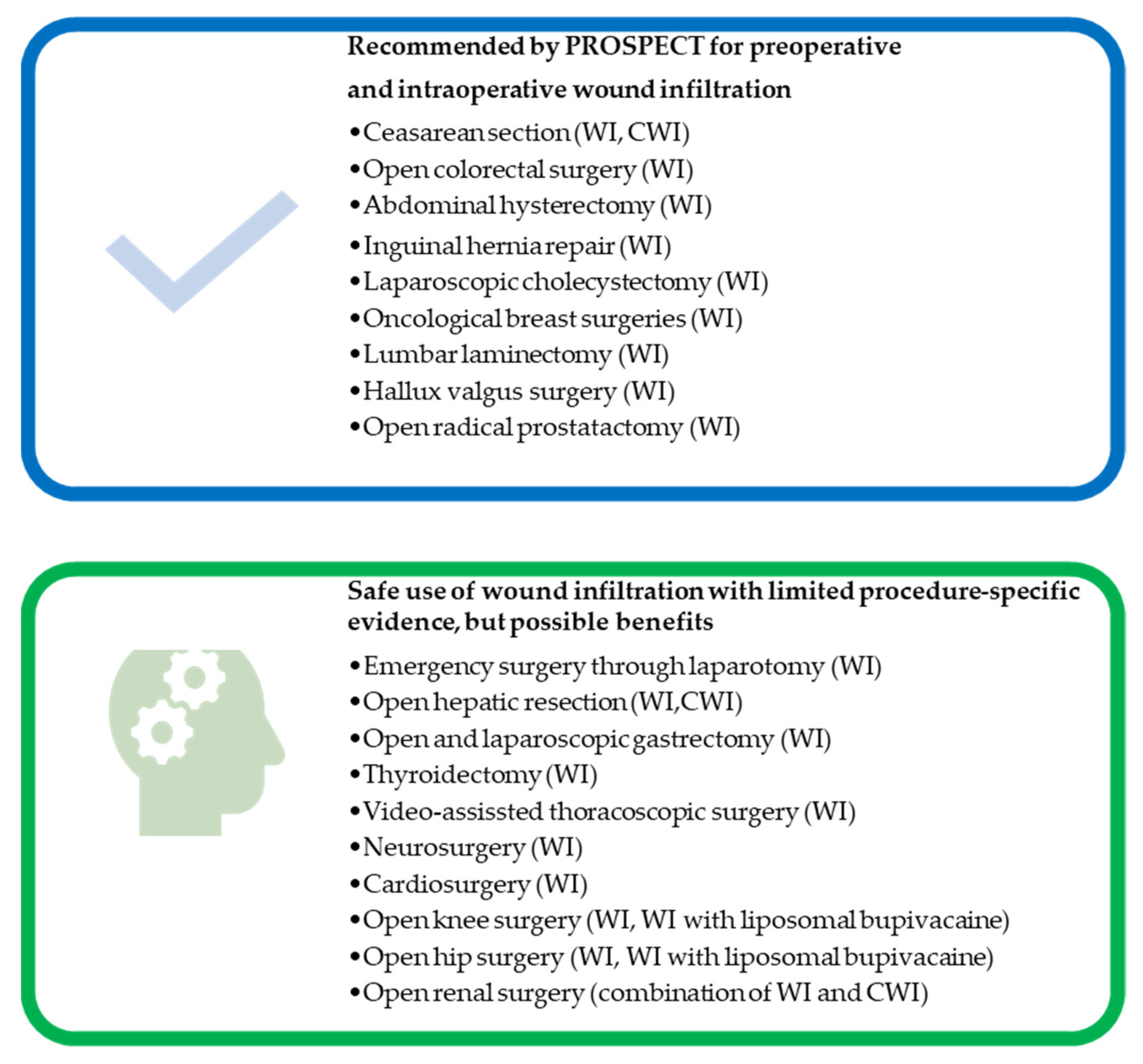
6. Application of Wound Infiltration in Different Surgical Types
6.1. Cardiac Surgery
6.2. Thoracic Surgery
6.3. Abdominal Surgery
6.3.1. Appendectomy
6.3.2. Laparoscopic Cholecystectomy
6.3.3. Inguinal Herniorrhaphy
6.3.4. Esophagogastric Surgery
6.3.5. Hepatic, Biliary, and Pancreatic Surgery
6.3.6. Colorectal Surgery
6.3.7. Reconstruction of the Abdominal Aorta
6.4. Breast Surgery
6.5. Thyroid Surgery
6.6. Neurosurgery
6.7. Urology
6.8. Gynecological Surgery
6.9. Orthopedic Surgery
6.10. Ambulatory Surgical Procedures
6.11. Trauma and Emergency Surgery
7. Wound Infiltration in Enhanced Recovery after Surgery Protocols
8. Future Directions
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Chou, R.; Gordon, D.B.; De Leon-Casasola, O.A.; Rosenberg, J.M.; Bickler, S.; Brennan, T.; Carter, T.; Cassidy, C.L.; Chittenden, E.H.; Degenhardt, E.; et al. Management of postoperative pain: A clinical practice guideline from the American pain society, the American society of regional anesthesia and pain medicine, and the American society of anesthesiologists’ committee on regional anesthesia, executive committee, and administrative council. J. Pain 2016, 17, 131–157. [Google Scholar] [CrossRef]
- Joshi, G.P.; Machi, A. Surgical site infiltration: A neuroanatomical approach. Best Pract. Res. Clin. Anaesthesiol. 2019, 33, 317–324. [Google Scholar] [CrossRef]
- NYSORA. Intra-Articular and Periarticular Infiltration of Local Anesthetics—NYSORA. Available online: https://www.nysora.com/regional-anesthesia-for-specific-surgical-procedures/lower-extremity-regional-anesthesia-for-specific-surgical-procedures/anesthesia-and-analgesia-for-hip-procedures/intra-articular-periarticular-infiltration-local-anesthetics/ (accessed on 12 June 2021).
- Borgeat, A.; Rawal, N. Surgical Site Catheter Analgesia, 2nd ed.; Darwin Healthcare Communications: Oxford, UK, 2012; pp. 22–163. [Google Scholar]
- Kerr, D.R.; Kohan, L. Local infiltration analgesia: A technique for the control of acute postoperative pain following knee and hip surgery—A case study of 325 patients. Acta Orthop. 2008, 79, 174–183. [Google Scholar] [CrossRef]
- Scott, N.B. Wound infiltration for surgery. Anaesthesia 2010, 65, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Kehlet, H.; Liu, S.S. Continuous local anesthetic wound infusion to improve postoperative outcome: Back to the periphery? Anesthesiology 2007, 107, 369–371. [Google Scholar] [CrossRef] [PubMed]
- ESRA. Better Postoperative Pain Management—ESRA. Available online: https://esraeurope.org/prospect/ (accessed on 26 January 2021).
- Jacobs, A.; Lemoine, A.; Joshi, G.P.; Van de Velde, M.; Bonnet, F.; Pogatzki-Zahn, E.; Schug, S.; Kehlet, H.; Rawal, N.; Delbos, A.; et al. PROSPECT guideline for oncological breast surgery: A systematic review and procedure-specific postoperative pain management recommendations. Anaesthesia 2020, 75, 664–673. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.S.; Richman, J.M.; Thirlby, R.C.; Wu, C.L. Efficacy of Continuous Wound Catheters Delivering Local Anesthetic for Postoperative Analgesia: A Quantitative and Qualitative Systematic Review of Randomized Controlled Trials. J. Am. Coll. Surg. 2006, 203, 914–932. [Google Scholar] [CrossRef]
- Sallnas, F.V.; Auyong, D.B. Local Anesthetics. In Anesthetic Pharmacology, 2nd ed.; Evers, A.S., Maze, M., Kharasch, E.D., Eds.; Cambridge University Press: Cambridge, UK, 2011; pp. 574–589. [Google Scholar]
- Gasanova, I.; Alexander, J.; Ogunnaike, B.; Hamid, C.; Rogers, D.; Minhajuddin, A.; Joshi, G.P. Transversus abdominis plane block versus surgical site infiltration for pain management after open total abdominal hysterectomy. Anesth. Analg. 2015, 121, 1383–1388. [Google Scholar] [CrossRef] [PubMed]
- Joshi, G.P.; Rawal, N.; Kehlet, H. Evidence-based management of postoperative pain in adults undergoing open inguinal hernia surgery. Br. J. Surg. 2012, 99, 168–185. [Google Scholar] [CrossRef]
- Barazanchi, A.W.H.; MacFater, W.S.; Rahiri, J.L.; Tutone, S.; Hill, A.G.; Joshi, G.P.; Kehlet, H.; Schug, S.; Van de Velde, M.; Vercauteren, M.; et al. Evidence-based management of pain after laparoscopic cholecystectomy: A PROSPECT review update. Br. J. Anaesth. 2018, 121, 787–803. [Google Scholar] [CrossRef] [PubMed]
- Saul, D.; Roch, J.; Lehmann, W.; Dresing, K. Infiltration anesthesia. Oper. Orthop. Traumatol. 2020, 32, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Thornton, P.C.; Buggy, D.J. Local anaesthetic wound infusion for acute postoperative pain: A viable option? Br. J. Anaesth. 2011, 107, 656–658. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gill, H.S.; Prausnitz, M.R. Does needle size matter? J. Diabetes Sci. Technol. 2007, 1, 725–729. [Google Scholar] [CrossRef] [PubMed]
- Arendt-Nielsen, L.; Egekvist, H.; Bjerring, P. Pain following controlled cutaneous insertion of needles with different diameters. Somatosens. Mot. Res. 2006, 23, 37–43. [Google Scholar] [CrossRef]
- Strazar, A.R.; Leynes, P.G.; Lalonde, D.H. Minimizing the pain of local anesthesia injection. Plast. Reconstr. Surg. 2013, 132, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Zilinsky, I.; Bar-Meir, E.; Zaslansky, R.; Mendes, D.; Winkler, E.; Orenstein, A. Ten Commandments for Minimal Pain during Administration of Local Anesthetics—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/15776779/ (accessed on 27 January 2021).
- Habif, T. Clinical Dermatology, 6th ed.; Elsevier: Philadelphia, PA, USA, 2015. [Google Scholar]
- Arndt, K.A.; Burton, C.; Noe, J.M. Minimizing the pain of local anesthesia. Plast. Reconstr. Surg. 1983, 72, 676–679. [Google Scholar] [CrossRef]
- Beaussier, M.; El’Ayoubi, H.; Schiffer, E.; Rollin, M.; Parc, Y.; Mazoit, J.X.; Azizi, L.; Gervaz, P.; Rohr, S.; Biermann, C.; et al. Continuous preperitoneal infusion of ropivacaine provides effective analgesia and accelerates recovery after colorectal surgery: A randomized, double-blind, placebo-controlled study. Anesthesiology 2007, 107, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Polglase, A.L.; McMurrick, P.J.; Simpson, P.J.B.; Wale, R.J.; Carne, P.W.G.; Johnson, W.; Chee, J.; Ooi, C.W.; Chong, J.W.D.; Kingsland, S.R.; et al. Continuous wound infusion of local anesthetic for the control of pain after elective abdominal colorectal surgery. Dis. Colon Rectum 2007, 50, 2158–2167. [Google Scholar] [CrossRef]
- Fredman, B.; Zohar, E.; Tarabykin, A.; Shapiro, A.; Mayo, A.; Klein, E.; Jedeikin, R. Bupivacaine wound instillation via an electronic patient-controlled analgesia device and a double-catheter system does not decrease postoperative pain or opioid requirements after major abdominal surgery. Anesth. Analg. 2001, 92, 189–193. [Google Scholar] [CrossRef]
- Sistla, S.C.; Dhanapal, B.; Badhe, A.S.; Ali, S.M.; Ravichandran, N.T.; Galidevara, I. Effectiveness of continuous wound infusion of local anesthetics after abdominal surgeries. J. Surg. Res. 2017, 212, 94–100. [Google Scholar] [CrossRef]
- Kristensen, B.B.; Christensen, D.S.; Østergaard, M.; Skjelsager, K.; Nielsen, D.; Mogensen, T.S. Lack of postoperative pain relief after hysterectomy using preperitoneally administered bupivacaine. Reg. Anesth. Pain Med. 1999, 24, 576–580. [Google Scholar] [CrossRef]
- Sistla, S.C.; Sibal, A.K.; Ravishankar, M. Intermittent wound perfusion for postoperative pain relief following upper abdominal surgery: A surgeon’s perspective. Pain Pract. 2009, 9, 65–70. [Google Scholar] [CrossRef]
- Raines, S.; Hedlund, C.; Franzon, M.; Lillieborg, S.; Kelleher, G.; Ahlén, K. Ropivacaine for continuous wound infusion for postoperative pain management: A systematic review and meta-analysis of randomized controlled trials. Eur. Surg. Res. 2014, 53, 43–60. [Google Scholar] [CrossRef] [PubMed]
- Mungroop, T.H.; Bond, M.J.; Lirk, P.; Busch, O.R.; Hollmann, M.W.; Veelo, D.P.; Besselink, M.G. Preperitoneal or Subcutaneous Wound Catheters as Alternative for Epidural Analgesia in Abdominal Surgery: A Systematic Review and Meta-analysis. Ann. Surg. 2019, 269, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Rao, Z.; Zhou, H.; Pan, X.; Chen, J.; Wang, Y.; Wang, Z.; Ding, Z. Ropivacaine wound infiltration: A fast-track approach in patients undergoing thoracotomy surgery. J. Surg. Res. 2017, 220, 379–384. [Google Scholar] [CrossRef]
- Fiorelli, A.; Vicidomini, G.; Laperuta, P.; Busiello, L.; Perrone, A.; Napolitano, F.; Messina, G.; Santini, M. Pre-emptive local analgesia in video-assisted thoracic surgery sympathectomy. Eur. J. Cardio-Thorac. Surg. 2010, 37, 588–593. [Google Scholar] [CrossRef]
- Sihoe, A.D.L.; Manlulu, A.V.; Lee, T.W.; Thung, K.H.; Yim, A.P.C. Pre-emptive local anesthesia for needlescopic video-assisted thoracic surgery: A randomized controlled trial. Eur. J. Cardiothorac. Surg. 2007, 31, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Leung, C.C.; Chan, Y.M.; Ngai, S.W.; Ng, K.F.J.; Tsui, S.L. Effect of pre-incision skin infiltration on post-hysterectomy pain—A double-blind randomized controlled trial. Anaesth. Intensive Care 2000, 28, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Dahl, J.B.; Møiniche, S. Relief of postoperative pain by local anaesthetic infiltration: Efficacy for major abdominal and orthopedic surgery. Pain 2009, 143, 7–11. [Google Scholar] [CrossRef]
- Paladini, G.; Carlo, S.D.; Musella, G.; Petrucci, E.; Scimia, P.; Ambrosoli, A.; Cofini, V.; Fusco, P. Continuous wound infiltration of local anesthetics in postoperative pain management: Safety, efficacy and current perspectives. J. Pain Res. 2020, 13, 285–294. [Google Scholar] [CrossRef]
- Neal, J.M.; Barrington, M.J.; Fettiplace, M.R.; Gitman, M.; Memtsoudis, S.G.; Mörwald, E.E.; Rubin, D.S.; Weinberg, G. The Third American Society of Regional Anesthesia and Pain Medicine Practice Advisory on Local Anesthetic Systemic Toxicity: Executive Summary 2017. Reg. Anesth. Pain Med. 2018, 43, 113–123. [Google Scholar] [CrossRef]
- Dickerson, D.M.; Apfelbaum, J.L. Local anesthetic systemic toxicity. Aesthet. Surg. J. 2014, 34, 1111–1119. [Google Scholar] [CrossRef]
- Steele, E.A.; Ng, J.D.; Poissant, T.M.; Campbell, N.M. Comparison of injection pain of articaine and lidocaine in eyelid surgery. Ophthal. Plast. Reconstr. Surg. 2009, 25, 13–15. [Google Scholar] [CrossRef] [PubMed]
- Cassuto, J.; Sinclair, R.; Bonderovic, M. Anti-inflammatory properties of local anesthetics and their present and potential clinical implications. Acta Anaesthesiol. Scand. 2006, 50, 265–282. [Google Scholar] [CrossRef]
- Hardman, J.G.; Limbird, L.E.; Gilman, A.G. Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 10th ed.; McGraw-Hill: New York, NY, USA, 2001. [Google Scholar]
- Kelly, A.M.; Cohen, M.; Richards, D. Minimizing the pain of local infiltration anesthesia for wounds by injection into the wound edges. J. Emerg. Med. 1994, 12, 593–595. [Google Scholar] [CrossRef]
- Burns, C.A.; Ferris, G.; Feng, C.; Cooper, J.Z.; Brown, M.D. Decreasing the pain of local anesthesia: A prospective, double-blind comparison of buffered, premixed 1% lidocaine with epinephrine versus 1% lidocaine freshly mixed with epinephrine. J. Am. Acad. Dermatol. 2006, 54, 128–131. [Google Scholar] [CrossRef] [PubMed]
- Hogan, M.E.; Vandervaart, S.; Perampaladas, K.; MacHado, M.; Einarson, T.R.; Taddio, A. Systematic review and meta-analysis of the effect of warming local anesthetics on injection pain. Ann. Emerg. Med. 2011, 58, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Whiteman, A.; Bajaj, S.; Hasan, M. Novel techniques of local anaesthetic infiltration. Contin. Educ. Anaesth. Crit. Care Pain 2011, 11, 167–171. [Google Scholar] [CrossRef]
- White, P.F.; Rawal, S.; Latham, P.; Markowitz, S.; Issioui, T.; Chi, L.; Dellaria, S.; Shi, C.; Morse, L.; Ing, C. Use of a continuous local anesthetic infusion for pain management after median sternotomy. Anesthesiology 2003, 99, 918–923. [Google Scholar] [CrossRef]
- Yadav, U.; Srivastava, S.; Srivastav, D. Postoperative analgesic effect of bupivacaine alone and with dexmedetomidine in wound instillation for lumbar laminectomy: A randomized control trial. Anesth. Essays Res. 2020, 14, 149. [Google Scholar] [CrossRef]
- Bharti, N.; Dontukurthy, S.; Bala, I.; Singh, G. Postoperative analgesic effect of intravenous (i.v.) clonidine compared with clonidine administration in wound infiltration for open cholecystectomy. Br. J. Anaesth. 2013, 111, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Kaki, A.M.; Al Marakbi, W. Post-herniorrhaphy infiltration of tramadol versus bupivacaine for postoperative pain relief: A randomized study. Ann. Saudi Med. 2008, 28, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Rømsing, J.; Mysager, S.; Vilmann, P.; Sonne, J.; Larsen, N.E.; Øtergaard, D. Postoperative analgesia is not different after local vs. systemic administration of meloxicam in patients undergoing inguinal hernia repair. Can. J. Anesth. 2001, 48, 978–984. [Google Scholar] [CrossRef] [PubMed]
- Karamanlioglu, B.; Turan, A.; Memis, D.; Kaya, G.; Ozata, S.; Ture, M. Infiltration with ropivacaine plus lornoxicam reduces postoperative pain and opioid consumption. Can. J. Anesth. 2005, 52, 1047–1053. [Google Scholar] [CrossRef]
- Loh, J.W.; Taib, N.A.; Cheong, Y.T.; Tin, T.S. A Double-Blind, Randomized Controlled Trial of Pre-incision Wound Infiltration Using Diclofenac Versus Bupivacaine for Post-operative Pain Relief in Open Thyroid and Parathyroid Surgery. World J. Surg. 2020, 44, 2656–2666. [Google Scholar] [CrossRef]
- Pace, V.; Gul, A.; Prakash, V.; Park, C.; Placella, G.; Raine, G. Wound Infiltration with Levobupivacaine, Ketorolac, and Adrenaline for Postoperative Pain Control after Spinal Fusion Surgery. Asian Spine J. 2020, 15, 539–544. [Google Scholar] [CrossRef]
- Mohamed, S.A.; Sayed, D.M.; El Sherif, F.A.; Abd El-Rahman, A.M. Effect of local wound infiltration with ketamine versus dexmedetomidine on postoperative pain and stress after abdominal hysterectomy, a randomized trial. Eur. J. Pain 2018, 22, 951–960. [Google Scholar] [CrossRef]
- Macfarlane, A.J.R.; Gitman, M.; Bornstein, K.J.; El-Boghdadly, K.; Weinberg, G. Updates in our understanding of local anaesthetic systemic toxicity: A narrative review. Anaesthesia 2021, 76, 27–39. [Google Scholar] [CrossRef]
- CP, H.; JP, T. Pharmacokinetics of anaesthetic drugs at extremes of body weight. BJA Educ. 2018, 18, 364–370. [Google Scholar] [CrossRef]
- Zink, W.; Graf, B.M. The toxicity of local anesthetics: The place of ropivacaine and levobupivacaine. Curr. Opin. Anaesthesiol. 2008, 21, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Casati, A.; Putzu, M. Bupivacaine, levobupivacaine and ropivacaine: Are they clinically different? Best Pract. Res. Clin. Anaesthesiol. 2005, 19, 247–268. [Google Scholar] [CrossRef]
- Park, K.K.; Sharon, V.R. A review of local anesthetics: Minimizing risk and side effects in cutaneous surgery. Dermatol. Surg. 2017, 43, 173–187. [Google Scholar] [CrossRef]
- Kouba, D.J.; Lopiccolo, M.C.; Alam, M.; Bordeaux, J.S.; Cohen, B.; Hanke, C.W.; Jellinek, N.; Maibach, H.I.; Tanner, J.W.; Vashi, N.; et al. Guidelines for the use of local anesthesia in office-based dermatologic surgery. J. Am. Acad. Dermatol. 2016, 74, 1201–1219. [Google Scholar] [CrossRef]
- Koay, J.; Orengo, I. Application of local anesthetics in dermatologic surgery. Dermatol. Surg. 2002, 28, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, P.H.; Veering, B.T.; Urmey, W.F. Maximum recommended doses of local anesthetics: A multifactorial concept. Reg. Anesth. Pain Med. 2004, 29, 564–575. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.; Gadsden, J.; Connery, C. Local Infiltration Anesthesia. In Textbook of Regional Anesthesia and Acute Pain Management, 1st ed.; Hadzic, A., Ed.; McGraw-Hill Medical: New York, NY, USA, 2007; pp. 181–192. [Google Scholar]
- Oxman, A.D. Grading quality of evidence and strength of recommendations. Br. Med. J. 2004, 328, 1490–1494. [Google Scholar]
- Gottschalk, A.; Burmeister, M.A.; Radtke, P.; Krieg, M.; Farokhzad, F.; Kreissl, S.; Strauss, M.; Standl, T. Continuous wound infiltration with ropivacaine reduces pain and analgesic requirement after shoulder surgery. Anesth. Analg. 2003, 97, 1086–1091. [Google Scholar] [CrossRef] [PubMed]
- Pacik, P.T. Pain management in augmentation mammaplasty: A randomized, comparative study of the use of a continuous infusion versus self-administration intermittent bolus of a local anesthetic. Aesthetic Surg. J. 2004, 24, 523–530. [Google Scholar] [CrossRef][Green Version]
- Andersen, L.; Husted, H.; Kristensen, B.B.; Otte, K.S.; Gaarn-Larsen, L.; Kehlet, H. Analgesic efficacy of subcutaneous local anaesthetic wound infiltration in bilateral knee arthroplasty: A randomised, placebo-controlled, double-blind trial. Acta Anaesthesiol. Scand. 2010, 54, 543–548. [Google Scholar] [CrossRef]
- Bianconi, M.; Ferraro, L.; Ricci, R.; Zanoli, G.; Antonelli, T.; Giulia, B.; Guberti, A.; Massari, L. The Pharmacokinetics and Efficacy of Ropivacaine Continuous Wound Instillation after Spine Fusion Surgery. Anesth. Analg. 2004, 98, 166–172. [Google Scholar] [CrossRef]
- Blumenthal, S.; Dullenkopf, A.; Rentsch, K.; Borgeat, A. Continuous infusion of ropivacaine for pain relief after iliac crest bone grafting for shoulder surgery. Anesthesiology 2005, 102, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Thörn, S.E.; Axelsson, K.; Larsson, L.G.; Ågren, G.; Holmström, B.; Rawal, N. Postoperative Pain Relief Using Intermittent Injections of 0.5% Ropivacaine Through a Catheter After Laparoscopic Cholecystectomy. Anesth. Analg. 2002, 95, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Ansaloni, L.; Agnoletti, V.; Bettini, D.; Caira, A.; Calli, M.; Catena, F.; Celotti, M.; De Cataldis, A.; Gagliardi, S.; Gasperoni, E.; et al. The analgesic efficacy of continuous elastomeric pump ropivacaine wound instillation after appendectomy. J. Clin. Anesth. 2007, 19, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Givens, V.A.; Lipscomb, G.H.; Meyer, N.L. A randomized trial of postoperative wound irrigation with local anesthetic for pain after cesarean delivery. Am. J. Obstet. Gynecol. 2002, 186, 1188–1191. [Google Scholar] [CrossRef]
- Gupta, A.; Perniola, A.; Axelsson, K.; Thörn, S.E.; Crafoord, K.; Rawal, N. Postoperative pain after abdominal hysterectomy: A double-blind comparison between placebo and local anesthetic infused intraperitoneally. Anesth. Analg. 2004, 99, 1173–1179. [Google Scholar] [CrossRef]
- Rawal, N.; Gupta, A.; Helsing, M.; Grell, K.; Allvin, R. Pain relief following breast augmentation surgery: A comparison between incisional patient-controlled regional analgesia and traditional oral analgesia. Eur. J. Anaesthesiol. 2006, 23, 1010–1017. [Google Scholar] [CrossRef]
- SafeLocal by Johns Hopkins Digital. Available online: https://appadvice.com/app/safelocal/1440999841 (accessed on 14 January 2021).
- Cousins, M.J.; Mather, L.E. Clinical pharmacology of local anaesthetics. Anaesth. Intensive Care 1980, 8, 257–277. [Google Scholar] [CrossRef]
- Abrão, J.; Fernandes, C.R.; White, P.F.; Shimano, A.C.; Okubo, R.; Lima, G.B.; Bachur, J.A.; Garcia, S.B. Effect of local anaesthetic infiltration with bupivacaine and ropivacaine on wound healing: A placebo-controlled study. Int. Wound J. 2014, 11, 379–385. [Google Scholar] [CrossRef]
- Kampe, S.; Poetter, C.; Buzello, S.; Wenchel, H.M.; Paul, M.; Kiencke, P.; Kasper, S.M. Ropivacaine 0.1% with sufentanil 1 μg/mL inhibits in vitro growth of Pseudomonas aeruginosa and does not promote multiplication of Staphylococcus aureus. Anesth. Analg. 2003, 97, 409–411. [Google Scholar] [CrossRef]
- Carvalho, B.; Clark, D.J.; Yeomans, D.C.; Angst, M.S. Continuous subcutaneous instillation of bupivacaine compared to saline reduces interleukin 10 and increases substance p in surgical wounds after cesarean delivery. Anesth. Analg. 2010, 111, 1452–1459. [Google Scholar] [CrossRef]
- Newton, D.J.; McLeod, G.A.; Khan, F.; Belch, J.J.F. Vasoactive characteristics of bupivacaine and levobupivacaine with and without adjuvant epinephrine in peripheral human skin. Br. J. Anaesth. 2005, 94, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Byager, N.; Hansen, M.S.; Mathiesen, O.; Dahl, J.B. The analgesic effect of wound infiltration with local anaesthetics after breast surgery: A qualitative systematic review. Acta Anaesthesiol. Scand. 2014, 58, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Kuan, Y.M.; Smith, S.; Miles, C.; Grigg, M. Effectiveness of intra-operative wound infiltration with long-acting local anaesthetic. ANZ J. Surg. 2002, 72, 18–20. [Google Scholar] [CrossRef] [PubMed]
- Campbell, I.; Cavanagh, S.; Creighton, J.; French, R.; Banerjee, S.; Kerr, E.; Shirley, R. To infiltrate or not? Acute effects of local anaesthetic in breast surgery. ANZ J. Surg. 2015, 85, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Schurr, M.J.; Gordon, D.B.; Pellino, T.A.; Scanlon, T.A. Continuous local anesthetic infusion for pain management after outpatient inguinal herniorrhaphy. Surgery 2004, 136, 761–769. [Google Scholar] [CrossRef]
- Claroni, C.; Marcelli, M.E.; Sofra, M.C.; Covotta, M.; Torregiani, G.; Giannarelli, D.; Forastiere, E. Preperitoneal continuous infusion of local anesthetics: What is the impact on surgical wound infections in humans? Pain Med. 2016, 17, 582–589. [Google Scholar] [CrossRef][Green Version]
- Shimizu, K.; Hoshi, T.; Iijima, T. Occlusion of multi-holed catheters used in continuous wound infusion in open gynecologic surgery: A pathological study. Saudi J. Anaesth. 2020, 14, 302–306. [Google Scholar] [CrossRef]
- Ventham, N.T.; Hughes, M.; O’Neill, S.; Johns, N.; Brady, R.R.; Wigmore, S.J. Systematic review and meta-analysis of continuous local anaesthetic wound infiltration versus epidural analgesia for postoperative pain following abdominal surgery. Br. J. Surg. 2013, 100, 1280–1289. [Google Scholar] [CrossRef]
- Tam, K.W.; Chen, S.Y.; Huang, T.W.; Lin, C.C.; Su, C.M.; Li, C.L.; Ho, Y.S.; Wang, W.Y.; Wu, C.H. Effect of wound infiltration with ropivacaine or bupivacaine analgesia in breast cancer surgery: A meta-analysis of randomized controlled trials. Int. J. Surg. 2015, 22, 79–85. [Google Scholar] [CrossRef]
- Hong, S.S.; Milross, M.A.; Alison, J.A. Effect of continuous local anesthetic in post-cardiac surgery patients: A systematic review. Pain Med. 2018, 19, 1077–1090. [Google Scholar] [CrossRef]
- Mazzeffi, M.; Khelemsky, Y. Poststernotomy pain: A clinical review. J. Cardiothorac. Vasc. Anesth. 2011, 25, 1163–1178. [Google Scholar] [CrossRef] [PubMed]
- Jellish, W.S. Opioid-Sparing Analgesia for Sternotomy: Do Surgical Site Continuous Local Anesthetics Actually Work? J. Cardiothorac. Vasc. Anesth. 2019, 33, 385–387. [Google Scholar] [CrossRef]
- Kar, P.; Ramachandran, G. Pain relief following sternotomy in conventional cardiac surgery: A review of non neuraxial regional nerve blocks. Ann. Card. Anaesth. 2020, 23, 200–208. [Google Scholar] [CrossRef]
- Eljezi, V.; Imhoff, E.; Bourdeaux, D.; Pereira, B.; Farhat, M.; Schoeffler, P.; Azarnoush, K.; Duale, C. Bilateral sternal infusion of ropivacaine and length of stay in ICU after cardiac surgery with increased respiratory risk A randomised controlled trial. Eur. J. Anaesthesiol. 2017, 34, 56–65. [Google Scholar] [CrossRef]
- Eljezi, V.; Dualé, C.; Azarnoush, K.; Skrzypczak, Y.; Sautou, V.; Pereira, B.; Tsokanis, I.; Schoeffler, P. The analgesic effects of a bilateral sternal infusion of ropivacaine after cardiac surgery. Reg. Anesth. Pain Med. 2012, 37, 166–174. [Google Scholar] [CrossRef]
- Kocabas, S.; Yedicocuklu, D.; Yuksel, E.; Uysallar, E.; Askar, F. Infiltration of the sternotomy wound and the mediastinal tube sites with 0.25% levobupivacaine as adjunctive treatment for postoperative pain after cardiac surgery. Eur. J. Anaesthesiol. 2008, 25, 842–849. [Google Scholar] [CrossRef] [PubMed]
- McDonald, S.B.; Jacobsohn, E.; Kopacz, D.J.; Desphande, S.; Helman, J.D.; Salinas, F.; Hall, R.A. Parasternal block and local anesthetic infiltration with levobupivacaine after cardiac surgery with desflurane: The effect on postoperative pain, pulmonary function, and tracheal extubation times. Anesth. Analg. 2005, 100, 25–32. [Google Scholar] [CrossRef]
- Bethenod, F.; Ellouze, O.; Berthoud, V.; Missaoui, A.; Cransac, A.; Aho, S.; Bouchot, O.; Girard, C.; Guinot, P.G.; Bouhemad, B. A single dose of tramadol in continuous wound analgesia with levobupivacaine does not reduce post-sternotomy pain: A randomized controlled trial. J. Pain Res. 2019, 12, 2733–2741. [Google Scholar] [CrossRef]
- Borges, M.F.; Coulson, A.S. Minimally invasive coronary bypass surgery: Postoperative pain management using intermittent bupivacaine infiltration. Br. J. Anaesth. 1998, 80, 519–520. [Google Scholar] [CrossRef]
- Nasr, D.; Abdelhamid, H.; Mohsen, M.; Aly, A. The analgesic efficacy of continuous presternal bupivacaine infusion through a single catheter after cardiac surgery. Ann. Card. Anaesth. 2015, 18, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Nuttall, G.A.; Johnson, M.E.; Hanson, A.C.; Oliver, W.C. A prospective, randomized, blinded study of continuous ropivacaine infusion in the median sternotomy incision following cardiac surgery. Reg. Anesth. Pain Med. 2013, 38, 145–150. [Google Scholar] [CrossRef]
- Mijovski, G.; Podbregar, M.; Kšela, J.; Jenko, M.; Šoštarič, M. Effectiveness of wound infusion of 0.2% ropivacaine by patient control analgesia pump after minithoracotomy aortic valve replacement: A randomized, double-blind, placebo-controlled trial. BMC Anesthesiol. 2020, 20, 1–9. [Google Scholar] [CrossRef]
- Pala, A.A.; Urcun, Y.S.; Çiçek, Ö.F.; Şahin, S. Can Continuous Local Anesthetic Infusion After Median Sternotomy Reduce Opioid Use? Cureus 2020, 12, e10711. [Google Scholar] [CrossRef]
- Magnano, D.; Montalbano, R.; Lamarra, M.; Ferri, F.; Lorini, L.; Clarizia, S.; Rescigno, G. Ineffectiveness of local wound anesthesia to reduce postoperative pain after median sternotomy. J. Card. Surg. 2005, 20, 314–318. [Google Scholar] [CrossRef]
- Dowling, R.; Thielmeier, K.; Ghaly, A.; Barber, D.; Boice, T.; Dine, A. Improved pain control after cardiac surgery: Results of a randomized, double-blind, clinical trial. J. Thorac. Cardiovasc. Surg. 2003, 126, 1271–1278. [Google Scholar] [CrossRef]
- Kamel, E.Z.; Abd-Elshafy, S.K.; Sayed, J.A.; Mostafa, M.M.; Seddik, M.I. Pain alleviation in patients undergoing cardiac surgery; Presternal local anesthetic and magnesium infiltration versus conventional intravenous analgesia: A randomized double-blind study. Korean J. Pain 2018, 31, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Chiu, K.M.; Wu, C.C.; Wang, M.J.; Lu, C.W.; Shieh, J.S.; Lin, T.Y.; Chu, S.H. Local infusion of bupivacaine combined with intravenous patient-controlled analgesia provides better pain relief than intravenous patient-controlled analgesia alone in patients undergoing minimally invasive cardiac surgery. J. Thorac. Cardiovasc. Surg. 2008, 135, 1348–1352. [Google Scholar] [CrossRef]
- Koukis, I.; Argiriou, M.; Dimakopoulou, A.; Panagiotakopoulos, V.; Theakos, N.; Charitos, C. Use of continuous subcutaneous anesthetic infusion in cardiac surgical patients after median sternotomy. J. Cardiothorac. Surg. 2008, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- FDA. FDA Strengthens Warning of Heart Attack and Stroke Risk for Non-Steroidal Anti-Inflammatory Drugs. Available online: https://www.fda.gov/consumers/consumer-updates/fda-strengthens-warning-heart-attack-and-stroke-risk-non-steroidal-anti-inflammatory-drugs (accessed on 6 June 2021).
- Kulik, A.; Bykov, K.; Choudhry, N.K.; Bateman, B.T. Non-steroidal anti-inflammatory drug administration after coronary artery bypass surgery: Utilization persists despite the boxed warning. Pharmacoepidemiol. Drug Saf. 2015, 24, 647–653. [Google Scholar] [CrossRef]
- Hodson, M.; Gajraj, R.; Scott, N.B. A comparison of the antibacterial activity of levobupivacaine vs. bupivacaine: An in vitro study with bacteria implicated in epidural infection. Anaesthesia 1999, 54, 699–702. [Google Scholar] [CrossRef] [PubMed]
- Gerner, P. Postthoracotomy Pain Management Problems. Anesthesiol. Clin. 2008, 26, 355–367. [Google Scholar] [CrossRef]
- Bayman, E.O.; Parekh, K.R.; Keech, J.; Selte, A.; Brennan, T.J. A Prospective Study of Chronic Pain after Thoracic Surgery. Anesthesiology 2017, 126, 938–951. [Google Scholar] [CrossRef]
- Batchelor, T.J.P.; Rasburn, N.J.; Abdelnour-Berchtold, E.; Brunelli, A.; Cerfolio, R.J.; Gonzalez, M.; Ljungqvist, O.; Petersen, R.H.; Popescu, W.M.; Slinger, P.D.; et al. Guidelines for enhanced recovery after lung surgery: Recommendations of the Enhanced Recovery after Surgery (ERAS®) Society and the European Society of Thoracic Surgeons (ESTS). Eur. J. Cardiothorac. Surg. 2019, 55, 91–115. [Google Scholar] [CrossRef]
- Gebhardt, R.; John Mehran, R.; Soliz, J.; Cata, J.P.; Smallwood, A.K.; Feeley, T.W. Epidural versus ON-Q local anesthetic-infiltrating catheter for post-thoracotomy pain control. J. Cardiothorac. Vasc. Anesth. 2013, 27, 423–426. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, G.; Wei, S.; He, Z.; Sun, L.; Zheng, H. Comparison of the effects of ultrasound-guided erector spinae plane block and wound infiltration on perioperative opioid consumption and postoperative pain in thoracotomy. J. Coll. Physicians Surg. Pak. 2019, 29, 1138–1143. [Google Scholar] [CrossRef]
- Yang, H.C.; Lee, J.Y.; Ahn, S.; Cho, S.; Kim, K.; Jheon, S.; Kim, J.S. Pain control of thoracoscopic major pulmonary resection: Is pre-emptive local bupivacaine injection able to replace the intravenous patient controlled analgesia? J. Thorac. Dis. 2015, 7, 1960–1969. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shu, L.; Lin, C.; Yang, P.; Zhou, Y.; Wang, Q.; Wu, Y.; Xu, X.; Cui, X.; Lin, X.; et al. Comparison between Intraoperative Two-Space Injection Thoracic Paravertebral Block and Wound Infiltration as a Component of Multimodal Analgesia for Postoperative Pain Management after Video-Assisted Thoracoscopic Lobectomy: A Randomized Controlled Trial. J. Cardiothorac. Vasc. Anesth. 2015, 29, 1550–1556. [Google Scholar] [CrossRef] [PubMed]
- Ganapathy, S.; Brookes, J.; Bourne, R. Local infiltration analgesia. Anesthesiol. Clin. 2011, 29, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Rawal, N. Epidural technique for postoperative pain: Gold standard no more? Reg. Anesth. Pain Med. 2012, 37, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Shi, W.; Chen, C.; Li, H.; Zheng, X.; Zheng, X.; Niu, C. Efficacy of dexmedetomidine as an adjuvant to local wound infiltration anaesthesia in abdominal surgery: A meta-analysis of randomised controlled trials. Int. Wound J. 2019, 16, 1206–1213. [Google Scholar] [CrossRef]
- Liddle, D.; Varghese, M.; Chander, R.; Kaur, B. Wound infiltration with plain bupivacaine as compared with bupivacaine fentanyl mixture for postoperative pain relief after abdominal surgery. Anesth. Essays Res. 2011, 5, 142. [Google Scholar] [CrossRef]
- Tilleul, P.; Aissou, M.; Bocquet, F.; Thiriat, N.; Le Grelle, O.; Burke, M.J.; Hutton, J.; Beaussier, M. Cost-effectiveness analysis comparing epidural, patient-controlled intravenous morphine, and continuous wound infiltration for postoperative pain management after open abdominal surgery. Br. J. Anaesth. 2012, 108, 998–1005. [Google Scholar] [CrossRef] [PubMed]
- Willard, P.T.; Blair, N. Is wound infiltration with anesthetic effective as pre-emptive analgesia? A clinical trial in appendectomy patients. Can. J. Surg. 1997, 40, 213. [Google Scholar] [PubMed]
- Ahn, S.R.; Kang, D.B.; Lee, C.; Park, W.C.; Lee, J.K. Postoperative pain relief using wound infiltration with 0.5% bupivacaine in single-incision laparoscopic surgery for an appendectomy. Ann. Coloproctol. 2013, 29, 238–242. [Google Scholar] [CrossRef]
- Lohsiriwat, V.; Lert-Akyamanee, N.; Rushatamukayanunt, W. Efficacy of pre-incisional bupivacaine infiltration on postoperative pain relief after appendectomy: Prospective double-blind randomized trial. World J. Surg. 2004, 28, 947–950. [Google Scholar] [CrossRef]
- Turner, G.A.; Chalkiadis, G. Comparison of preoperative with postoperative lignocaine infiltration on postoperative analgesic requirements. Br. J. Anaesth. 1994, 72, 541–543. [Google Scholar] [CrossRef]
- Cervini, P.; Smith, L.C.; Urbach, D.R. The effect of intraoperative bupivacaine administration on parenteral narcotic use after laparoscopic appendectomy. Surg. Endosc. Other Interv. Tech. 2002, 16, 1579–1582. [Google Scholar] [CrossRef] [PubMed]
- Loizides, S.; Gurusamy, K.S.; Nagendran, M.; Rossi, M.; Guerrini, G.P.; Davidson, B.R. Wound infiltration with local anaesthetic agents for laparoscopic cholecystectomy. Cochrane Database Syst. Rev. 2014, 3, CD007049. [Google Scholar] [CrossRef]
- Liang, M.; Chen, Y.; Zhu, W.; Zhou, D. Efficacy and safety of different doses of ropivacaine for laparoscopy-assisted infiltration analgesia in patients undergoing laparoscopic cholecystectomy: A prospective randomized control trial. Medicine 2020, 99, e22540. [Google Scholar] [CrossRef] [PubMed]
- Pavlidis, T.E.; Atmatzidis, K.S.; Papaziogas, B.T.; Makris, J.G.; Lazaridis, C.N.; Papaziogas, T.B. The effect of preincisional periportal infiltration with ropivacaine in pain relief after laparoscopic procedures: A prospective, randomized controlled trial. JSLS J. Soc. Laparoendosc. Surg. 2003, 7, 305. [Google Scholar]
- Barczyński, M.; Konturek, A.; Herman, R.M. Superiority of preemptive analgesia with intraperitoneal instillation of bupivacaine before rather than after the creation of pneumoperitoneum for laparoscopic cholecystectomy: A randomized, double-blind, placebo-controlled study. Surg. Endosc. Other Interv. Tech. 2006, 20, 1088–1093. [Google Scholar] [CrossRef]
- Fassoulaki, A.; Vassi, E.; Korkolis, D.; Zotou, M. Perioperative continuous ropivacaine wound infusion in laparoscopic cholecystectomy: A randomized controlled double-blind trial. Surg. Laparosc. Endosc. Percutaneous Tech. 2016, 26, 25–30. [Google Scholar] [CrossRef]
- Altuntaş, G.; Akkaya, Ö.T.; Özkan, D.; Sayın, M.M.; Balas, Ş.; Özlü, E. Comparison of intraabdominal and trocar site local anaesthetic infiltration on postoperative analgesia after laparoscopic cholecystectomy. Turk Anesteziyoloji Reanimasyon Dern. Derg. 2016, 44, 306–311. [Google Scholar] [CrossRef]
- Lepner, U.; Goroshina, J.; Samarütel, J. Postoperative pain relief after laparoscopic cholecystectomy: A randomised prospective double-blind clinical trial. Scand. J. Surg. 2003, 92, 121–124. [Google Scholar]
- Souto, M.M.; Radaelli, E.; Giordani, A.E.; Savaris, A.; Bassols, G.F. Effectiveness of Local Anesthetics in Laparoscopic Cholecystectomy: A Randomized Clinical Trial. Surg. Laparosc. Endosc. Percutaneous Tech. 2015, 25, 317–320. [Google Scholar] [CrossRef]
- Pappas-Gogos, G.; Tsimogiannis, K.E.; Zikos, N.; Nikas, K.; Manataki, A.; Tsimoyiannis, E.C. Preincisional and intraperitoneal ropivacaine plus normal saline infusion for postoperative pain relief after laparoscopic cholecystectomy: A randomized double-blind controlled trial. Surg. Endosc. Other Interv. Tech. 2008, 22, 2036–2045. [Google Scholar] [CrossRef]
- Feroci, F.; Kröning, K.C.; Scatizzi, M. Effectiveness for pain after laparoscopic cholecystectomy of 0.5% bupivacaine-soaked Tabotamp® placed in the gallbladder bed: A prospective, randomized, clinical trial. Surg. Endosc. 2009, 23, 2214–2220. [Google Scholar] [CrossRef]
- Abet, E.; Orion, F.; Denimal, F.; Brau-Weber, A.G.; de Kerviler, B.; Jean, M.H.; Boulanger, G.; Dimet, J.; Comy, M. Interest of Using Ropivacaine for Outpatient Laparoscopic Cholecystectomy: Prospective Randomized Trial. World J. Surg. 2017, 41, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Zayas-González, H.; González-Hernández, A.; Manzano-García, A.; Hernández-Rivero, D.; García-Cuevas, M.A.; Granados-Mortera, J.C.; Vaca-Aguirre, L.; Flores-Fierro, S.; Martínez-Lorenzana, G.; Condés-Lara, M. Effect of local infiltration with oxytocin on hemodynamic response to surgical incision and postoperative pain in patients having open laparoscopic surgery under general anesthesia. Eur. J. Pain 2019, 23, 1519–1526. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Navarro, A.J.; Berde, C.B.; Wiedmaier, G.; Mercado, A.; Garcia, C.; Iglesias, V.; Zurakowski, D. Comparison of neosaxitoxin versus bupivacaine via port infiltration for postoperative analgesia following laparoscopic cholecystectomy: A randomized, double-blind trial. Reg. Anesth. Pain Med. 2011, 36, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Ellakany, M.; Elatter, A.I.; Moustafa Teima, M.A.; Megahed, N.A. Comparison between analgesic effect of bupivacaine thoracic epidural and ketamine infusion plus wound infiltration with local anesthetics in open cholecystectomy. Anesth. Essays Res. 2014, 8, 162. [Google Scholar] [CrossRef]
- Kadam, V.R.; Howell, S.; Kadam, V. Evaluation of postoperative pain scores following ultrasound guided transversus abdominis plane block versus local infiltration following day surgery laparoscopic cholecystectomy-retrospective study. J. Anaesthesiol. Clin. Pharmacol. 2016, 32, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Elamin, G.; Waters, P.S.; Hamid, H.; O’Keeffe, H.M.; Waldron, R.M.; Duggan, M.; Khan, W.; Barry, M.K.; Khan, I.Z. Efficacy of a Laparoscopically Delivered Transversus Abdominis Plane Block Technique during Elective Laparoscopic Cholecystectomy: A Prospective, Double-Blind Randomized Trial. J. Am. Coll. Surg. 2015, 221, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.H.; Hamade, A.; Choudhry, M.N.; Hamza, N.; Nadeem, R.; Ammori, B.J. Infiltration of wounds and extraperitoneal space with local anesthetic in patients undergoing laparoscopic totally extraperitoneal repair of unilateral inguinal hernias: A randomized double-blind placebo-controlled trial. Scand. J. Surg. 2010, 99, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Ausems, M.E.; Hulsewé, K.W.; Hooymans, P.M.; Hoofwijk, A.G. Postoperative analgesia requirements at home after inguinal hernia repair: Effects of wound infiltration on postoperative pain. Anaesthesia 2007, 62, 325–331. [Google Scholar] [CrossRef]
- Qureshi, R.; Khan, F. Effects of bupivacaine infiltration on postoperative tramadol consumption in elective day care unilateral inguinal hernia repair. J. Pak. Med. Assoc. 2016, 66, 256. [Google Scholar]
- Song, Y.; Han, B.; Lei, W.; Kou, Y.; Liu, Y.; Gong, Y.; Ma, D. Low concentrations of lidocaine for inguinal hernia repair under local infiltration anaesthesia. J. Int. Med. Res. 2013, 41, 371–377. [Google Scholar] [CrossRef]
- Kingsnorth, A.N.; Cummings, C.G.; Bennett, D.H. Local anaesthesia in elective inguinal hernia repair: A randomised, double-blind study comparing the efficacy of levobupivacaine with racemic bupivacaine. Eur. J. Surg. 2002, 168, 391–396. [Google Scholar] [CrossRef]
- LeBlanc, K.A.; Bellanger, D.; Rhynes, V.K.; Hausmann, M. Evaluation of continuous infusion of 0.5% bupivacaine by elastomeric pump for postoperative pain management after open inguinal hernia repair. J. Am. Coll. Surg. 2005, 200, 198–202. [Google Scholar] [CrossRef]
- Velanovich, V.; Rider, P.; Deck, K.; Minkowitz, H.S.; Leiman, D.; Jones, N.; Niebler, G. Safety and Efficacy of Bupivacaine HCl Collagen-Matrix Implant (INL-001) in Open Inguinal Hernia Repair: Results from Two Randomized Controlled Trials. Adv. Ther. 2019, 36, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Giraldes, A.L.A.; Sousa, A.M.; Slullitel, A.; Guimarães, G.M.N.; Santos, M.G.M.E.; Pinto, R.E.; Ashmawi, H.A.; Sakata, R.K. Tramadol wound infiltration is not different from intravenous tramadol in children: A randomized controlled trial. J. Clin. Anesth. 2016, 28, 62–66. [Google Scholar] [CrossRef]
- Zheng, X.; Feng, X.; Cai, X.J. Effectiveness and safety of continuous wound infiltration for postoperative pain management after open gastrectomy. World J. Gastroenterol. 2016, 22, 1902–1910. [Google Scholar] [CrossRef]
- Zhu, Z.P.; Chen, B.R.; Ye, W.P.; Wang, S.J.; Xu, G.X.; Pan, Z.R.; Zeng, J.J.; Luo, Q.; Jun, Y.; Huang, Z.J. Clinical significance of wound infiltration with ropivacaine for elderly patients in china underwent total laparoscopic radical gastrectomy: A retrospective cohort study. Medicine 2019, 98, e15115. [Google Scholar] [CrossRef]
- Kostic, T.; Bojic, S. Modified intermitent subfascial wound infiltration with local anaesthetic after open gastric bypass. Serb. J. Anesth. Intensive Ther. 2017, 39, 81–85. [Google Scholar] [CrossRef]
- Moncada, R.; Martinaitis, L.; Landecho, M.; Rotellar, F.; Sanchez-Justicia, C.; Bellver, M.; de la Higuera, M.; Silva, C.; Osés, B.; Martín, E.; et al. Does Preincisional Infiltration with Bupivacaine Reduce Postoperative Pain in Laparoscopic Bariatric Surgery? Obes. Surg. 2016, 26, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Luan, H.; Zhu, P.; Zhang, X.; Tian, L.; Feng, J.; Wu, Y.; Yan, Y.; Zhao, Z.; Gu, X. Effect of dexmedetomidine as an adjuvant to ropivacaine for wound infiltration in patients undergoing open gastrectomy: A prospective randomized controlled trial. Medicine 2017, 96, e7950. [Google Scholar] [CrossRef] [PubMed]
- Abdelsalam, K.; Mohamdin, O. Ultrasound-guided rectus sheath and transversus abdominis plane blocks for perioperative analgesia in upper abdominal surgery: A randomized controlled study. Saudi J. Anaesth. 2016, 10, 25–28. [Google Scholar] [CrossRef]
- Xin, Y.; Hong, Y.; Yong, L.Z. Efficacy of postoperative continuous wound infiltration with local anesthesia after open hepatectomy. Clin. J. Pain 2014, 30, 571–576. [Google Scholar] [CrossRef]
- Sun, J.X.; Bai, K.Y.; Liu, Y.F.; Du, G.; Fu, Z.H.; Zhang, H.; Yang, J.H.; Wang, B.; Wang, X.Y.; Jin, B. Effect of local wound infiltration with ropivacaine on postoperative pain relief and stress response reduction after open hepatectomy. World J. Gastroenterol. 2017, 23, 6733–6740. [Google Scholar] [CrossRef] [PubMed]
- Peres-Bachelot, V.; Blanc, E.; Oussaid, N.; Pérol, D.; Daunizeau-Walker, A.L.; Pouderoux, S.; Peyrat, P.; Rivoire, M.; Dupré, A. A 96-hour continuous wound infiltration with ropivacaine reduces analgesic consumption after liver resection: A randomized, double-blind, controlled trial. J. Surg. Oncol. 2019, 119, 47–55. [Google Scholar] [CrossRef]
- Mungroop, T.H.; Veelo, D.P.; Busch, O.R.; van Dieren, S.; van Gulik, T.M.; Karsten, T.M.; de Castro, S.M.; Godfried, M.B.; Thiel, B.; Hollmann, M.W.; et al. Continuous wound infiltration versus epidural analgesia after hepato-pancreato-biliary surgery (POP-UP): A randomised controlled, open-label, non-inferiority trial. Lancet Gastroenterol. Hepatol. 2016, 1, 105–113. [Google Scholar] [CrossRef]
- Che, L.; Lu, X.; Pei, L. Efficacy and Safety of a Continuous Wound Catheter in Open Abdominal Partial Hepatectomy. Chin. Med. Sci. J. 2017, 32, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Du, G.; Liu, Y.F.; Yang, J.H.; A-Niu, M.G.; Zhai, X.Y.; Jin, B. Overlay of a sponge soaked with ropivacaine and multisite infiltration analgesia result in faster recovery after laparoscopic hepatectomy. World J. Gastroenterol. 2019, 25, 5185–5196. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.; Pandanaboyana, S.; Prasad, K.R. Epidural versus local anaesthetic infiltration via wound catheters in open liver resection: A meta-analysis. ANZ J. Surg. 2015, 85, 16–21. [Google Scholar] [CrossRef]
- Gavriilidis, P.; Roberts, K.J.; Sutcliffe, R.P. Local anaesthetic infiltration via wound catheter versus epidural analgesia in open hepatectomy: A systematic review and meta-analysis of randomised controlled trials. HPB 2019, 21, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.; Ward, D.; Jeffery, J.; Toogood, G.J.; Lodge, J.A.; Rao, K.; Lotia, S.; Hidalgo, E. A Randomized Controlled Trial Comparing Epidural Analgesia Versus Continuous Local Anesthetic Infiltration Via Abdominal Wound Catheter in Open Liver Resection. Ann. Surg. 2019, 269, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Grass, F.; Cachemaille, M.; Martin, D.; Fournier, N.; Hahnloser, D.; Blanc, C.; Demartines, N.; Hübner, M. Pain perception after colorectal surgery: A propensity score matched prospective cohort study. Biosci. Trends 2018, 12, 47–53. [Google Scholar] [CrossRef]
- Telletxea, S.; Gonzalez, J.; Portugal, V.; Alvarez, R.; Aguirre, U.; Anton, A.; Arizaga, A. Analgesia basada en infusión continua de anestésico local a nivel interfascial tras cirugía de colon laparoscópico: Ensayo clínico. Rev. Esp. Anestesiol. Reanim. 2016, 63, 197–206. [Google Scholar] [CrossRef]
- Keller, D.S.; Pedraza, R.; Tahilramani, R.N.; Flores-Gonzalez, J.R.; Ibarra, S.; Haas, E.M. Impact of long-acting local anesthesia on clinical and financial outcomes in laparoscopic colorectal surgery. Am. J. Surg. 2017, 214, 53–58. [Google Scholar] [CrossRef]
- Gorfine, S.R.; Onel, E.; Patou, G.; Krivokapic, Z.V. Bupivacaine extended-release liposome injection for prolonged postsurgical analgesia in patients undergoing hemorrhoidectomy: A multicenter, randomized, double-blind, placebo-controlled trial. Dis. Colon Rectum 2011, 54, 1552–1559. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, N.K.; Baik, S.H.; Min, B.S.; Hur, H.; Lee, J.; Noh, H.Y.; Lee, J.H.; Koo, B.N. Effects of postoperative pain management on immune function after laparoscopic resection of colorectal cancer: A randomized study. Medicine 2016, 95, e3602. [Google Scholar] [CrossRef] [PubMed]
- Beaussier, M.; Parc, Y.; Guechot, J.; Cachanado, M.; Rousseau, A.; Lescot, T.; Rollin, M.; Aissou, M.; Lefevre, J.H.; Restoux, A.; et al. Ropivacaine preperitoneal wound infusion for pain relief and prevention of incisional hyperalgesia after laparoscopic colorectal surgery: A randomized, triple-arm, double-blind controlled evaluation vs. intravenous lidocaine infusion, the CATCH study. Color. Dis. 2018, 20, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.C.; Lu, C.C.; Lin, S.E.; Chang, C.L.; Chen, H.H. Infiltration of Local Anesthesia at Wound Site after Single-Incision Laparoscopic Colectomy Reduces Postoperative Pain and Analgesic Usage. Hepatogastroenterology 2015, 62, 811–816. [Google Scholar] [PubMed]
- Li, H.; Chen, R.; Yang, Z.; Nie, C.; Yang, S. Comparison of the postoperative effect between epidural anesthesia and continuous wound infiltration on patients with open surgeries: A meta-analysis. J. Clin. Anesth. 2018, 51, 20–31. [Google Scholar] [CrossRef]
- Pedrazzani, C.; Menestrina, N.; Moro, M.; Brazzo, G.; Mantovani, G.; Polati, E.; Guglielmi, A. Local wound infiltration plus transversus abdominis plane (TAP) block versus local wound infiltration in laparoscopic colorectal surgery and ERAS program. Surg. Endosc. 2016, 30, 5117–5125. [Google Scholar] [CrossRef]
- Pedrazzani, C.; Park, S.Y.; Scotton, G.; Park, J.S.; Kim, H.J.; Polati, E.; Guglielmi, A.; Choi, G.S. Analgesic efficacy of preemptive local wound infiltration plus laparoscopic-assisted transversus abdominis plane block versus wound infiltration in patients undergoing laparoscopic colorectal resection: Study protocol for a randomized, multicenter, single-blind, noninferiority trial. Trials 2019, 20, 1–8. [Google Scholar]
- Yu, N.; Long, X.; Lujan-Hernandez, J.R.; Succar, J.; Xin, X.; Wang, X. Transversus abdominis-plane block versus local anesthetic wound infiltration in lower abdominal surgery: A systematic review and meta-analysis of randomized controlled trials. BMC Anesthesiol. 2014, 14, 121. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Choi, G.S.; Kwak, K.H.; Jung, H.; Jeon, Y.; Park, S.; Yeo, J. Effect of local wound infiltration and transversus abdominis plane block on morphine use after laparoscopic colectomy: A nonrandomized, single-blind prospective study. J. Surg. Res. 2015, 195, 61–66. [Google Scholar] [CrossRef] [PubMed]
- De León-Ballesteros, G.P.; Ramírez-Del Val, A.; Romero-Vélez, G.; Perez-Soto, R.H.; Moctezuma, P.; Santes, O.; Ponce de León-Felix, F.; Salgado-Nesme, N. LAW Trial–The Impact of Local Anesthetics Infiltration in Surgical Wound for Gastrointestinal Procedures (LAW): A Double-Blind, Randomized Controlled Trial. J. Investig. Surg. 2020, 15, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ball, L.; Pellerano, G.; Corsi, L.; Giudici, N.; Pellegrino, A.; Cannata, D.; Santori, G.; Palombo, D.; Pelosi, P.; Gratarola, A. Continuous epidural versus wound infusion plus single morphine bolus as postoperative analgesia in open abdominal aortic aneurysm repair: A randomized non-inferiority trial. Minerva Anestesiol. 2016, 82, 1296–1305. [Google Scholar]
- Cheng, G.S.; Ilfeld, B.M. An Evidence-Based Review of the Efficacy of Perioperative Analgesic Techniques for Breast Cancer-Related Surgery. Pain Med. 2017, 18, 1344–1365. [Google Scholar] [CrossRef]
- Beguinot, M.; Monrigal, E.; Kwiatkowski, F.; Ginzac, A.; Joly, D.; Gayraud, G.; Le Bouedec, G.; Gimbergues, P. Continuous Wound Infiltration With Ropivacaine After Mastectomy: A Randomized Controlled Trial. J. Surg. Res. 2020, 254, 318–326. [Google Scholar] [CrossRef]
- Rica, M.A.I.; Norlia, A.; Rohaizak, M.; Naqiyah, I. Preemptive ropivacaine local anaesthetic infiltration versus postoperative ropivacaine wound infiltration in mastectomy: Postoperative pain and drain outputs. Asian J. Surg. 2007, 30, 34–39. [Google Scholar] [CrossRef][Green Version]
- Kang, R.; Read, J.T.; Glaser, A.C.; Barth, R.J. Eliminating Opioids from Breast Conserving Surgery: Perioperative Pain Management Pathway. J. Am. Coll. Surg. 2020, 230, 975–982. [Google Scholar] [CrossRef]
- Vigneau, A.; Salengro, A.; Berger, J.; Rouzier, R.; Barranger, E.; Marret, E.; Bonnet, F. A double blind randomized trial of wound infiltration with ropivacaine after breast cancer surgery with axillary nodes dissection. BMC Anesthesiol. 2011, 11, 23. [Google Scholar] [CrossRef]
- Albi-Feldzer, A.; Mouret-Fourme, E.E.; Hamouda, S.; Motamed, C.; Dubois, P.Y.; Jouanneau, L.; Jayr, C. A double-blind randomized trial of wound and intercostal space infiltration with ropivacaine during breast cancer surgery: Effects on chronic postoperative pain. Anesthesiology 2013, 118, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.J.; Chen, J.H.; Hsu, H.M.; Wu, C.T.; Yu, J.C. Efficiency of infiltration with bupivacain after modified radical mastectomy. Acta Chir. Belg. 2011, 111, 360–363. [Google Scholar] [CrossRef] [PubMed]
- Sidiropoulou, T.; Buonomo, O.; Fabbi, E.; Silvi, M.B.; Kostopanagiotou, G.; Sabato, A.F.; Dauri, M. A prospective comparison of continuous wound infiltration with ropivacaine versus single-injection paravertebral block after modified radical mastectomy. Anesth. Analg. 2008, 106, 997–1001. [Google Scholar] [CrossRef] [PubMed]
- Bouman, E.A.C.; Theunissen, M.; Kessels, A.G.H.; Keymeulen, K.B.M.I.; Joosten, E.A.J.; Marcus, M.A.E.; Buhre, W.F.; Gramke, H.F. Continuous paravertebral block for postoperative pain compared to general anaesthesia and wound infiltration for major oncological breast surgery. Springerplus 2014, 3, 517. [Google Scholar] [CrossRef]
- Syal, K.; Chandel, A. Comparison of the post-operative analgesic effect of paravertebral block, pectoral nerve block and local infiltration in patients undergoing modified radical mastectomy: A randomised double-blind trial. Indian J. Anaesth. 2017, 61, 643–648. [Google Scholar] [CrossRef]
- Hards, M.; Harada, A.; Neville, I.; Harwell, S.; Babar, M.; Ravalia, A.; Davies, G. The effect of serratus plane block performed under direct vision on postoperative pain in breast surgery. J. Clin. Anesth. 2016, 34, 427–431. [Google Scholar] [CrossRef]
- Nadeem, M.; Sahu, A. Ultrasound guided surgery under Dilutional Local Anaesthesia and no sedation in breast cancer patients. Surgeon 2020, 18, 91–94. [Google Scholar] [CrossRef]
- Bolletta, A.; Dessy, L.A.; Fiorot, L.; Tronci, A.; Rusciani, A.; Ciudad, P.; Trignano, E. Sub-muscular Breast Augmentation Using Tumescent Local Anesthesia. Aesthetic Plast. Surg. 2019, 43, 7–13. [Google Scholar] [CrossRef]
- Baudry, G.; Steghens, A.; Laplaza, D.; Koeberle, P.; Bachour, K.; Bettinger, G.; Combier, F.; Samain, E. Infiltration de ropivacaïne en chirurgie carcinologique du sein:Effet sur la douleur postopératoire aiguë et chronique. Ann. Fr. Anesth. Reanim. 2008, 27, 979–986. [Google Scholar] [CrossRef]
- Johansson, A.; Kornfält, J.; Nordin, L.; Svensson, L.; Ingvar, C.; Lundberg, J. Wound infiltration with ropivacaine and fentanyl: Effects on postoperative pain and PONV after breast surgery. J. Clin. Anesth. 2003, 15, 113–118. [Google Scholar] [CrossRef]
- Gozal, Y.; Shapira, S.; Gozal, D.; Magora, F. Bupivacaine wound infiltration in thyroid surgery reduces postoperative pain and opioid demand. Acta Anaesthesiol. Scand. 1994, 38, 813. [Google Scholar] [CrossRef]
- Bagul, A.; Taha, R.; Metcalfe, M.S.; Brook, N.R.; Nicholson, M.L. Pre-incision infiltration of local anesthetic reduces postoperative pain with no effects on bruising and wound cosmesis after thyroid surgery. Thyroid 2005, 15, 1245–1248. [Google Scholar] [CrossRef]
- Sellami, M.; Feki, S.; Triki, Z.; Zghal, J.; Zouche, I.; Hammami, B.; Charfeddine, I.; Chaari, M.; Ghorbel, A. Bupivacaine wound infiltration reduces postoperative pain and analgesic requirement after thyroid surgery. Eur. Arch. Oto-Rhino-Laryngol. 2018, 275, 1265–1270. [Google Scholar] [CrossRef] [PubMed]
- Miu, M.; Royer, C.; Gaillat, C.; Schaup, B.; Menegaux, F.; Langeron, O.; Riou, B.; Aubrun, F. Lack of analgesic effect induced by ropivacaine wound infiltration in thyroid surgery: A randomized, double-blind, placebo-controlled trial. Anesth. Analg. 2016, 122, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.E.; Kim, E. Local anesthesia with monitored anesthesia care for patients undergoing thyroidectomy—A case series. Korean J. Anesthesiol. 2016, 69, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Eti, Z.; Irmak, P.; Gulluoglu, B.M.; Manukyan, M.N.; Gogus, F.Y. Does bilateral superficial cervical plexus block decrease analgesic requirement after thyroid surgery? Anesth. Analg. 2006, 102, 1174–1176. [Google Scholar] [CrossRef]
- Hoh, S.Y.; Doon, Y.K.; Chong, S.S.; Ng, K.L. Randomized controlled trial comparing bilateral superficial cervical plexus block and local wound infiltration for pain control in thyroid surgery. Asian J. Surg. 2019, 42, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Li, L.; Yu, P.; Gao, T.; Liu, K. Preemptive scalp infiltration with 0.5 % ropivacaine and 1 % lidocaine reduces postoperative pain after craniotomy. Acta Neurochir. 2015, 157, 993–998. [Google Scholar] [CrossRef]
- Verchère, E.; Grenier, B.; Mesli, A.; Siao, D.; Sesay, M.; Maurette, P. Postoperative pain management after supratentorial craniotomy. J. Neurosurg. Anesthesiol. 2002, 14, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Akcil, E.F.; Dilmen, O.K.; Vehid, H.; Ibısoglu, L.S.; Tunali, Y. Which one is more effective for analgesia in infratentorial craniotomy? The scalp block or local anesthetic infiltration. Clin. Neurol. Neurosurg. 2017, 154, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Ma, J.; Li, K.; Chen, L.; Dong, R.; Lu, Y.; Zhang, Z.; Peng, M. A comparison of effects of scalp nerve block and local anesthetic infiltration on inflammatory response, hemodynamic response, and postoperative pain in patients undergoing craniotomy for cerebral aneurysms: A randomized controlled trial. BMC Anesthesiol. 2019, 19, 94. [Google Scholar] [CrossRef] [PubMed]
- Geze, S.; Yilmaz, A.A.; Tuzuner, F. The effect of scalp block and local infiltration on the haemodynamic and stress response to skull-pin placement for craniotomy. Eur. J. Anaesthesiol. 2009, 26, 298–303. [Google Scholar] [CrossRef]
- Saringcarinkul, A.; Boonsri, S. Effect of scalp infiltration on postoperative pain relief in elective supratentorial craniotomy with 0.5% bupivacaine with adrenaline 1:400,000. J. Med. Assoc. Thai. 2008, 91, 1518. [Google Scholar]
- Batoz, H.; Verdonck, O.; Pellerin, C.; Roux, G.; Maurette, P. The analgesic properties of scalp infiltrations with ropivacaine after intracranial tumoral resection. Anesth. Analg. 2009, 109, 240–244. [Google Scholar] [CrossRef]
- Biswas, B.K.; Bithal, P.K. Preincision 0.25% Bupivacaine scalp infiltration and postcraniotomy pain: A randomized double-blind, placebo-controlled study. J. Neurosurg. Anesthesiol. 2003, 15, 234–239. [Google Scholar] [CrossRef]
- Zhou, H.; Ou, M.; Yang, Y.; Ruan, Q.; Pan, Y.; Li, Y. Effect of skin infiltration with ropivacaine on postoperative pain in patients undergoing craniotomy. Springerplus 2016, 5, 1180. [Google Scholar] [CrossRef]
- Pakulski, C.; Nowicki, R.; Badowicz, B.; Bak, P.; Mikulski, K.; Wojnarska, B. Effect of scalp infiltration with lidocaine on the circulatory response to craniotomy. Med. Sci. Monit. 2001, 7, 725–728. [Google Scholar] [PubMed]
- Theerth, K.A.; Sriganesh, K.; Reddy, K.M.; Chakrabarti, D.; Umamaheswara Rao, G.S. Analgesia Nociception Index-guided intraoperative fentanyl consumption and postoperative analgesia in patients receiving scalp block versus incision-site infiltration for craniotomy. Minerva Anestesiol. 2018, 84, 1361–1368. [Google Scholar] [CrossRef] [PubMed]
- El-Dawlatly, A.; Abbas, S.; Turkistani, A.; Elwatidy, S.; Elgamal, E.; Jamjoom, Z.; Sheta, S.; Alshaer, A. Use Of Tenoxicam For Post Craniotomy Pain Relief With Or Without Bupivacaine Scalp Infiltration: A Randomized Study. Internet J. Anesthesiol. 2007, 15, 1–6. [Google Scholar]
- Law-Koune, J.D.; Szekely, B.; Fermanian, C.; Peuch, C.; Liu, N.; Fischler, M. Scalp infiltration with bupivacaine plus epinephrine or plain ropivacaine reduces postoperative pain after supratentorial craniotomy. J. Neurosurg. Anesthesiol. 2005, 17, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Ban, V.S.; Bhoja, R.; McDonagh, D.L. Multimodal analgesia for craniotomy. Curr. Opin. Anaesthesiol. 2019, 32, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Thompson, E.; Green, A.; Hartsilver, E.; Stott, M.; Meikle, K.; Ormerod, V.; Lilaonitkul, M.; Powell, R. A Comparison of Continuous Wound Infiltration Plus Patient Controlled Analgesia Versus Epidural Analgesia after Open Renal Surgery. Urol. Androl. Open J. 2020, 4, 20–26. [Google Scholar] [CrossRef]
- Al-Kaabneh, A.; Qamar, A.A.; Al-Hammouri, F.; Al-Majali, A.; Alasmar, A.; Al-Sayedeh, N.; Khori, F.; Qapaha, A.; Beidas, M. The effect of anaesthesia on flank incisional pain: Infiltration versus intercostal nerve block, a comparative study. Pan Afr. Med. J. 2020, 36, 356. [Google Scholar] [CrossRef]
- Cook, T.M.; Counsell, D.; Wildsmith, J.A.W. Major complications of central neuraxial block: Report on the Third National Audit Project of the Royal College of Anaesthetists. Br. J. Anaesth. 2009, 102, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Skjelsager, A.; Ruhnau, B.; Kistorp, T.K.; Kridina, I.; Hvarness, H.; Mathiesen, O.; Dahl, J.B. Transversus abdominis plane block or subcutaneous wound infiltration after open radical prostatectomy: A randomized study. Acta Anaesthesiol. Scand. 2013, 57, 502–508. [Google Scholar] [CrossRef]
- Alessandri, F.; Lijoi, D.; Mistrangelo, E.; Nicoletti, A.; Ragni, N. Effect of presurgical local infiltration of levobupivacaine in the surgical field on postsurgical wound pain in laparoscopic gynecological surgery. Acta Obstet. Gynecol. Scand. 2006, 85, 844–849. [Google Scholar] [CrossRef]
- Sugihara, M.; Miyake, T.; Miyagi, Y.; Oda, T.; Hazama, Y.; Sano, R.; Nakamura, T.; Shiota, M.; Shimoya, K. Does local infiltration anesthesia on laparoscopic surgical wounds reduce postoperative pain? Randomized control study. Reprod. Med. Biol. 2018, 17, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Hannibal, K.; Galatius, H.; Hansen, A.; Obel, E.; Ejlersen, E. Preoperative wound infiltration with bupivacaine reduces early and late opioid requirement after hysterectomy. Anesth. Analg. 1996, 83, 376–381. [Google Scholar] [CrossRef]
- Barron, K.I.; Lamvu, G.M.; Schmidt, R.C.; Fisk, M.; Blanton, E.; Patanwala, I. Wound Infiltration With Extended-Release Versus Short-Acting Bupivacaine Before Laparoscopic Hysterectomy: A Randomized Controlled Trial. J. Minim. Invasive Gynecol. 2017, 24, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Kong, T.W.; Park, H.; Cheong, J.Y.; Min, S.K.; Ryu, H.S. Efficacy of continuous wound infiltration of local anesthetic for pain relief after gynecologic laparoscopy. Int. J. Gynecol. Obstet. 2014, 124, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Osaheni, O.; Idehen, H.O.; Imarengiaye, C.O. Analgesia for postoperative myomectomy pain: A comparison of ultrasound-guided transversus abdominis plane block and wound infiltration. Niger. J. Clin. Pract. 2020, 23, 1523–1529. [Google Scholar] [CrossRef] [PubMed]
- Ranjit, S.; Shrestha, S.K. Comparison of ultrasound guided transversus abdominis plane block versus local wound infiltration for post operative analgesia in patients undergoing gynaecological surgery under general anaesthesia. Kathmandu Univ. Med. J. 2014, 12, 94–97. [Google Scholar] [CrossRef]
- Atim, A.; Bilgin, F.; Kilickaya, O.; Purtuloglu, T.; Alanbay, I.; Orhan, M.E.; Kurt, E. The efficacy of ultrasound-guided transversus abdominis plane block in patients undergoing hysterectomy. Anaesth. Intensive Care 2011, 39, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Kim, K.; Ahn, S.; Shin, H.J.; Suh, D.H.; No, J.H.; Kim, Y.B. Continuous wound infiltration system for postoperative pain management in gynecologic oncology patients. Arch. Gynecol. Obstet. 2017, 295, 1219–1226. [Google Scholar] [CrossRef]
- Yu, W.; Wu, X.; Liu, L.; Long, B.; Tian, Y.; Ma, C.; Dong, Y. The median effective dose of one intravenous bolus of oxycodone for postoperative analgesia after myomectomy and hysterectomy with local ropivacaine wound infiltration: An up-down dose-finding study. Anesth. Analg. 2020, 131, 1599–1606. [Google Scholar] [CrossRef]
- McCarthy, D.; Iohom, G. Local infiltration analgesia for postoperative pain control following total hip arthroplasty: A systematic review. Anesthesiol. Res. Pract. 2012, 2012, 709531. [Google Scholar] [CrossRef]
- Herbst, S.A. Local infiltration of liposome bupivacaine in foot and ankle surgery: Case-based reviews. Am. J. Orthop. 2014, 43, S10–S12. [Google Scholar]
- Ganapathy, S. Wound/intra-articular infiltration or peripheral nerve blocks for orthopedic joint surgery: Efficacy and safety issues. Curr. Opin. Anaesthesiol. 2012, 25, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Fusco, P.; Cofini, V.; Petrucci, E.; Scimia, P.; Fiorenzi, M.; Paladini, G.; Behr, A.U.; Borghi, B.; Flamini, S.; Pizzoferrato, R.; et al. Continuous wound infusion and local infiltration analgesia for postoperative pain and rehabilitation after total hip arthroplasty. Minerva Anestesiol. 2018, 84, 556–564. [Google Scholar] [CrossRef]
- Bech, R.D.; Ovesen, O.; Lauritsen, J.; Emmeluth, C.; Lindholm, P.; Overgaard, S. Local Anesthetic Wound Infiltration after Osteosynthesis of Extracapsular Hip Fracture Does Not Reduce Pain or Opioid Requirements: A Randomized, Placebo-Controlled, Double-Blind Clinical Trial in 49 Patients. Pain Res. Manag. 2018, 2018, 6398424. [Google Scholar] [CrossRef]
- Li, B.L.; Liu, X.; Cui, L.; Zhang, W.; Pang, H.; Wang, M.; Wang, H.Q. Local Infiltration Analgesia with Ropivacaine Improves Postoperative Pain Control in Ankle Fracture Patients: A Retrospective Cohort Study. Pain Res. Manag. 2020, 2020, 8542849. [Google Scholar] [CrossRef]
- Chahar, P.; Cummings, K.C. Liposomal bupivacaine: A review of a new bupivacaine formulation. J. Pain Res. 2012, 5, 257–264. [Google Scholar]
- Amin, N.H.; Hutchinson, H.L.; Sanzone, A.G. Infiltration Techniques for Local Infiltration Analgesia with Liposomal Bupivacaine in Extracapsular and Intracapsular Hip Fracture Surgery: Expert Panel Opinion. J. Orthop. Trauma 2018, 32, S5–S10. [Google Scholar] [CrossRef]
- Hutchinson, H.L.; Jaekel, D.J.; Lovald, S.T.; Watson, H.N.; Ong, K.L. Multimodal Pain Management of Femoral Neck Fractures Treated With Hemiarthroplasty. J. Surg. Orthop. Adv. 2019, 28, 58. [Google Scholar] [CrossRef]
- Berninger, M.T.; Friederichs, J.; Leidinger, W.; Augat, P.; Bühren, V.; Fulghum, C.; Reng, W. Effect of local infiltration analgesia, peripheral nerve blocks, general and spinal anesthesia on early functional recovery and pain control in total knee arthroplasty. BMC Musculoskelet. Disord. 2018, 19, 232. [Google Scholar] [CrossRef]
- Seangleulur, A.; Vanasbodeekul, P.; Prapaitrakool, S.; Worathongchai, S.; Anothaisintawee, T.; McEvoy, M.; Vendittoli, P.A.; Attia, J.; Thakkinstian, A. The efficacy of local infiltration analgesia in the early postoperative period after total knee arthroplasty: A systematic review and meta-analysis. Eur. J. Anaesthesiol. 2016, 33, 816–831. [Google Scholar] [CrossRef]
- Li, C.; Qu, J.; Pan, S.; Qu, Y. Local infiltration anesthesia versus epidural analgesia for postoperative pain control in total knee arthroplasty: A systematic review and meta-analysis. J. Orthop. Surg. Res. 2018, 13, 112. [Google Scholar] [CrossRef]
- Essving, P.; Axelsson, K.; Åberg, E.; Spännar, H.; Gupta, A.; Lundin, A. Local infiltration analgesia versus intrathecal morphine for postoperative pain management after total knee arthroplasty: A randomized controlled trial. Anesth. Analg. 2011, 113, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Cipollaro, L.; Trucillo, P.; Bragazzi, N.L.; Porta, G.D.; Reverchon, E.; Maffulli, N. Liposomes for intra-articular analgesic drug delivery in orthopedics: State-of-art and future perspectives. insights from a systematic mini-review of the literature. Medicina 2020, 56, 423. [Google Scholar] [CrossRef] [PubMed]
- Emerson, R.H.; Barrington, J.W.; Olugbode, O.; Lovald, S.; Watson, H.; Ong, K. Femoral nerve block versus long-acting wound infiltration in total knee arthroplasty. Orthopedics 2016, 39, e449–e455. [Google Scholar] [CrossRef]
- Jiménez-Almonte, J.H.; Wyles, C.C.; Wyles, S.P.; Norambuena-Morales, G.A.; Báez, P.J.; Murad, M.H.; Sierra, R.J. Is Local Infiltration Analgesia Superior to Peripheral Nerve Blockade for Pain Management After THA: A Network Meta-analysis. Clin. Orthop. Relat. Res. 2016, 474, 495–516. [Google Scholar] [CrossRef]
- Updegrove, G.F.; Stauch, C.M.; Ponnuru, P.; Kunselman, A.R.; Armstrong, A.D. Efficacy of local infiltration anesthesia versus interscalene nerve blockade for total shoulder arthroplasty. JSES Int. 2020, 4, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Tang, S.; Sun, S.; Zhang, Y.; Chen, L.; Xia, D.; Wang, Y.; Ren, L.; Huang, Y. Comparison between local infiltration analgesia with combined femoral and sciatic nerve block for pain management after total knee arthroplasty. J. Orthop. Surg. Res. 2020, 15, 41. [Google Scholar] [CrossRef]
- Harrop-Griffiths, W.; Cook, T.; Gill, H.; Hill, D.; Ingram, M.; Makris, M.; Malhotra, S.; Nicholls, B.; Popat, M.; Swales, H.; et al. Regional anaesthesia and patients with abnormalities of coagulation: The Association of Anaesthetists of Great Britain & Ireland the Obstetric Anaesthetists’ Association Regional Anaesthesia UK. Anaesthesia 2013, 68, 966–972. [Google Scholar] [CrossRef]
- Keene, D.D.; Rea, W.E.; Aldington, D. Acute pain management in trauma. Trauma 2011, 13, 167–179. [Google Scholar] [CrossRef]
- Whipple, J.K.; Lewis, K.S.; Quebbeman, E.J.; Wolff, M.; Gottlieb, M.S.; Medicus-Bringa, M.; Hartnett, K.R.; Graf, M.; Ausman, R.K. Analysis of Pain Management in Critically Ill Patients. Pharmacother. J. Hum. Pharmacol. Drug Ther. 1995, 15, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Koehler, D.; Marsh, J.L.; Karam, M.; Fruehling, C.; Willey, M. Efficacy of surgical-site, multimodal drug injection following operative management of femoral fractures: A randomized controlled trial. J. Bone Jt. Surg. Am. Vol. 2017, 99, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Wick, E.C.; Grant, M.C.; Wu, C.L. Postoperative Multimodal Analgesia Pain Management With Nonopioid Analgesics and Techniques. JAMA Surg. 2017, 152, 691. [Google Scholar] [CrossRef] [PubMed]
- Engelman, D.T.; Ali, W.A.; Wiliams, J.B.; Perrault, L.P.; Reddy, V.S.; Arora, R.C.; Roselli, E.E.; Khaynezhad, A.K.; Gerdisch, M.; Levy, J.H.; et al. Guidelines for Perioperative Care in Cardiac Surgery: Enhanced Recovery After Surgery Society Recommendations. JAMA Surg. 2019, 154, 755–766. [Google Scholar] [CrossRef]
- Low, D.E.; Allum, W.; De Manzoni, G.; Ferri, L.; Immanuel, A.; Kuppusamy, M.; Law, S.; Lindblad, M.; Maynard, N.; Neal, J.; et al. Guidelines for Perioperative Care in Esophagectomy: Enhanced Recovery After Surgery (ERAS®) Society Recommendations. World J. Surg. 2019, 43, 299–330. [Google Scholar] [CrossRef]
- Thorell, A.; MacCormick, A.D.; Awad, S.; Reynolds, N.; Roulin, D.; Demartines, N.; Vignaud, M.; Alvarez, A.; Singh, P.M.; Lobo, D.N. Guidelines for Perioperative Care in Bariatric Surgery: Enhanced Recovery After Surgery (ERAS) Society Recommendations. World J. Surg. 2016, 40, 2065–2083. [Google Scholar] [CrossRef]
- Alkhamesi, N.A.; Kane, J.M.; Guske, P.J.; Wallace, J.W.; Rantis, P.C. Intraperitoneal aerosolization of bupivacaine is a safe and effective method in controlling postoperative pain in laparoscopic Roux-en-Y gastric bypass. J. Pain Res. 2008, 1, 9. [Google Scholar] [CrossRef]
- Gustafsson, U.O.; Scott, M.J.; Hubner, M.; Nygren, J.; Demartines, N.; Francis, N.; Rockall, T.A.; Young-Fadok, T.M.; Hill, A.G.; Soop, M.; et al. Guidelines for Perioperative Care in Elective Colorectal Surgery: Enhanced Recovery After Surgery (ERAS®) Society Recommendations: 2018. World J. Surg. 2019, 43, 659–695. [Google Scholar] [CrossRef] [PubMed]
- Nygren, J.; Thacker, J.; Carli, F.; Fearon, K.C.; Norderval, S.; Lobo, D.N.; Ljungqvist, O.; Soop, M.; Ramirez, J.; Enhanced Recovery After Surgery Society. Guidelines for perioperative care in elective rectal/pelvic surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Clin. Nutr. 2012, 31, 801–816. [Google Scholar] [CrossRef]
- Melloul, E.; Lassen, K.; Roulin, D.; Grass, F.; Perinel, J.; Adham, M.; Wellge, E.B.; Kunzler, F.; Besselink, M.G.; Asbun, H.; et al. Guidelines for Perioperative Care for Pancreatoduodenectomy: Enhanced Recovery After Surgery (ERAS) Recommendations 2019. World J. Surg. 2020, 44, 2056–2084. [Google Scholar] [CrossRef]
- Dort, J.C.; Farwell, D.G.; Findlay, M.; Huber, G.F.; Kerr, P.; Shea-Budgell, M.A.; Simon, C.; Uppington, J.; Zygun, D.; Ljungqvist, O.; et al. Optimal Perioperative Care in Major Head and Neck Cancer Surgery With Free Flap Reconstruction: A Consensus Review and Recommendations From the Enhanced Recovery After Surgery Society. JAMA Otolaryngol. Head Neck Surg. 2017, 143, 292–303. [Google Scholar] [CrossRef]
- Hani, U.; Bakhshi, S.K.; Shamim, M.S. Enhanced Recovery after Elective Craniotomy for Brain Tumours. J. Pak. Med. Assoc. 2019, 69, 749–751. [Google Scholar]
- Hagan, K.B.; Bhavsar, S.; Raza, S.M.; Arnold, B.; Arunkumar, R.; Dang, A.; Gottumukkala, V.; Popat, K.; Pratt, G.; Rahlfs, T.; et al. Enhanced recovery after surgery for oncological craniotomies. J. Clin. Neurosci. 2016, 24, 10–16. [Google Scholar] [CrossRef]
- Cerantola, Y.; Valerio, M.; Persson, B.; Jichlinski, P.; Ljungqvist, O.; Hubner, M.; Kassouf, W.; Muller, S.; Baldini, G.; Carli, F.; et al. Guidelines for perioperative care after radical cystectomy for bladder cancer: Enhanced Recovery After Surgery (ERAS®) society recommendations. Clin. Nutr. 2013, 32, 879–887. [Google Scholar] [CrossRef]
- Nelson, G.; Bakkum-Gamez, J.; Kalogera, E.; Glaser, G.; Altman, A.; Meyer, L.A.; Taylor, J.S.; Iniesta, M.; Lasala, J.; Mena, G.; et al. Guidelines for perioperative care in gynecologic/oncology: Enhanced Recovery after Surgery (ERAS) Society recommendations—2019 update. Int. J. Gynecol. Cancer 2019, 29, 651–668. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, T.W.; Gill, M.; McDonald, D.A.; Middleton, R.G.; Reed, M.; Sahota, O.; Yates, P.; Ljungqvist, O. Consensus statement for perioperative care in total hip replacement and total knee replacement surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Acta Orthop. 2020, 91, 3–19. [Google Scholar] [CrossRef] [PubMed]
- McGinigle, K.L.; Eldrup-Jorgensen, J.; McCall, R.; Freeman, N.L.; Pascarella, L.; Farber, M.A.; Marston, W.A.; Crowner, J.R. A systematic review of enhanced recovery after surgery for vascular operations. J. Vasc. Surg. 2019, 70, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Renghi, A.; Gramaglia, L.; Casella, F.; Moniaci, D.; Gaboli, K.; Brustia, P. Local versus epidural anesthesia in fast-track abdominal aortic surgery. J. Cardiothorac. Vasc. Anesth. 2013, 27, 451–458. [Google Scholar] [CrossRef]
- Kranke, P.; Jokinen, J.; Pace, N.L.; Schnabel, A.; Hollmann, M.W.; Hahnenkamp, K.; Eberhart, L.H.; Poepping, D.M.; Weibel, S. Continuous intravenous perioperative lidocaine infusion for postoperative pain and recovery. Cochrane Database Syst. Rev. 2015, CD009642. [Google Scholar] [CrossRef]
- Dunn, L.K.; Durieux, M.E. Perioperative Use of Intravenous Lidocaine. Anesthesiology 2017, 126, 729–737. [Google Scholar] [CrossRef]
- Khan, J.S.; Yousuf, M.; Victor, J.C.; Sharma, A.; Siddiqui, N. An estimation for an appropriate end time for an intraoperative intravenous lidocaine infusion in bowel surgery: A comparative meta-analysis. J. Clin. Anesth. 2016, 28, 95–104. [Google Scholar] [CrossRef] [PubMed]

| Local Anesthetic | Adult Dosing without Epinephrine | Adult Dosing with Epinephrine | Duration without Epinephrine (min) | Duration with Epinephrine (min) | Strength of Recommendation | Level of Evidence |
|---|---|---|---|---|---|---|
| Lidocaine [59] | 4.5 mg/kg (max:300 mg) | <7 mg/kg (max 500 mg) | 30–120 | 60–400 | C | III |
| Mepivacaine [59,60] | 6 mg/kg (max < 300 mg) | 7 mg/kg (max < 500 mg) | 30–120 | 60–400 | No data | No data |
| Bupivacaine [59,60,61] | 2 mg/kg (max 400 mg) | 3 mg/kg (max 225 mg) | 120–240 | 240–480 | No data | No data |
| Ropivacaine [59] | 2.9 mg/kg (max 200 mg) | - | No data | No data | No data | No data |
| Procaine [59,60] | 10 mg/kg (max 350–500 mg) | 16 mg/kg | 15–30 | 30–90 | No data | No data |
| Surgery | Catheter Location and Type | Pre-Closure Infiltration | Local Anesthetic | Postoperatively Dosing Regimen | Duration of Infusion * |
|---|---|---|---|---|---|
| Shoulder surgery [4] Arthroscopic Open | Subacromial (High) Multi-orifice/epidural (Moderate) | - | Bupivacaine 0.25% Ropivacaine 0.2% (Moderate) | 2–5 mL/h (High) | 48 h (Moderate) |
| Subacromial Multi-orifice (Low) | Ropivacaine 0.5%/0.75%, 30 mL [65] (Low) | Ropivacaine 0.375% (Low) | 5 mL/h (Low) | 48 h (Low) | |
| Knee surgery [4] Anterior cruciate ligament reconstruction Total knee arthroplasty | Intra-articular/combination with subcutaneous (Low) Multi-orifice (Moderate) | - | Bupivacaine 0.25% Ropivacaine 0.25% (Moderate) | 4–10 mL/h (Moderate) | 48 h (Moderate) |
| Intra-articular/combination with subcutaneous parapatellar area (Low) Multi-orifice (Low) | Ropivacaine (0.2%) + epinephrine (1 mg/mL) + ketorolac (30 mg/mL) WI along all layers [66] (Low) | Ropivacaine 0.2% (Low) | 5 mL/h (Low) | 48 h (Low) | |
| Hip surgery [4] Total hip arthroplasty Minimally invasive approach to total hip arthroplasty | Subcutaneous all along wound + epicapsullary (Double catheter technique) (Low) Multi-orifice (Low) | Ropivacaine 0.3%, 20 mL (Low) | Ropivacaine 0.2% (Low) | 5 mL/h (Low) | 48 h (Low) |
| Epicapsullary (Low) Multi-orifice | - | Ropivacaine 0.3% (Low) | 8 mL/h (Low) | 48 h (Low) | |
| Spine surgery [4] Iliac crest bone harvesting | Above the fascia Double catheter technique- “one catheter tip opposite to other” (Low) Multi-orifice (Low) | Ropivacaine 0.5%, 40 mL bolus [67] (Low) | Ropivacaine 0.2% (Low) | 5 mL/h (Low) | 48 h (Low) |
| Close to the bone (Moderate) Multi-orifice (Moderate) | Ropivacaine 0.3%, 20 mL bolus [68] (Low) | Bupivacaine 0.5% Ropivacaine 0.3–0.5% (Low) | 8–10 mL/h (Low) | 60–72 h (Moderate) | |
| Open major digestive tract surgery (colorectal) [4] | Preperitoneal space Cephalad catheter orientation (Moderate) Multi-orifice (Moderate) | Ropivacaine 0.2%, 10 mL (Moderate) | Ropivacaine 0.2% Bupivacaine 0.25% Levobupivacaine 0.25% (Moderate) | 10 mL/h (Moderate) or intermittent bolus 8–10 mL repeated at 5 to 12 h (Moderate) | 48 h (Moderate) |
| Open hepatobiliary surgery (subcostal incision) [4] | Preperitoneal or in a musculo-fascial layer (Moderate) Multi-orifice (Moderate) | Bupivacaine 0.5%, 10 mL Ropivacaine 0.25%, 20 mL (Low) | Ropivacaine 0.25% Bupivacaine 0.5% (Moderate) | 4 mL/h (High) intermittent bolus 10 mL repeated at 4 or 12 h (Moderate) | At least 48 h (Moderate) |
| Laparoscopic cholecystectomy [4] | Gall bladder bed and trocar sites (Low) Epidural/Multi-orifice (Moderate) | Ropivacaine 0.5%, 20 mL Intraperitoneally and at trocar sites [69] (Low) | Ropivacaine 0.5% (Low) | Incremental doses of 10 mL (Low) | Not given |
| Open appendectomy [4] | Preperitoneal (Moderate) Epidural (Low) | Ropivacaine 0.2%, 10 mL [70] (Moderate) | Ropivacaine 0.2% (Low) | 5 mL/h (Moderate) | 24 h (Low) |
| Nephrectomy [4] | Between transverse and oblique intern muscles (Low) Epidural/Multi-orifice (Low) | Bupivacaine 0.25%, 20 mL Ropivacaine 1%, 10 mL (low) | Bupivacaine 0.25% Ropivacaine 0.5% (Very low) | At least 4 mL/h (Very low) | 48 h (Very low) |
| Inguinal herniotomy [4] | Subfascial (Moderate) Epidural/Multi-orifice (Low) | Bupivacaine 0.25%, 20 mL Bupivacaine 0.5%, 10 mL (High) | Bupivacaine 0.5% (Low) | 2 mL/h (Low) | 48 h (Moderate) |
| Cesarean section [4] | Above/below fascia (Moderate) Multi-orifice (Moderate) | Bupivacaine 0.125–0.25%, 25 mL [71] Ropivacaine 0.2% Levobupivacaine 0.125% (Moderate) | Bupivacaine 0.125–0.25%, 25 mLRopivacaine 0.2% Levobupivacaine 0.125% (Moderate) | 5 mL/h - | 72 h (Moderate) |
| Abdominal hysterectomy with bilateral salpingo-oophorectomy [4] | Above fascia (Moderate) Multi-orifice (Moderate) | Levobupivacaine 0.25%, 20 mL [72] (Moderate) | Bupivacaine 0.25–0.5% Ropivacaine 0.1–0.2% Levobupivacaine 0.25% (Moderate) | 5 mL/h [72] - | 52 h (Moderate) |
| Retropubic prostatectomy [4] | Subfascial (beneath rectus muscle) (Very low) Multi-orifice (Very low) | - | Ropivacaine 0.2% Bupivacaine 0.5% (Very low) | 5 mL/h (Very low) | 48 h - |
| Median sternotomy [4] | Two catheters in different wound layers (subfascial plane and subcutaneous) (Moderate) Multi-orifice (Moderate) | - | Ropivacaine 0.2% (Moderate) | At least 4 mL/h (2 mL/h for each catheter) or intermittent boli 5 mL/h per catheter (Low) | 48 h (Moderate) |
| Thoracotomy [4] | Subcutaneous (Low) Epidural (Low) | - | Ropivacaine 0.2% (Low) | At least 2 mL/h (Low) | 48 h (Low) |
| Breast surgery [4] Modified radical mastectomy and axillary node dissection Elective cosmetic breast augmentation Bilateral breast augmentation | Axillary wound cavity (Moderate) Multi-orifice (Moderate) | - | Bupivacaine 0.5% (Moderate) | 0.5 mL/h (Moderate) | 5 days (Moderate) |
| Subcutaneous (Moderate) Multi-orifice (Moderate) | Suggested not evaluated | Ropivacaine 0.25% (Moderate) | Intermittent 10 mL on demand [73] (Moderate) | 48 h (Moderate) | |
| Catheter tip superior to the prothesis (Moderate) Multi-orifice (Moderate) | Suggested not evaluated | Bupivacaine 0.25% (Moderate) | 2 mL/h at catheter for each breast [74] (Moderate) | 48 h (Moderate) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stamenkovic, D.M.; Bezmarevic, M.; Bojic, S.; Unic-Stojanovic, D.; Stojkovic, D.; Slavkovic, D.Z.; Bancevic, V.; Maric, N.; Karanikolas, M. Updates on Wound Infiltration Use for Postoperative Pain Management: A Narrative Review. J. Clin. Med. 2021, 10, 4659. https://doi.org/10.3390/jcm10204659
Stamenkovic DM, Bezmarevic M, Bojic S, Unic-Stojanovic D, Stojkovic D, Slavkovic DZ, Bancevic V, Maric N, Karanikolas M. Updates on Wound Infiltration Use for Postoperative Pain Management: A Narrative Review. Journal of Clinical Medicine. 2021; 10(20):4659. https://doi.org/10.3390/jcm10204659
Chicago/Turabian StyleStamenkovic, Dusica M., Mihailo Bezmarevic, Suzana Bojic, Dragana Unic-Stojanovic, Dejan Stojkovic, Damjan Z. Slavkovic, Vladimir Bancevic, Nebojsa Maric, and Menelaos Karanikolas. 2021. "Updates on Wound Infiltration Use for Postoperative Pain Management: A Narrative Review" Journal of Clinical Medicine 10, no. 20: 4659. https://doi.org/10.3390/jcm10204659
APA StyleStamenkovic, D. M., Bezmarevic, M., Bojic, S., Unic-Stojanovic, D., Stojkovic, D., Slavkovic, D. Z., Bancevic, V., Maric, N., & Karanikolas, M. (2021). Updates on Wound Infiltration Use for Postoperative Pain Management: A Narrative Review. Journal of Clinical Medicine, 10(20), 4659. https://doi.org/10.3390/jcm10204659






