Coronary Artery Disease in Women: A Comprehensive Appraisal
Abstract
:1. Introduction
2. Pathophysiology of CAD in Women
2.1. CAD Risk Factors
2.2. Traditional CAD Risk Factors
- Smoking is more detrimental to women than men, increasing the risk of myocardial infarction in women 6-fold (as opposed to men where the risk is mitigated 3-fold) [15]. There is also a compounded CAD risk between smoking and the use of hormonal treatment. This is thought to be due to prothrombotic effects. Rosenberg et al. found that heavy smoking (more than 25 cigarettes a day) increased a women’s risk of myocardial infarction by 12-fold, and this risk was compounded 32-fold in women using oral contraception [16]. Therefore, combined estrogen-progestin oral contraceptive pills should be avoided in women with a history of cardiovascular disease [17]. This compounded risk is of such concern that the use of combined oral contraceptive pills is contraindicated in women over the age of 35 who smoke and in women with severe dyslipidemia or obesity [18].
- The prevalence and incidence of hypertension are higher in women over 60 years old. Women are less likely to receive medical treatment for hypertension and have poorer blood pressure control [5]. Furthermore, the incidence of hypertension is increased 2- to 3-fold in those taking oral contraception [19].
- Diabetes mellitus has a more potent risk for CAD in women compared to men [5]. Diabetes mellitus also confers a higher adjusted hazard ratio (HR) of fatal CAD in diabetic women (HR = 14.74; 95% CI, 6.16–35.27) compared with diabetic men (HR = 3.77; 95% CI, 2.52–5.65). There is also a sex disparity in the intensity of cardiovascular risk reduction in women with diabetes—including poorer glycated hemoglobin levels and lower use of lipid-lowering pharmacotherapy [20].
- Dyslipidemia is common in women. Adverse changes in the lipid profile are associated with menopausal transition [21]. SWAN (Study of Women’s Health Across the Nation) found that during menopause, women have substantial increases in total cholesterol, low-density lipoprotein, and apolipoprotein B [22] Furthermore, increases in high-density lipoprotein (HDL), which is associated with athero-protective properties in the premenopausal phase, have been found to be paradoxically associated with an increase in atherosclerosis progression in the postmenopausal phase [22]. This has been postulated to be due to changes in HDL function due to the hormonal alterative in this phase of life [23].
- Age is a powerful predictor of CAD. While the prevalence of CAD increases with age in both men and women, the clinical presentation of CVD in women lags, on average, ~10 years behind their male counterparts [20]. Post-menopausal women more frequently have many traditional vascular disease risk conditions and these conditions cluster more frequently in them than men. These findings support the hypothesis that differences in endogenous sex hormones contribute to sex differences in CAD.
3. Women-Specific CAD Risk Factors
3.1. CAD Risk Factors Associated with Pregnancy
3.2. Hypertensive Disorders of Pregnancy
3.3. Gestational Diabetes
3.4. Gynecological Conditions Unrelated to Pregnancy Polycystic Ovary Syndrome
3.5. Menopause
3.6. Menopausal Hormone Therapy (MHT)
3.7. Breast Cancer
4. Clinical Presentation of CAD in Women
4.1. Obstructive Versus Non-Obstructive CAD
4.2. Non-Obstructive CAD
4.3. Obstructive CAD in Women
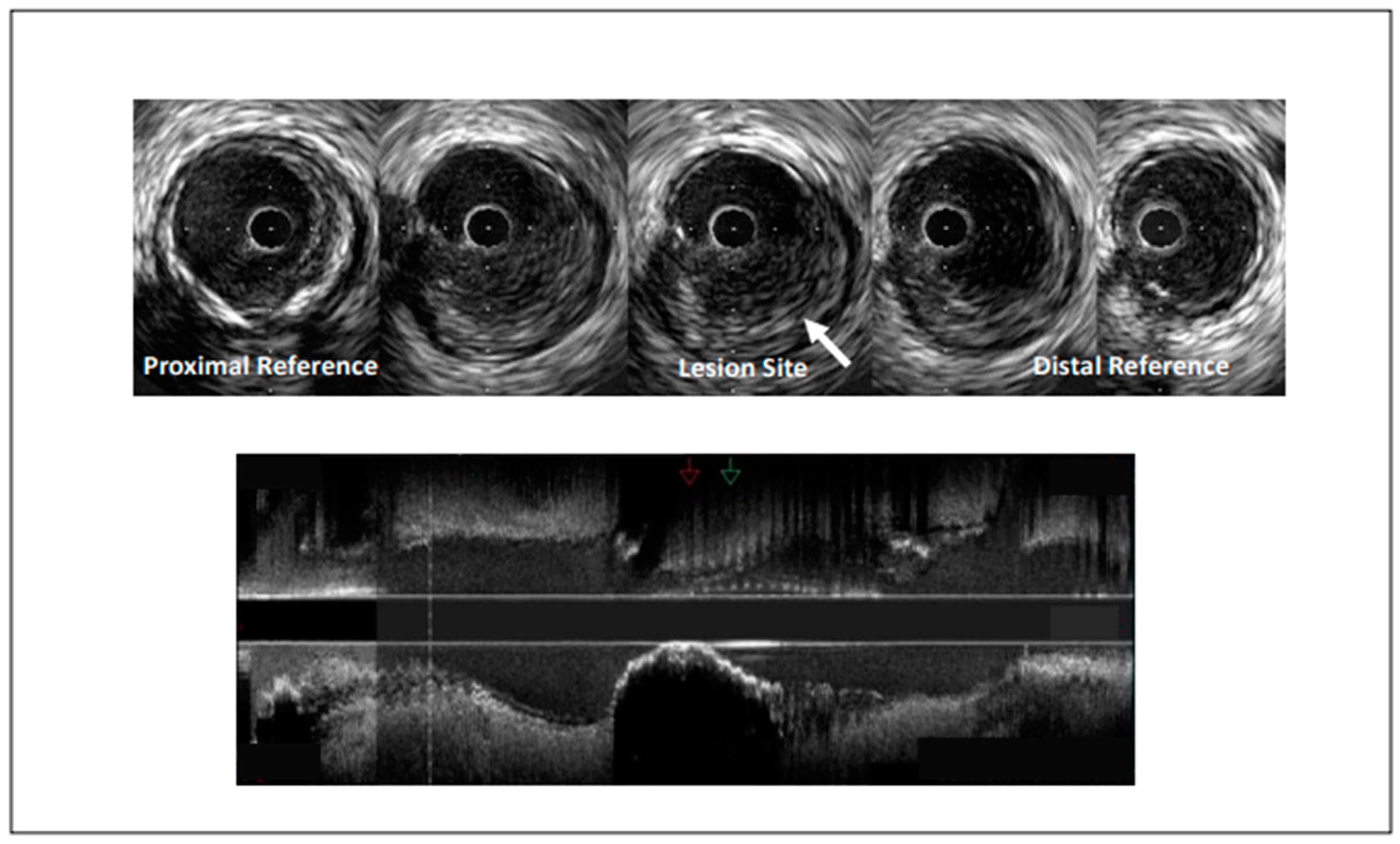
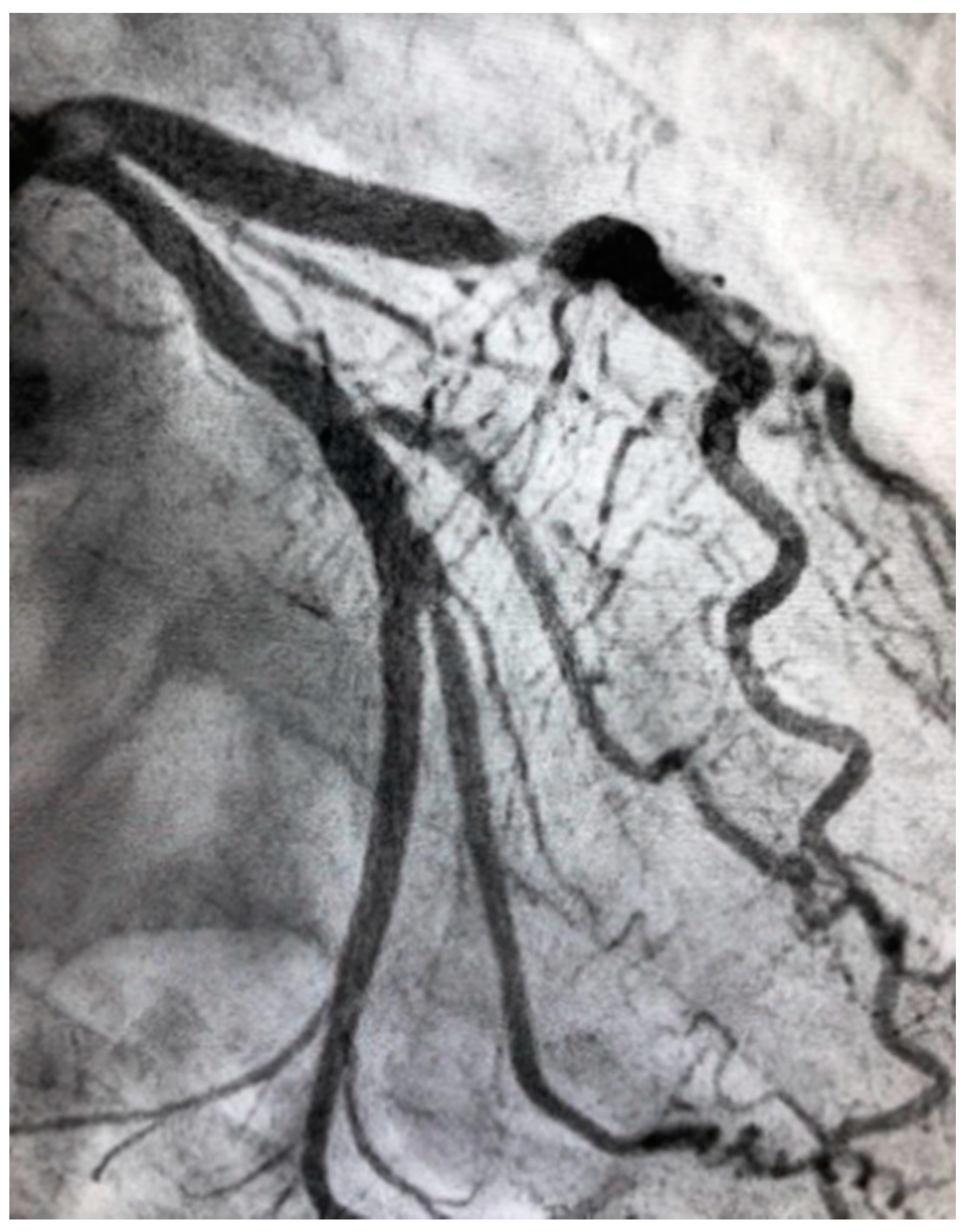
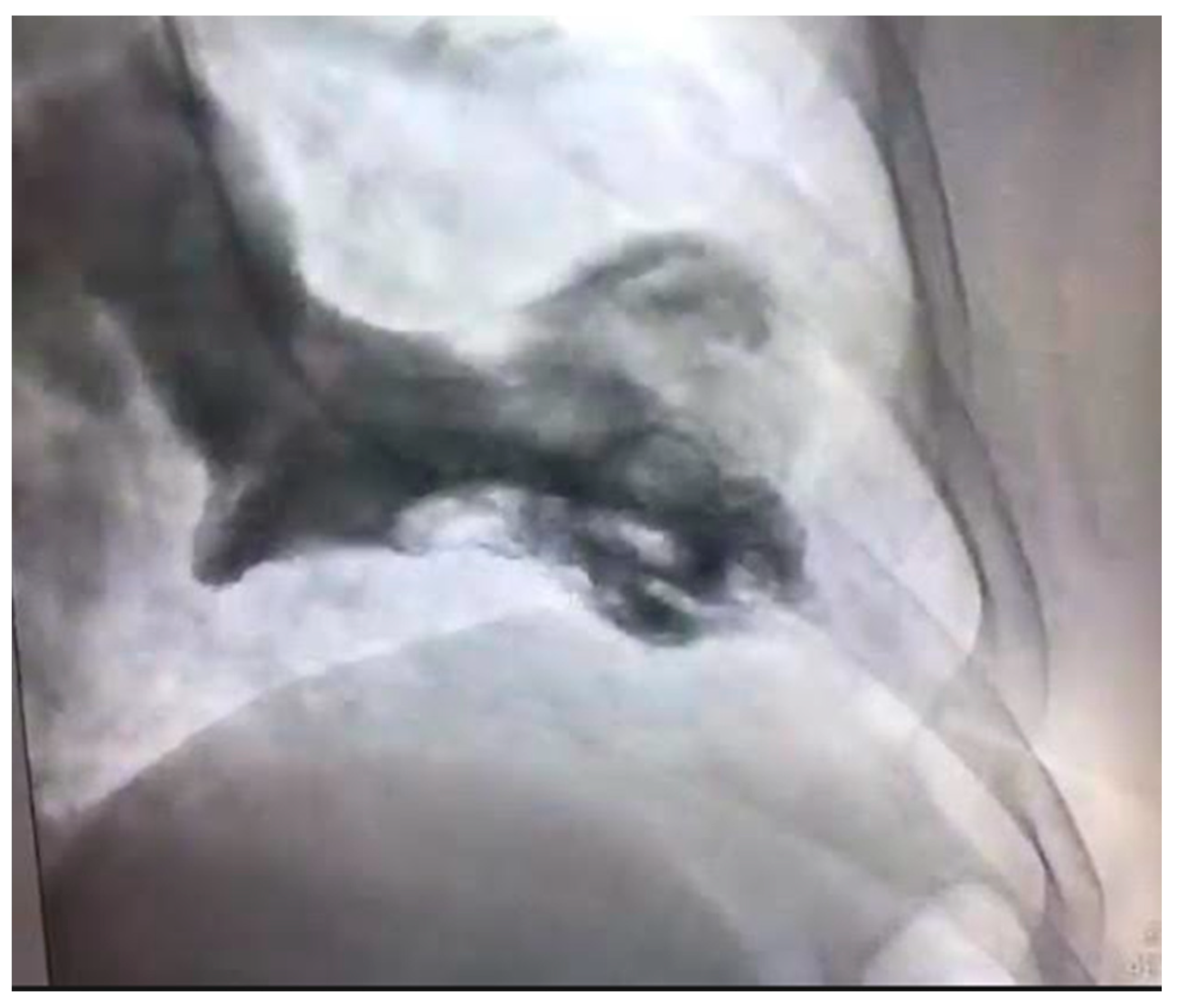
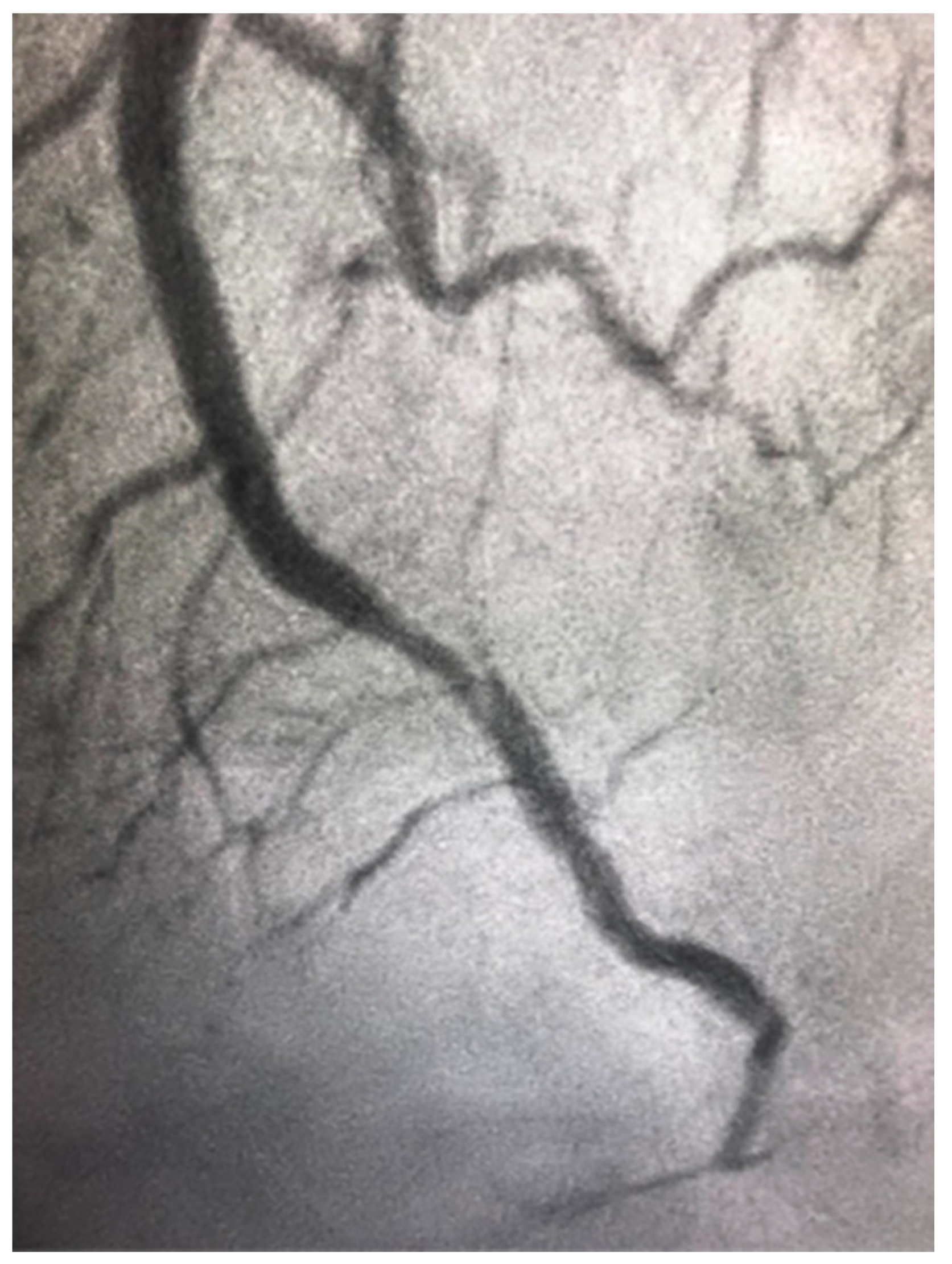
4.4. Spontaneous Coronary Artery Dissection
5. Treatment Gaps
5.1. Bleeding Risk in Women
5.2. Coronary Artery Bypass Surgery
5.3. Cardiac Rehabilitation Programs
5.4. Prognosis
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cho, L.; Davis, M.; Elgendy, I.; Epps, K.; Lindley, K.J.; Mehta, P.K.; Michos, E.D.; Minissian, M.; Pepine, C.; Vaccarino, V.; et al. Summary of Updated Recommendations for Primary Prevention of Cardiovascular Disease in Women. J. Am. Coll. Cardiol. 2020, 75, 2602–2618. [Google Scholar] [CrossRef]
- Mehta, L.S.; Beckie, T.M.; DeVon, H.; Grines, C.L.; Krumholz, H.M.; Johnson, M.N.; Lindley, K.J.; Vaccarino, V.; Wang, T.Y.; Watson, K.E.; et al. Acute Myocardial Infarction in Women. Circulation 2016, 133, 916–947. [Google Scholar] [CrossRef]
- Mosca, L.; Grundy, S.M.; Judelson, D.; King, K.; Limacher, M.; Oparil, S.; Pasternak, R.; Pearson, T.A.; Redberg, R.F.; Smith, S.C.; et al. Guide to Preventive Cardiology for Women. Circulation 1999, 99, 2480–2484. [Google Scholar] [CrossRef] [Green Version]
- Merz, C.N.B.; Andersen, H.; Sprague, E.; Burns, A.; Keida, M.; Walsh, M.N.; Greenberger, P.; Campbell, S.; Pollin, I.; McCullough, C.; et al. Knowledge, Attitudes, and Beliefs Regarding Cardiovascular Disease in Women. J. Am. Coll. Cardiol. 2017, 70, 123–132. [Google Scholar] [CrossRef]
- Norris, C.M.; Yip, C.Y.; Nerenberg, K.A.; Clavel, M.A.; Pacheco, C.; Foulds, H.J.; Hardy, M.; Gonsalves, C.A.; Jaffer, S.; Parry, M. State of the Science in Women’s Cardiovascular Disease: A Canadian Perspective on the Influence of Sex and Gender. J. Am. Heart Assoc. 2020, 9, e015634. [Google Scholar] [CrossRef]
- Pinn, V.W. Sex and Gender Factors in Medical Studies. JAMA 2003, 289, 397–400. [Google Scholar] [CrossRef]
- Pepine, C.J.; Kerensky, R.A.; Lambert, C.R.; Smith, K.M.; von Mering, G.O.; Sopko, G.; Merz, C.N.B. Some Thoughts on the Vasculopathy of Women with Ischemic Heart Disease. J. Am. Coll. Cardiol. 2006, 47, S30–S35. [Google Scholar] [CrossRef] [Green Version]
- Vogel, B.; Acevedo, M.; Appelman, Y.; Merz, C.N.B.; Chieffo, A.; Figtree, G.A.; Guerrero, M.; Kunadian, V.; Lam, C.S.P.; Maas, A.H.E.M.; et al. The Lancet women and cardiovascular disease Commission: Reducing the global burden by 2030. Lancet 2021, 397, 2385–2438. [Google Scholar] [CrossRef]
- Reynolds, H.; Shaw, L.J.; Min, J.K.; Spertus, J.A.; Chaitman, B.R.; Berman, D.S.; Picard, M.H.; Kwong, R.Y.; Bairey-Merz, C.N.; Cyr, D.D.; et al. Association of Sex with Severity of Coronary Artery Disease, Ischemia, and Symptom Burden in Patients with Moderate or Severe Ischemia. JAMA Cardiol. 2020, 5, 773–786. [Google Scholar] [CrossRef]
- Kornowski, R.; Lansky, A.J.; Mintz, G.S.; Kent, K.M.; Pichard, A.D.; Satler, L.F.; Bucher, T.A.; Popma, J.J.; Leon, M.B. Comparison of Men Versus Women in Cross-Sectional Area Luminal Narrowing, Quantity of Plaque, Presence of Calcium in Plaque, and Lumen Location in Coronary Arteries by Intravascular Ultrasound in Patients with Stable Angina Pectoris. Am. J. Cardiol. 1997, 79, 1601–1605. [Google Scholar] [CrossRef]
- Williams, M.C.; Kwiecinski, J.; Doris, M.; McElhinney, P.; D’Souza, M.S.; Cadet, S.; Adamson, P.D.; Moss, A.J.; Alam, S.; Hunter, A.; et al. Sex-Specific Computed Tomography Coronary Plaque Characterization and Risk of Myocardial Infarction. JACC Cardiovasc. Imaging 2021, 14, 1804–1814. [Google Scholar] [CrossRef]
- Orshal, J.M.; Khalil, R.A. Gender, sex hormones, and vascular tone. Am. J. Physiol. Integr. Comp. Physiol. 2004, 286, R233–R249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shufelt, C.L. Statin therapy in midlife women. Menopause 2021, 28, 1067–1069. [Google Scholar] [CrossRef] [PubMed]
- Von Mering, G.O.; Arant, C.B.; Wessel, T.R.; McGorray, S.P.; Merz, C.N.B.; Sharaf, B.L.; Smith, K.M.; Olson, M.B.; Johnson, B.D.; Sopko, G.; et al. Abnormal Coronary Vasomotion as a Prognostic Indicator of Cardiovascular Events in Women. Circulation 2004, 109, 722–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Njølstad, I.; Arnesen, E.; Lund-Larsen, P.G. Smoking, Serum Lipids, Blood Pressure, and Sex Differences in Myocardial Infarction. Circulation 1996, 93, 450–456. [Google Scholar] [CrossRef]
- Rosenberg, L.; Palmer, J.R.; Rao, R.S.; Shapiro, S. Low-Dose Oral Contraceptive Use and the Risk of Myocardial Infarction. Arch. Intern. Med. 2001, 161, 1065–1070. [Google Scholar] [CrossRef] [Green Version]
- Plu-Bureau, G.; Hugon-Rodin, J.; Maitrot-Mantelet, L.; Canonico, M. Hormonal contraceptives and arterial disease: An epidemiological update. Best Pr. Res. Clin. Endocrinol. Metab. 2013, 27, 35–45. [Google Scholar] [CrossRef]
- Maas, A.H.E.M.; Rosano, G.; Cifkova, R.; Chieffo, A.; van Dijken, D.; Hamoda, H.; Kunadian, V.; Laan, E.; Lambrinoudaki, I.; Maclaran, K.; et al. Cardiovascular health after menopause transition, pregnancy disorders, and other gynaecologic conditions: A consensus document from European cardiologists, gynaecologists, and endocrinologists. Eur. Heart J. 2021, 42, 967–984. [Google Scholar] [CrossRef]
- Shufelt, C.L.; Merz, C.N.B. Contraceptive Hormone Use and Cardiovascular Disease. J. Am. Coll. Cardiol. 2009, 53, 221–231. [Google Scholar] [CrossRef] [Green Version]
- Regensteiner, J.G.; Golden, S.; Huebschmann, A.G.; Barrett-Connor, E.; Chang, A.Y.; Chyun, D.; Fox, C.S.; Kim, C.; Mehta, N.; Reckelhoff, J.F.; et al. Sex Differences in the Cardiovascular Consequences of Diabetes Mellitus. Circulation 2015, 132, 2424–2447. [Google Scholar] [CrossRef]
- El Khoudary, S.R.; Aggarwal, B.; Beckie, T.M.; Hodis, H.N.; Johnson, A.E.; Langer, R.D.; Limacher, M.C.; Manson, J.E.; Stefanick, M.L.; Allison, M.A.; et al. Menopause Transition and Cardiovascular Disease Risk: Implications for Timing of Early Prevention: A Scientific Statement From the American Heart Association. Circulation 2020, 142, 506–532. [Google Scholar] [CrossRef]
- Matthews, K.A.; Crawford, S.L.; Chae, C.U.; Everson-Rose, S.; Sowers, M.F.; Sternfeld, B.; Sutton-Tyrrell, K. Are Changes in Cardiovascular Disease Risk Factors in Midlife Women Due to Chronological Aging or to the Menopausal Transition? J. Am. Coll. Cardiol. 2009, 54, 2366–2373. [Google Scholar] [CrossRef] [Green Version]
- El Khoudary, S.R.; Wang, L.; Brooks, M.; Thurston, R.C.; Derby, C.A.; Matthews, K.A. Increase HDL-C level over the menopausal transition is associated with greater atherosclerotic progression. J. Clin. Lipidol. 2016, 10, 962–969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.-X.; Arvizu, M.; Rich-Edwards, J.W.; Wang, L.; Rosner, B.; Stuart, J.J.; Rexrode, K.M.; Chavarro, J.E. Hypertensive Disorders of Pregnancy and Subsequent Risk of Premature Mortality. J. Am. Coll. Cardiol. 2021, 77, 1302–1312. [Google Scholar] [CrossRef] [PubMed]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, e596–e646. [Google Scholar] [CrossRef] [PubMed]
- Azziz, R.; Woods, K.S.; Reyna, R.; Key, T.J.; Knochenhauer, E.S.; Yildiz, B.O. The Prevalence and Features of the Polycystic Ovary Syndrome in an Unselected Population. J. Clin. Endocrinol. Metab. 2004, 89, 2745–2749. [Google Scholar] [CrossRef]
- Leeners, B.; Geary, N.; Tobler, P.; Asarian, L. Ovarian hormones and obesity. Hum. Reprod. Update 2017, 23, 300–321. [Google Scholar] [CrossRef]
- Dam, V.; Van Der Schouw, Y.T.; Onland-Moret, N.C.; Groenwold, R.H.H.; Peters, S.; Burgess, S.; Wood, A.M.; Chirlaque, M.-D.; Moons, K.G.M.; Oliver-Williams, C.; et al. Association of menopausal characteristics and risk of coronary heart disease: A pan-European case–cohort analysis. Int. J. Epidemiol. 2019, 48, 1275–1285. [Google Scholar] [CrossRef] [Green Version]
- Parikh, N.I.; Gonzalez, J.M.; Anderson, C.A.; Judd, S.E.; Rexrode, K.M.; Hlatky, M.A.; Gunderson, E.P.; Stuart, J.J.; Vaidya, D. Adverse Pregnancy Outcomes and Cardiovascular Disease Risk: Unique Opportunities for Cardiovascular Disease Prevention in Women: A Scientific Statement From the American Heart Association. Circulation 2021, 143, e902–e916. [Google Scholar] [CrossRef]
- Park, K.; Quesada, O.; Cook-Wiens, G.; Wei, J.; Minissian, M.; Handberg, E.M.; Merz, N.B.; Pepine, C.J. Adverse Pregnancy Outcomes Are Associated with Reduced Coronary Flow Reserve in Women with Signs and Symptoms of Ischemia without Obstructive Coronary Artery Disease: A Report from the Women’s Ischemia Syndrome Evaluation-Coronary Vascular Dysfunction Study. J. Women’s Health 2020, 29, 487–492. [Google Scholar] [CrossRef]
- Zoet, G.A.; Benschop, L.; Boersma, E.; Budde, R.P.; Fauser, B.C.; Van Der Graaf, Y.; De Groot, C.J.; Maas, A.H.; Van Lennep, J.E.R.; Steegers, E.A.; et al. Prevalence of Subclinical Coronary Artery Disease Assessed by Coronary Computed Tomography Angiography in 45- to 55-Year-Old Women with a History of Preeclampsia. Circulation 2018, 137, 877–879. [Google Scholar] [CrossRef]
- Gunderson, E.P.; Sun, B.; Catov, J.M.; Carnethon, M.; Lewis, C.E.; Allen, N.B.; Sidney, S.; Wellons, M.; Rana, J.S.; Hou, L.; et al. Gestational Diabetes History and Glucose Tolerance after Pregnancy Associated with Coronary Artery Calcium in Women During Midlife. Circulation 2021, 143, 974–987. [Google Scholar] [CrossRef]
- Talmor-Barkan, Y.; Chezar-Azerrad, C.; Kruchin, B.; Leshem-Lev, D.; Levi, A.; Hadar, E.; Kornowski, R.; Tenenbaum-Gavish, K.; Porter, A. Elevated galectin-3 in women with gestational diabetes mellitus, a new surrogate for cardiovascular disease in women. PLoS ONE 2020, 15, e0234732. [Google Scholar] [CrossRef] [PubMed]
- Grandi, S.M.; Filion, K.B.; Yoon, S.; Ayele, H.T.; Doyle, C.M.; Hutcheon, J.A.; Smith, G.; Gore, G.C.; Ray, J.G.; Nerenberg, K.; et al. Cardiovascular Disease-Related Morbidity and Mortality in Women with a History of Pregnancy Complications. Circulation 2019, 139, 1069–1079. [Google Scholar] [CrossRef] [PubMed]
- Oliver-Williams, C.; Vassard, D.; Pinborg, A.; Schmidt, L. Risk of cardiovascular disease for women with polycystic ovary syndrome: Results from a national Danish registry cohort study. Eur. J. Prev. Cardiol. 2020, 2047487320939674. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.M.; Zalos, G.; Halcox, J.P.; Schenke, W.H.; Waclawiw, M.A.; Quyyumi, A.A.; Finkel, T. Circulating Endothelial Progenitor Cells, Vascular Function, and Cardiovascular Risk. N. Engl. J. Med. 2003, 348, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Chang, Y.; Kim, B.-K.; Kang, D.; Kwon, M.-J.; Kim, C.-W.; Jeong, C.; Ahn, Y.; Park, H.-Y.; Ryu, S.; et al. Menopausal stages and serum lipid and lipoprotein abnormalities in middle-aged women. Maturitas 2015, 80, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Muka, T.; Oliver-Williams, C.; Kunutsor, S.; Laven, J.S.E.; Fauser, B.C.J.M.; Chowdhury, R.; Kavousi, M.; Franco, O. Association of Age at Onset of Menopause and Time Since Onset of Menopause with Cardiovascular Outcomes, Intermediate Vascular Traits, and All-Cause Mortality. JAMA Cardiol. 2016, 1, 767–776. [Google Scholar] [CrossRef] [Green Version]
- Thurston, R.C.; Chang, Y.; Barinas-Mitchell, E.; Jennings, J.R.; von Känel, R.; Landsittel, D.P.; Matthews, K.A. Physiologically assessed hot flashes and endothelial function among midlife women. Menopause 2018, 25, 1354–1361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manson, J.E.; Hsia, J.; Johnson, K.C.; Rossouw, J.E.; Assaf, A.R.; Lasser, N.L.; Trevisan, M.; Black, H.R.; Heckbert, S.R.; Detrano, R.; et al. Estrogen plus Progestin and the Risk of Coronary Heart Disease. N. Engl. J. Med. 2003, 349, 523–534. [Google Scholar] [CrossRef]
- Lyon, A.R. Cardiovascular disease following breast cancer treatment: Can we predict who will be affected? Eur. Heart J. 2019, 40, 3921–3923. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, G.T.; Oeffinger, K.C.; Chen, Y.; Kawashima, T.; Yasui, Y.; Leisenring, W.; Stovall, M.; Chow, E.; Sklar, C.A.; Mulrooney, D.A.; et al. Modifiable Risk Factors and Major Cardiac Events Among Adult Survivors of Childhood Cancer. J. Clin. Oncol. 2013, 31, 3673–3680. [Google Scholar] [CrossRef]
- Zamorano, J.L.; Lancellotti, P.; Muñoz, D.R.; Aboyans, V.; Asteggiano, R.; Galderisi, M.; Habib, G.; Lenihan, D.J.; Lip, G.Y.H.; Lyon, A.R.; et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 2768–2801. [Google Scholar] [CrossRef]
- Humphries, K.; Izadnegahdar, M.; Sedlak, T.; Saw, J.; Johnston, N.; Schenck-Gustafsson, K.; Shah, R.; Regitz-Zagrosek, V.; Grewal, J.; Vaccarino, V.; et al. Sex differences in cardiovascular disease—Impact on care and outcomes. Front. Neuroendocrinol. 2017, 46, 46–70. [Google Scholar] [CrossRef]
- Lichtman, J.H.; Leifheit, E.C.; Safdar, B.; Bao, H.; Krumholz, H.M.; Lorenze, N.P.; Daneshvar, M.; Spertus, J.A.; D’Onofrio, G. Sex Differences in the Presentation and Perception of Symptoms Among Young Patients with Myocardial Infarction. Circulation 2018, 137, 781–790. [Google Scholar] [CrossRef]
- DeFilippis, E.M.; Collins, B.L.; Singh, A.; Biery, D.W.; Fatima, A.; Qamar, A.; Berman, A.N.; Gupta, A.; Cawley, M.; Wood, M.J.; et al. Women who experience a myocardial infarction at a young age have worse outcomes compared with men: The Mass General Brigham YOUNG-MI registry. Eur. Heart J. 2020, 41, 4127–4137. [Google Scholar] [CrossRef]
- Canto, J.G.; Rogers, W.J.; Goldberg, R.J.; Peterson, E.D.; Wenger, N.K.; Vaccarino, V.; Kiefe, C.I.; Frederick, P.; Sopko, G.; Zheng, Z.-J.; et al. Association of Age and Sex with Myocardial Infarction Symptom Presentation and In-Hospital Mortality. JAMA 2012, 307, 813–822. [Google Scholar] [CrossRef]
- Kirchberger, I.; Heier, M.; Wende, R.; Von Scheidt, W.; Meisinger, C. The patient’s interpretation of myocardial infarction symptoms and its role in the decision process to seek treatment: The MONICA/KORA Myocardial Infarction Registry. Clin. Res. Cardiol. 2012, 101, 909–916. [Google Scholar] [CrossRef]
- Stehli, J.; Martin, C.; Brennan, A.; Dinh, D.T.; Lefkovits, J.; Zaman, S. Sex Differences Persist in Time to Presentation, Revascularization, and Mortality in Myocardial Infarction Treated with Percutaneous Coronary Intervention. J. Am. Heart Assoc. 2019, 8, e012161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mefford, M.T.; Li, B.H.; Qian, L.; Reading, S.R.; Harrison, T.N.; Scott, R.D.; Cavendish, J.J.; Jacobsen, S.J.; Kanter, M.H.; Woodward, M.; et al. Sex-Specific Trends in Acute Myocardial Infarction within an Integrated Healthcare Network, 2000 through 2014. Circulation 2020, 141, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Vaccarino, V.; Parsons, L.; Every, N.R.; Barron, H.V.; Krumholz, H.M. Sex-Based Differences in Early Mortality after Myocardial Infarction. N. Engl. J. Med. 1999, 341, 217–225. [Google Scholar] [CrossRef]
- Kenkre, T.S.; Malhotra, P.; Johnson, B.D.; Handberg, E.M.; Thompson, D.V.; Marroquin, O.C.; Rogers, W.J.; Pepine, C.J.; Merz, C.N.B.; Kelsey, S.F. Ten-Year Mortality in the WISE Study (Women’s Ischemia Syndrome Evaluation). Circ. Cardiovasc. Qual. Outcomes 2017, 10, 003863. [Google Scholar] [CrossRef]
- Sara, J.D.; Widmer, R.J.; Matsuzawa, Y.; Lennon, R.J.; Lerman, L.O.; Lerman, A. Prevalence of Coronary Microvascular Dysfunction Among Patients with Chest Pain and Nonobstructive Coronary Artery Disease. JACC Cardiovasc. Interv. 2015, 8, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Mangion, K.; Adamson, P.D.; Williams, M.C.; Hunter, A.; Pawade, T.; Shah, A.S.V.; Lewis, S.; Boon, N.A.; Flather, M.; Forbes, J.; et al. Sex associations and computed tomography coronary angiography-guided management in patients with stable chest pain. Eur. Heart J. 2019, 41, 1337–1345. [Google Scholar] [CrossRef] [PubMed]
- Sidik, N.P.; McEntegart, M.; Roditi, G.; Ford, T.J.; McDermott, M.; Morrow, A.; Byrne, J.; Adams, J.; Hargreaves, A.; Oldroyd, K.G.; et al. Rationale and design of the British Heart Foundation (BHF) Coronary Microvascular Function and CT Coronary Angiogram (CorCTCA) study. Am. Heart J. 2020, 221, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Claudio, C.P.; Quesada, O.; Pepine, C.J.; Merz, C.N.B. Why names matter for women: MINOCA/INOCA (myocardial infarction/ischemia and no obstructive coronary artery disease). Clin. Cardiol. 2018, 41, 185–193. [Google Scholar] [CrossRef]
- Dreyer, R.P.; Tavella, R.; Curtis, J.P.; Wang, Y.; Pauspathy, S.; Messenger, J.; Rumsfeld, J.S.; Maddox, T.M.; Krumholz, H.M.; Spertus, J.A.; et al. Myocardial infarction with non-obstructive coronary arteries as compared with myocardial infarction and obstructive coronary disease: Outcomes in a Medicare population. Eur. Heart J. 2020, 41, 870–878. [Google Scholar] [CrossRef]
- Smilowitz, N.R.; Mahajan, A.M.; Roe, M.T.; Hellkamp, A.S.; Chiswell, K.; Gulati, M.; Reynolds, H.R. Mortality of Myocardial Infarction by Sex, Age, and Obstructive Coronary Artery Disease Status in the ACTION Registry–GWTG (Acute Coronary Treatment and Intervention Outcomes Network Registry–Get with the Guidelines). Circ. Cardiovasc. Qual. Outcomes 2017, 10, e003443. [Google Scholar] [CrossRef]
- Reynolds, H.R.; Maehara, A.; Kwong, R.Y.; Sedlak, T.; Saw, J.; Smilowitz, N.R.; Mahmud, E.; Wei, J.; Marzo, K.; Matsumura, M.; et al. Coronary Optical Coherence Tomography and Cardiac Magnetic Resonance Imaging to Determine Underlying Causes of Myocardial Infarction with Nonobstructive Coronary Arteries in Women. Circulation 2021, 143, 624–640. [Google Scholar] [CrossRef] [PubMed]
- Taqueti, V.R.; Di Carli, M.F. Coronary Microvascular Disease Pathogenic Mechanisms and Therapeutic Options. J. Am. Coll. Cardiol. 2018, 72, 2625–2641. [Google Scholar] [CrossRef]
- Maddox, T.M.; Ho, P.M.; Roe, M.; Dai, D.; Tsai, T.T.; Rumsfeld, J.S. Utilization of Secondary Prevention Therapies in Patients with Nonobstructive Coronary Artery Disease Identified During Cardiac Catheterization. Circ. Cardiovasc. Qual. Outcomes 2010, 3, 632–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghadri, J.R.; Kato, K.; Cammann, V.L.; Gili, S.; Jurisic, S.; Di Vece, D.; Candreva, A.; Ding, K.J.; Micek, J.; Szawan, K.A.; et al. Long-Term Prognosis of Patients with Takotsubo Syndrome. J. Am. Coll. Cardiol. 2018, 72, 874–882. [Google Scholar] [CrossRef]
- Napp, L.C.; Cammann, V.L.; Jaguszewski, M.; Szawan, K.A.; Wischnewsky, M.; Gili, S.; Knorr, M.; Heiner, S.; Citro, R.; Bossone, E.; et al. Coexistence and outcome of coronary artery disease in Takotsubo syndrome. Eur. Heart J. 2020, 41, 3255–3268. [Google Scholar] [CrossRef]
- Pelliccia, F.; Kaski, J.C.; Crea, F.; Camici, P.G. Pathophysiology of Takotsubo Syndrome. Circulation 2017, 135, 2426–2441. [Google Scholar] [CrossRef]
- Udell, J.A.; Koh, M.; Qiu, F.; Austin, P.C.; Wijeysundera, H.C.; Bagai, A.; Yan, A.T.; Goodman, S.G.; Tu, J.V.; Ko, D.T. Outcomes of Women and Men with Acute Coronary Syndrome Treated with and without Percutaneous Coronary Revascularization. J. Am. Heart Assoc. 2017, 6, e004319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, R.G.; Parlow, S.; Simard, T.; Chen, C.; Ghataura, H.; Kishore, A.; Perera, A.; Moreland, R.; Hughes, I.; Tavella, R.; et al. Clinical features, sex differences and outcomes of myocardial infarction with nonobstructive coronary arteries: A registry analysis. Coron. Artery Dis. 2021, 32, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Hayes, S.N.; Kim, E.S.; Saw, J.; Adlam, D.; Arslanian-Engoren, C.; Economy, K.E.; Ganesh, S.K.; Gulati, R.; Lindsay, M.E.; Mieres, J.H.; et al. Spontaneous Coronary Artery Dissection: Current State of the Science: A Scientific Statement From the American Heart Association. Circulation 2018, 137, e523–e557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pagidipati, N.J.; Coles, A.; Hemal, K.; Lee, K.L.; Dolor, R.J.; Pellikka, P.A.; Mark, D.B.; Patel, M.R.; Litwin, S.E.; Daubert, M.A.; et al. Sex differences in management and outcomes of patients with stable symptoms suggestive of coronary artery disease: Insights from the PROMISE trial. Am. Heart J. 2019, 208, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Perl, L.; Bental, T.; Assali, A.; Vaknin-Assa, H.; Lev, E.; Kornowski, R.; Porter, A. Impact of female sex on long-term acute coronary syndrome outcomes. Coron. Artery Dis. 2015, 26, 11–16. [Google Scholar] [CrossRef]
- Patel, M.R.; Chen, A.Y.; Peterson, E.D.; Newby, L.K.; Pollack, C.V.; Brindis, R.G.; Gibson, C.M.; Kleiman, N.S.; Saucedo, J.F.; Bhatt, D.L.; et al. Prevalence, predictors, and outcomes of patients with non–ST-segment elevation myocardial infarction and insignificant coronary artery disease: Results from the Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA Guidelines (CRUSADE) initiative. Am. Heart J. 2006, 152, 641–647. [Google Scholar] [CrossRef]
- Smolina, K.; Ball, L.; Humphries, K.H.; Khan, N.; Morgan, S.G. Sex Disparities in Post-Acute Myocardial Infarction Pharmacologic Treatment Initiation and Adherence. Circ. Cardiovasc. Qual. Outcomes 2015, 8, 586–592. [Google Scholar] [CrossRef] [Green Version]
- Collet, J.-P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef]
- Vogel, B.; Baber, U.; Cohen, D.J.; Sartori, S.; Sharma, S.K.; Angiolillo, D.J.; Farhan, S.; Goel, R.; Zhang, Z.; Briguori, C.; et al. Sex Differences Among Patients with High Risk Receiving Ticagrelor with or without Aspirin after Percutaneous Coronary Intervention. JAMA Cardiol. 2021, 6, 1032–1041. [Google Scholar] [CrossRef]
- Vaccarino, V.; Abramson, J.L.; Veledar, E.; Weintraub, W.S. Sex Differences in Hospital Mortality after Coronary Artery Bypass Surgery. Circulation 2002, 105, 1176–1181. [Google Scholar] [CrossRef] [Green Version]
- Rauch, B.; Davos, C.; Doherty, P.; Saure, D.; Metzendorf, M.-I.; Salzwedel, A.; Völler, H.; Jensen, K.; Schmid, J.-P. The prognostic effect of cardiac rehabilitation in the era of acute revascularisation and statin therapy: A systematic review and meta-analysis of randomized and non-randomized studies—The Cardiac Rehabilitation Outcome Study (CROS). Eur. J. Prev. Cardiol. 2016, 23, 1914–1939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, L.; Thompson, D.; Oldridge, N.; Zwisler, A.-D.; Rees, K.; Martin, N.; Taylor, R.S. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst. Rev. 2016, 1, CD001800. [Google Scholar] [CrossRef] [Green Version]
- Anjo, D.; Santos, M.; Rodrigues, P.; Brochado, B.; Sousa, M.J.; Barreira, A.; Viamonte, S.; Fernandes, P.; Reis, A.H.; Gomes, J.L.; et al. Os benefícios da reabilitação cardíaca na doença coronária: Uma questão de género? Rev. Port. Cardiol. 2014, 33, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Hyun, K.; Negrone, A.; Redfern, J.; Atkins, E.; Chow, C.; Kilian, J.; Rajaratnam, R.; Brieger, D. Gender Difference in Secondary Prevention of Cardiovascular Disease and Outcomes Following the Survival of Acute Coronary Syndrome. Heart Lung Circ. 2021, 30, 121–127. [Google Scholar] [CrossRef]
- Allen, J.K.; Scott, L.B.; Stewart, K.J.; Young, D.R. Disparities in women’s referral to and enrollment in outpatient cardiac rehabilitation. J. Gen. Intern. Med. 2004, 19, 747–753. [Google Scholar] [CrossRef] [Green Version]
- Porter, A.; Paradkar, A.; Goldenberg, I.; Shlomo, N.; Cohen, T.; Kornowski, R.; Eisen, A. Temporal Trends Analysis of the Characteristics, Management, and Outcomes of Women with Acute Coronary Syndrome (ACS): ACS Israeli Survey Registry 2000–2016. J. Am. Heart Assoc. 2020, 9, e014721. [Google Scholar] [CrossRef]
| CAD risk factors associated with Pregnancy | Adverse pregnancy outcomes (APOs) | APOs include the hypertensive disorders of pregnancy, pre-term birth and intra-uterine growth restriction. APOs are associated with microvascular dysfunction and a higher risk of cardiovascular events later in life [19] |
| Hypertensive disorders of pregnancy | Women with pre-eclampsia have an increased risk of future subclinical coronary artery atherosclerosis [21]. | |
| Gestational Diabetes (GD) | Women with a history of GD have also been found to have a 2-fold increased risk of CAD later in life [23]. | |
| Gynecological conditions unrelated to pregnancy | Polycystic ovary syndrome (PCOS) | PCOS is associated with a greater cardiovascular risk [24]. |
| Menopause | The risk of CVD is higher in the postmenopausal period. Surgical menopause and earlier age at natural menopause are associated with an increased cardiovascular risk [25,26]. | |
| Menopausal Hormone Therapy (MHT) | MHT could have a potential cardiovascular benefit in women younger than 60 years old and when started within 10 years of menopause but can increase the cardiovascular risk in women with higher cardiovascular risk and after a prior cardiovascular event [16]. | |
| Breast Cancer | cardio-toxic effects of the chemotherapy and radiation-induced cardiotoxicity as well as due to accelerated development of CAD [27,28]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schamroth Pravda, N.; Karny-Rahkovich, O.; Shiyovich, A.; Schamroth Pravda, M.; Rapeport, N.; Vaknin-Assa, H.; Eisen, A.; Kornowski, R.; Porter, A. Coronary Artery Disease in Women: A Comprehensive Appraisal. J. Clin. Med. 2021, 10, 4664. https://doi.org/10.3390/jcm10204664
Schamroth Pravda N, Karny-Rahkovich O, Shiyovich A, Schamroth Pravda M, Rapeport N, Vaknin-Assa H, Eisen A, Kornowski R, Porter A. Coronary Artery Disease in Women: A Comprehensive Appraisal. Journal of Clinical Medicine. 2021; 10(20):4664. https://doi.org/10.3390/jcm10204664
Chicago/Turabian StyleSchamroth Pravda, Nili, Orith Karny-Rahkovich, Arthur Shiyovich, Miri Schamroth Pravda, Naomi Rapeport, Hana Vaknin-Assa, Alon Eisen, Ran Kornowski, and Avital Porter. 2021. "Coronary Artery Disease in Women: A Comprehensive Appraisal" Journal of Clinical Medicine 10, no. 20: 4664. https://doi.org/10.3390/jcm10204664






