Effect of Intraoral Drainage after Impacted Mandibular Third Molar Extraction on Non-Infectious Postoperative Complications
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Group
- -
- A (n = 30)—group with latex drain used;
- -
- B (n = 30)—group with a sodium–calcium alginate drain applied;
- -
- C (n = 30)—control group, in which the wound was secured with standard knotted sutures.
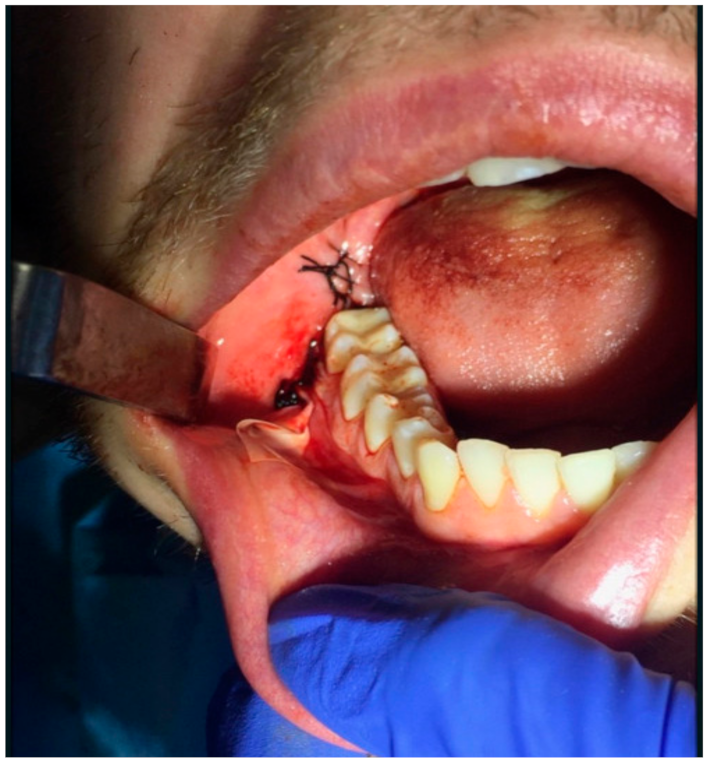
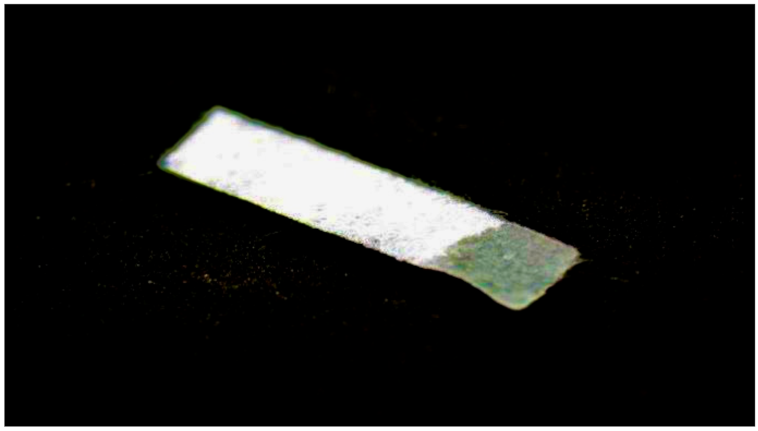
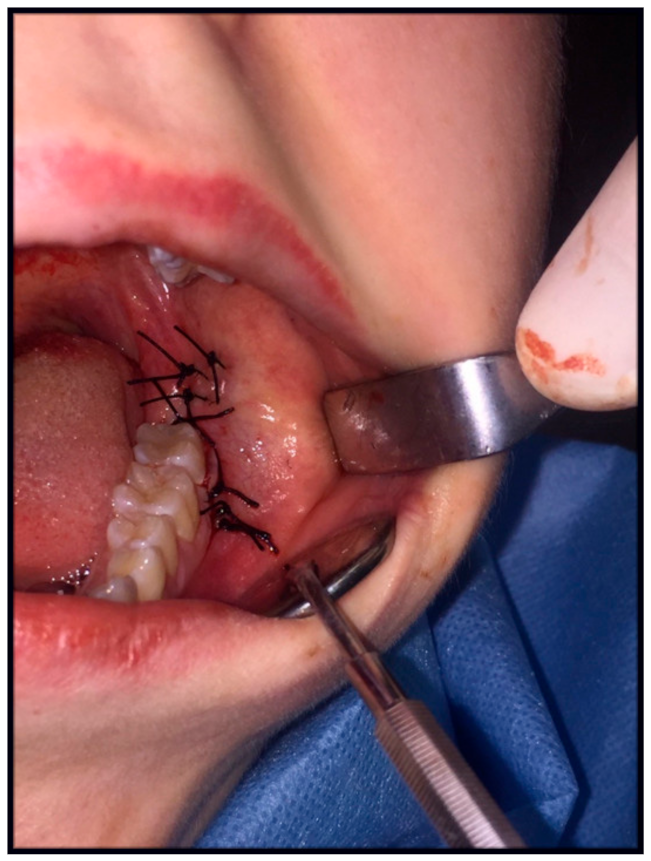
2.2. Surgery
2.3. Method of Assessing Postoperative Pain
2.4. Method for Assessing the Trismus
2.5. Method of Assessing Postoperative Soft Tissue Swelling
- -
- distance between the outer angle of the eye (Exocanthion—Ex) and the angle of the mandible (Gonion—Go)—line AB;
- -
- The distance between tragus (Tragus—T) and the angle of the mouth (Chelion—Ch)—line BC;
- -
- The distance between the tragus (Tragus—T) and the skin point (Pogonion—WPg)—line CD;
- -
- The distance between the medial upper incisor’s medial surface and the posterior edge of the mandibular ramus—line DE.
2.6. Methodology of Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Analysis of the Frequency of Retention Classes According to Pell and Gregory in the Study Groups
3.3. Comparative Analysis of the Procedure Difficulty Scale According to Pederson Classification between Study Groups
3.4. Comparative Analysis of the Level of Pain before and from Days 1 to 7 after Surgery between the Study Groups
3.5. Comparative Analysis of the Level of Jaw Opening before Surgery and on Day 1, 2, and 7 after Surgery between Groups
3.6. Comparative Analysis of AB Line Length before Surgery and on Day 1, 2, and 7 after Surgery between Study Groups
3.7. Comparative Analysis of BC Line Length before Surger, and on Day 1, 2 and 7 after Surgery between Study Groups
3.8. Comparative Analysis of CD Line Length before Surgery and on Day 1, 2, and 7 after Surgery between Study Groups
3.9. Analysis of Changes in the Length of the DE Line before Surgery and on Day 1, 2, and 7 after Surgery between Study Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Waite, P.D.; Reynolds, R.R. Surgical management of impacted third molars. Semin. Orthod. 1998, 4, 113–123. [Google Scholar] [CrossRef]
- Zawilska, A.; Koszkowski, R.; Waśkowska, J. Ocena budowy oraz typów retencji zatrzymanych trzecich trzonowców w obrazie pantomograficznym. Ann. Acad. Med. Stet. 2007, 53, 165–171. [Google Scholar]
- Czerniuk, M.R. Zęby mądrości jako potencjalne ognisko infekcji pochodzącej z jamy ustnej—Opis przypadku. Kardiol. Dypl. 2009, 8, 92–96. [Google Scholar]
- Lorè, B.; Gargari, M.; Ventucci, E.; Cagioli, A.; Nicolai, G.; Calabrese, L. A complication following tooth extraction: Chronic suppurative osteomyelitis. Oral Implantol. 2013, 6, 43–47. [Google Scholar] [CrossRef]
- Trybek, G.; Chamarczuk, A.; Falkowska, J.; Grzegorzewska, M.; Preuss, O.; Aniko-Włodarczyk, M. Intraoral odontogenic abscesses in patients of The Department of Oral Surgery at the Pomeranian Medical University in Szczecin: 7 years of observation. Postep. Hig. Med. Dosw. 2018, 72, 491–498. [Google Scholar] [CrossRef]
- Kilinc, A.; Ataol, M. How effective is collagen resorbable membrane placement after partially impacted mandibular third molar surgery on postoperative morbidity? A prospective randomized comparative study. BMC Oral Health 2017, 17, 1–8. [Google Scholar] [CrossRef]
- Kaczmarzyk, T. Poekstrakcyjne zapalenie zębodołu. Med. Prakt. 2012, 2, 45–50. [Google Scholar]
- Szubert, P.; Jankowski, M.; Krajecki, M.; Jankowska-Wika, A.; Sokalski, J. Analiza czynników predysponujących do powikłań po chirurgicznym usunięciu zębów mądrości w żuchwie. Dent. Forum 2015, 63, 45–50. [Google Scholar]
- de Brabander, E.C.; Cattaneo, G. Effectiveness of cold therapy in reducing pain, trismus, and oedema after impacted mandibular third molar surgery: A randomized, self-controlled, observer-blind, split-mouth clinical trial. Int. J. Oral Maxillofac. Surg. 2016, 45, 118–123. [Google Scholar]
- Xavier, R.L.; Vasconcelos, B.C.; Caubi, A.F.; Porto, G.G.; Maurette, M.A. Passive drainage through the vestibular oblique incision in impacted inferior third molar surgery: A preliminary study. Acta Odontol. Latinoam. 2008, 21, 57–63. [Google Scholar]
- Rullo, R.; Addabbo, F.; Papaccio, G.; D’Aquino, R.; Festa, V.M. Piezoelectric device vs. conventional rotative instruments in impacted third molar surgery: Relationships between surgical difficulty and postoperative pain with histological evaluations. J. Cranio-Maxillofac. Surg. 2013, 41, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Uyanik, L.O.; Bilginaylar, K.; Etikan, I. Effects of platelet-rich fibrin and piezosurgery on impacted mandibular third molar surgery outcomes. Head Face Med. 2015, 11, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Zhang, J.; Ma, J.Z.; Shao, L.N.; Gu, Y.F.; Li, D.Q.; Liang, L.; Yang, Y.Q. A novel method in the removal of impacted mandibular third molar: Buccal drainage. Sci. Rep. 2017, 7, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Matthew, I.R.; Browne, R.M.; Frame, J.W.; Millar, B.G. Tissue response to a haemostatic alginate wound dressing in tooth extraction sockets. Br. J. Oral Maxillofac. Surg. 1993, 31, 165–169. [Google Scholar] [CrossRef]
- Dominiak, M.; Gedrange, T.; Zapała, T. Podstawy Chirurgii Stomatologicznej; Urban & Partner: Wrocław, Poland, 2013. [Google Scholar]
- Zandi, M. Comparison of corticosteroids and rubber drain for reduction of sequelae after third molar surgery. Oral Maxillofac. Surg. 2008, 12, 29–33. [Google Scholar] [CrossRef]
- Chukwuneke, F.N.; Oji, C.; Saheeb, D.B. A comparative study of the effect of using a rubber drain on postoperative discomfort following lower third molar surgery. J. Oral Maxillofac. Surg. 2008, 37, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Osunde, O.D.; Adebola, R.A.; Omeje, U.K. Management of inflammatory complications in third molar surgery: A review of the literature. Afr. Health Sci. 2011, 11, 530–537. [Google Scholar] [PubMed]
- Rakprasitkul, S.; Pairuchvej, V. Mandibular third molar surgery with primary closure and tube drain. Int. J. Oral Maxillofac. Surg. 1997, 26, 187–190. [Google Scholar] [CrossRef]
- Saglam, A.A. Effects of tube drain with primary closure technique on postoperative trismus and swelling after removal of fully impacted mandibular third molars. Quintessence Int. 2003, 34, 143–147. [Google Scholar]
- Koyuncu, B.O.; Zeytinoglu, M.; Tetik, A.; Gomel, M.M. Effect of tube drainage compared with conventional suturing on postoperative discomfort after extraction of impacted mandibular third molars. Oral Maxillofac. Surg. 2014, 53, 63–67. [Google Scholar] [CrossRef]
- Garajei, A.; Emami, A. Effect of surgical drain on the control of swelling in impacted lower third molar surgery. J. Craniomaxillofacial Res. 2016, 3, 264–267. [Google Scholar]
- Gay-Escoda, C.; Gómez-Santos, L.; Sánchez-Torres, A.; Herráez-Vilas, J.M. Effect of the suture technique on postoperative pain, swelling and trismus after removal of lower third molars: A randomized clinical trial. Med. Oral Patol. Oral Cir. Bucal 2015, 20, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Sortino, F.; Cicciu, M. Strategies used to inhibit postoperative swelling following removal of impacted lower third molar. Dent. Res. J. 2011, 8, 162–171. [Google Scholar]
- Dolanmaz, D.; Esen, A.; Isik, K.; Candirli, C. Effect of 2 flap designs on postoperative pain and swelling after impacted third molar surgery. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 116, 244–246. [Google Scholar] [CrossRef] [PubMed]
- Danda, A.K.; Krishna Tatiparthi, M.; Narayanan, V.; Siddareddi, A. Influence of primary and secondary closure of surgical wound after impacted mandibular third molar removal on postoperative pain and swelling-A comparative and split mouth study. J. Oral Maxillofac. Surg. 2010, 68, 309–312. [Google Scholar] [CrossRef] [PubMed]
- Khande, K.; Saluja, H.; Mahindra, U. Primary and secondary closure of the surgical wound after removal of impacted mandibular third molars. J. Maxillofac. Oral Surg. 2011, 10, 112–117. [Google Scholar] [CrossRef]
- Hashemi, H.M.; Beshkar, M.; Aghajani, R. The effect of sutureless wound closure on postoperative pain and swelling after impacted mandibular third molar surgery. Br. J. Oral Maxillofac. Surg. 2012, 50, 256–258. [Google Scholar] [CrossRef]
- Maria, A.; Malik, M.; Virang, P. Comparison of primary and secondary closure of the surgical wound after removal of impacted mandibular third molars. J. Maxillofac. Oral Surg. 2012, 11, 276–283. [Google Scholar] [CrossRef][Green Version]
- Chaudhary, M.; Singh, M.; Singh, S.; Singh, S.P.; Kaur, G. Primary and secondary closure technique following removal of impacted mandibular third molars: A comparative study. Natl. J. Maxillofac. Surg. 2012, 3, 10–14. [Google Scholar]
- Skoracka, J.; Torlińska-Walkowiak, N.; Torlińska, T.; Woźniak, W. Podstawy anatomiczne i fizjologiczne zespołów bólowych układu stomatognatycznego. Now. Lek. 2006, 75, 80–89. [Google Scholar]
- Pathak, H.M.; Kumari, S.; Prasad, S.; Singh, N.; Pathak, P. Suture-less third molar surgery: Review of 30 cases. Int. J. Sci. Res. Publ. 2013, 3, 1–5. [Google Scholar]
- Osunde, O.D.; Adebola, R.A.; Saheeb, B.D. A comparative study of the effect of suture-less and multiple suture techniques on inflammatory complications following third molar surgery. Int. J. Oral Maxillofac. Surg. 2012, 41, 1275–1279. [Google Scholar] [CrossRef]
- Dąbrowiecki, S. Fizjologia i patofizjologia procesu gojenia ran. Pol. Med. Paliat. 2003, 2, 283. [Google Scholar]
- Dominiak, M.; Łysiak, K. Naprawa i/lub regeneracja poresekcyjnych i pocystektomijnych ubytków śródkostnych wyrostka zębodołowego–ocena uwarunkowań na podstawie piśmiennictwa i doświadczeń własnych. Dent. Med. Probl. 2005, 42, 341–350. [Google Scholar]
- Dawson, C.; Armstrong, M.W.; Fulford, S.C.; Farugi, R.M.; Galland, R.B. Use of calcium alginate to pack abscess cavities: A controlled clinical trial. J. R. Coll. Physicians Edinb. 1992, 37, 177–179. [Google Scholar]
- Matthew, I.R.; Browne, R.M.; Frame, J.W.; Millar, B.M. Subperiosteal behaviour of alginate and cellulose wound dressing materials. Biomaterials 1995, 16, 265–274. [Google Scholar] [CrossRef]
- Burrow, B.A.; Linday, A. A limited evaluation of alginates and a small scale comparision between Kaltostat and a standard non-adherent dressing, Ultraplast Alginate, in the treatment of nail avulsion by matrix phenolisation. Chiropodist 1989, 3, 211–218. [Google Scholar]
- Jaroń, A.; Preuss, O.; Grzywacz, E.; Trybek, G. The Impact of Using Kinesio Tape on Non-Infectious Complications after Impacted Mandibular Third Molar Surgery. Int. J. Environ. Res. Public Health 2021, 18, 399. [Google Scholar] [CrossRef]
- Jaroń, A.; Jedliński, M.; Grzywacz, E.; Mazur, M.; Trybek, G. Kinesiology Taping as an Innovative Measure against Post-Operative Complications after Third Molar Extraction-Systematic Review. J. Clin. Med. 2020, 9, 3988. [Google Scholar] [CrossRef]
- Falci, S.G.M.; Lima, T.C.; Martins, C.C.; Santos, C.R.R.D.; Pinheiro, M.L.P. Preemptive Effect of Dexamethasone in Third-Molar Surgery: A Meta-Analysis. Anesth. Prog. 2017, 64, 136–143. [Google Scholar] [CrossRef]
- do Nascimento-Júnior, E.M.; Dos Santos, G.M.S.; Tavares Mendes, M.L.; Cenci, M.; Correa, M.B.; Pereira-Cenci, T.; Martins-Filho, P.R.S. Cryotherapy in reducing pain, trismus, and facial swelling after third-molar surgery: Systematic review and meta-analysis of randomized clinical trials. J. Am. Dent. Assoc. 2019, 150, 269–277.e1. [Google Scholar] [CrossRef] [PubMed]
- Eshghpour, M.; Ahrari, F.; Takallu, M. Is Low-Level Laser Therapy Effective in the Management of Pain and Swelling After Mandibular Third Molar Surgery? J. Oral Maxillofac. Surg. 2016, 74, 1322.e1–1322.e8. [Google Scholar] [CrossRef] [PubMed]
- Deliverska, E.G.; Petkova, M. Complications after extraction of impacted third molars—Literature review. IMAB 2016, 22, 1202–1211. [Google Scholar] [CrossRef]
- Seymour, R.A.; Meechan, J.G.; Blair, G.S. An investigation into post-operative pain after third molar surgery under local analgesia. Br. J. Oral Maxillofac. Surg. 1985, 23, 410–418. [Google Scholar] [CrossRef]
- Grossi, G.B.; Maiorana, C.; Giarramone, R.A.; Borgonovo, A.; Beretta, M.; Farronato, D. Effects of submucosal injection of dexamethasone on postoperative discomfort after third molar surgery: A prospective study. J. Oral Maxillofac. Surg. 2007, 65, 2218–2226. [Google Scholar] [CrossRef]
- Ayaz, H.; Rehman, A.U.; Din, F.U. Post-operative complications associated with impacted mandibular third molar removal. Pak. Oral Dent. J. 2012, 32, 389–392. [Google Scholar]
- Darawade, D.A.; Kumar, S.; Mehta, R.; Sharma, A.R.; Reddy, G.S. In search of a better option: Dexamethasone versus methylprednisolone in third molar impaction surgery. J. Int. Oral Health 2014, 6, 14–17. [Google Scholar]
- Kumar, B.; Bhate, K.; Dolas, R.S.; Kumar, S.; Waknis, P. Comparative evaluation of immediate post-operative sequelae after surgical removal of impacted mandibular third molar with or without tube drain—Split-mouth study. J. Clin. Diagn Res. 2016, 10, 46–49. [Google Scholar] [CrossRef]
- Pedersen, A. Interrelation of complaints after removal of impacted mandibular third molars. Int. J. Oral Surg. 1985, 14, 241–244. [Google Scholar] [CrossRef]
- Handa, A.; Agwani, M.K.; Surendra, S.S.; Rana, S.S. Effects of tube drain with primary closure techniques on postoperative trismus and swelling after removal of impacted mandibular third molars. J. Dent. Med. Sci. 2016, 15, 92–100. [Google Scholar]
- Cerqueira, P.R.; Vasconcelos, B.C.; Bessa-Nogueira, R.V. Comparative Study of the Effect of a Tube Drain in Impacted Lower Third Molar Surgery. J. Oral Maxillofac. Surg. 2004, 62, 57–61. [Google Scholar] [CrossRef]
- Genc, A.; Cakarer, S.; Yalcin, B.K.; Kilic, B.B.; Isler, S.C.; Keskin, C. A comparative study of surgical drain placement and the use of kinesiologic tape to reduce postoperative morbidity after third molar surgery. Clin. Oral Investig. 2018, 23, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Metlerski, M.; Grocholewicz, K.; Jaroń, A.; Lipski, M.; Trybek, G. Comparison of Presurgical Dental Models Manufactured with Two Different Three-Dimensional Printing Techniques. J. Healthc. Eng. 2020, 2020, 8893338. [Google Scholar] [CrossRef] [PubMed]





| Group A (n = 30) | Group B (n = 30) | Group C (n = 30) | Groups A,B,C (n = 90) | p | ||
|---|---|---|---|---|---|---|
| Pell and Gregory | A1 | 5 (16.67%) | 1 (3.33%) | 5 (16.67%) | 11 (12.22%) | 0.184 |
| A2 | 8 (26.67%) | 10 (33.33%) | 6 (20.00%) | 24 (26.67%) | ||
| A3 | 0 (0.00%) | 1 (3.33%) | 0 (0.00%) | 1 (1.11%) | ||
| B1 | 5 (16.67%) | 4 (13.33%) | 3 (10.00%) | 12 (13.33%) | ||
| B2 | 8 (26.67%) | 7 (23.33%) | 4 (13.33%) | 19 (21.11%) | ||
| B3 | 0 (0.00%) | 2 (6.67%) | 1 (3.33%) | 3 (3.33%) | ||
| C2 | 0 (0.00%) | 1 (3.33%) | 6 (20.00%) | 7 (7.78%) | ||
| C3 | 4 (13.33%) | 4 (13.33%) | 5 (16.67%) | 13 (14.44%) | ||
| Group A (n = 30) | Group B (n = 30) | Group C (n = 30) | Groups A,B,C (n = 90) | p | ||
|---|---|---|---|---|---|---|
| Procedure difficulty according to Pederson | slightly difficult | 9 (30.00%) | 6 (20.00%) | 6 (20.00%) | 21 (23.33%) | 0.8 |
| moderately difficult | 12 (40.00%) | 16 (53.33%) | 14 (46.67%) | 42 (46.67%) | ||
| very difficult | 9 (30.00%) | 8 (26.67%) | 10 (33.33%) | 27 (30.00%) | ||
| Pain Intensity | Group A (n = 30) | Group B (n = 30) | Group C (n = 30) | p | |
|---|---|---|---|---|---|
| Before surgery | Mean ± SD | 0 ± 0 | 0 ± 0 | 0 ± 0 | 1 |
| median | 0 | 0 | 0 | P | |
| quartile | 0–0 | 0–0 | 0–0 | ||
| 1. day | Mean ± SD | 58.33 ± 17.98 | 63.1 ± 24.77 | 62.1 ± 26.09 | 0.583 |
| median | 64.5 | 66.5 | 60.5 | NP | |
| quartile | 46.25–72 | 55–75.75 | 45.25–85.5 | ||
| 2. day | Mean ± SD | 48.07 ± 18.54 | 53.37 ± 24.2 | 53.8 ± 21.08 | 0.516 |
| median | 52 | 55.5 | 54.5 | P | |
| quartile | 34.5–62 | 39.75–72 | 39–69 | ||
| 3. day | Mean ± SD | 33.8 ± 20.81 | 40.93 ± 22.36 | 45.37 ± 21.32 | 0.116 |
| median | 37 | 41.5 | 48 | P | |
| quartile | 15.25–49.75 | 25.5–54.75 | 29.25–60.5 | ||
| 4. day | Mean ± SD | 22.7 ± 18.48 | 30.27 ± 23.31 | 31.13 ± 21.6 | 0.336 |
| median | 20 | 24.5 | 30 | NP | |
| quartile | 6.75–34 | 14.5–48 | 12.5–48 | ||
| 5. day | Mean ± SD | 14.07 ± 11.84 | 24.17 ± 21.22 | 23.1 ± 21.02 | 0.211 |
| median | 12 | 19 | 15.5 | NP | |
| quartile | 3.25–22.75 | 8.5–39.25 | 6–41 | ||
| 6. day | Mean ± SD | 8.53 ± 11.05 | 17.37 ± 18.92 | 15.77 ± 17.72 | 0.154 |
| Median | 3.5 | 13 | 13 | NP | |
| Quartile | 0–14.5 | 1–28 | 0–24 | ||
| 7. day | Mean ± SD | 3.87 ± 5.34 | 10.47 ± 14.98 | 9.43 ± 13.67 | 0.159 |
| median | 1 | 5 | 3.5 | NP | |
| quartile | 0–6 | 0–11.75 | 0–15 | ||
| Jaw Opening [mm] | Group A (n = 30) | Group B (n = 30) | Group C (n = 30) | p | |
|---|---|---|---|---|---|
| Before surgery | Mean ± SD | 45.87 ± 5.09 | 45.74 ± 6.77 | 47.94 ± 6.83 | 0.277 |
| median | 45.89 | 46.86 | 49.22 | NP | |
| quartile | 42.05–49.69 | 44–50 | 43.27–51.99 | ||
| 1. day | Mean ± SD | 30.79 ± 8.72 | 25.87 ± 8.7 | 29.53 ± 9.06 | 0.059 |
| median | 30 | 24.88 | 28.57 | NP | |
| quartile | 23.46–34.5 | 20.3–31.12 | 24.02–34.74 | ||
| 2. day | Mean ± SD | 32.56 ± 8.23 | 27.76 ± 7.89 | 30.47 ± 9.58 | 0.101 |
| median | 31.16 | 28.75 | 29.3 | P | |
| quartile | 28.06–37.85 | 21.92–32.97 | 24.24–34.69 | ||
| 7. day | Mean ± SD | 39.6 ± 6.32 | 35.05 ± 6.65 | 39.07 ± 7.29 | 0.021 |
| median | 40.37 | 34.5 | 38.56 | P | |
| quartile | 36.52–43.14 | 31.59–39.88 | 33.04–44.75 | A, C > B | |
| Line AB [cm] | Group A (n = 30) | Group B (n = 30) | Group C (n = 30) | p | |
|---|---|---|---|---|---|
| Before surgery | Mean ± SD | 9.94 ± 0.98 | 9.98 ± 0.93 | 10.14 ± 1.05 | 0.701 |
| median | 10 | 10 | 10 | P | |
| quartile | 9.12–10.5 | 9.5–10.43 | 9.5–10.95 | ||
| 1. day | Mean ± SD | 10.32 ± 1.06 | 10.75 ± 1.16 | 11.02 ± 1.11 | 0.057 |
| median | 10.25 | 11 | 11 | P | |
| quartile | 9.5–11 | 10–11.3 | 10.12–12 | ||
| 2. day | Mean ± SD | 10.27 ± 1.04 | 10.46 ± 1 | 11.07 ± 1.08 | 0.011 |
| Median | 10 | 10.25 | 11 | NP | |
| quartile | 9.5–10.5 | 10–11 | 10–11.88 | C > B, A | |
| 7. day | Mean ± SD | 10.07 ± 1 | 10.11 ± 0.83 | 10.63 ± 0.82 | 0.012 |
| median | 10 | 10 | 10.5 | NP | |
| quartile | 9.5–10.5 | 9.5–10.5 | 10–11 | C > B, A | |
| Line BC [cm] | Group A (n = 30) | Group B (n = 30) | Group C (n = 30) | p | |
|---|---|---|---|---|---|
| Before surgery | Mean ± SD | 11.14 ± 0.73 | 10.84 ± 0.68 | 11.19 ± 0.9 | 0.169 |
| median | 11.35 | 10.9 | 11 | P | |
| quartile | 10.5–11.5 | 10.5–11.45 | 10.5–11.73 | ||
| 1. day | Mean ± SD | 11.51 ± 0.76 | 11.49 ± 0.64 | 11.79 ± 0.86 | 0.239 |
| Median | 11.5 | 11.5 | 11.6 | P | |
| quartile | 11–12 | 11–12 | 11–12.5 | ||
| 2. day | Mean ± SD | 11.52 ± 0.87 | 11.45 ± 0.64 | 11.77 ± 0.98 | 0.302 |
| median | 11.5 | 11.45 | 11.9 | P | |
| quartile | 11–12.07 | 11–11.95 | 11–12.5 | ||
| 7. day | Mean ± SD | 11.28 ± 0.77 | 11.12 ± 0.66 | 11.5 ± 0.91 | 0.18 |
| median | 11.45 | 11 | 11.5 | P | |
| quartile | 11–12 | 10.53–11.5 | 11–12 | ||
| Line CD [cm] | Group A(n = 30) | Group B (n = 30) | Group C (n = 30) | p | |
|---|---|---|---|---|---|
| Before surgery | Mean ± SD | 14.85 ± 0.97 | 14.39 ± 0.99 | 14.51 ± 0.93 | 0.174 |
| median | 14.5 | 14.45 | 14.5 | P | |
| quartile | 14.1–15.5 | 13.5–15 | 14–15 | ||
| 1. day | Mean ± SD | 15.39 ± 1.05 | 15.16 ± 0.9 | 15.26 ± 0.94 | 0.661 |
| median | 15.4 | 15 | 15.1 | P | |
| quartile | 14.62–16 | 14.5–15.57 | 15–16 | ||
| 2. day | Mean ± SD | 15.28 ± 0.96 | 15.07 ± 0.87 | 15.19 ± 0.97 | 0.686 |
| median | 15.35 | 15 | 15 | P | |
| quartile | 14.5–15.5 | 14.5–15.65 | 14.5–15.5 | ||
| 7. day | Mean ± SD | 15.01 ± 1.01 | 14.62 ± 0.97 | 14.77 ± 0.88 | 0.296 |
| median | 15 | 14.5 | 15 | P | |
| quartile | 14.5–15.5 | 14–15.38 | 14.5–15.15 | ||
| Line DE [cm] | Group A (n = 30) | Group B (n = 30) | Group C (n = 30) | p | |
|---|---|---|---|---|---|
| Before surgery | Mean ± SD | 12.45 ± 1 | 12.09 ± 0.64 | 12.62 ± 1.04 | 0.127 |
| median | 12.5 | 12 | 12.5 | NP | |
| quartile | 12–12.95 | 11.5–12.5 | 12–13 | ||
| 1. day | Mean ± SD | 12.91 ± 1 | 12.53 ± 0.65 | 13.25 ± 1.11 | 0.015 |
| median | 13 | 12.5 | 13 | P | |
| quartile | 12.5–13.5 | 12.25–13 | 12.5–14 | C > B | |
| 2. day | Mean ± SD | 12.91 ± 1.05 | 12.52 ± 0.63 | 13.29 ± 1.13 | 0.01 |
| median | 12.9 | 12.5 | 13 | P | |
| quartile | 12.2–13.5 | 12.1–12.7 | 12.5–14 | C > B | |
| 7. day | Mean ± SD | 12.74 ± 1.04 | 12.29 ± 0.63 | 12.89 ± 1.05 | 0.076 |
| median | 12.65 | 12.5 | 12.6 | NP | |
| quartile | 12–13.5 | 12–12.65 | 12.05–13.45 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trybek, G.; Jarzęcka, J.; Preuss, O.; Jaroń, A. Effect of Intraoral Drainage after Impacted Mandibular Third Molar Extraction on Non-Infectious Postoperative Complications. J. Clin. Med. 2021, 10, 4705. https://doi.org/10.3390/jcm10204705
Trybek G, Jarzęcka J, Preuss O, Jaroń A. Effect of Intraoral Drainage after Impacted Mandibular Third Molar Extraction on Non-Infectious Postoperative Complications. Journal of Clinical Medicine. 2021; 10(20):4705. https://doi.org/10.3390/jcm10204705
Chicago/Turabian StyleTrybek, Grzegorz, Joanna Jarzęcka, Olga Preuss, and Aleksandra Jaroń. 2021. "Effect of Intraoral Drainage after Impacted Mandibular Third Molar Extraction on Non-Infectious Postoperative Complications" Journal of Clinical Medicine 10, no. 20: 4705. https://doi.org/10.3390/jcm10204705
APA StyleTrybek, G., Jarzęcka, J., Preuss, O., & Jaroń, A. (2021). Effect of Intraoral Drainage after Impacted Mandibular Third Molar Extraction on Non-Infectious Postoperative Complications. Journal of Clinical Medicine, 10(20), 4705. https://doi.org/10.3390/jcm10204705








