Altered mRNA Expression of Interleukin-1 Receptors in Myocardial Tissue of Patients with Left Ventricular Assist Device Support
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. mRNA-Isolation and Real-Time PCR
2.3. Statistical Analysis
3. Results
3.1. Patient Demographics
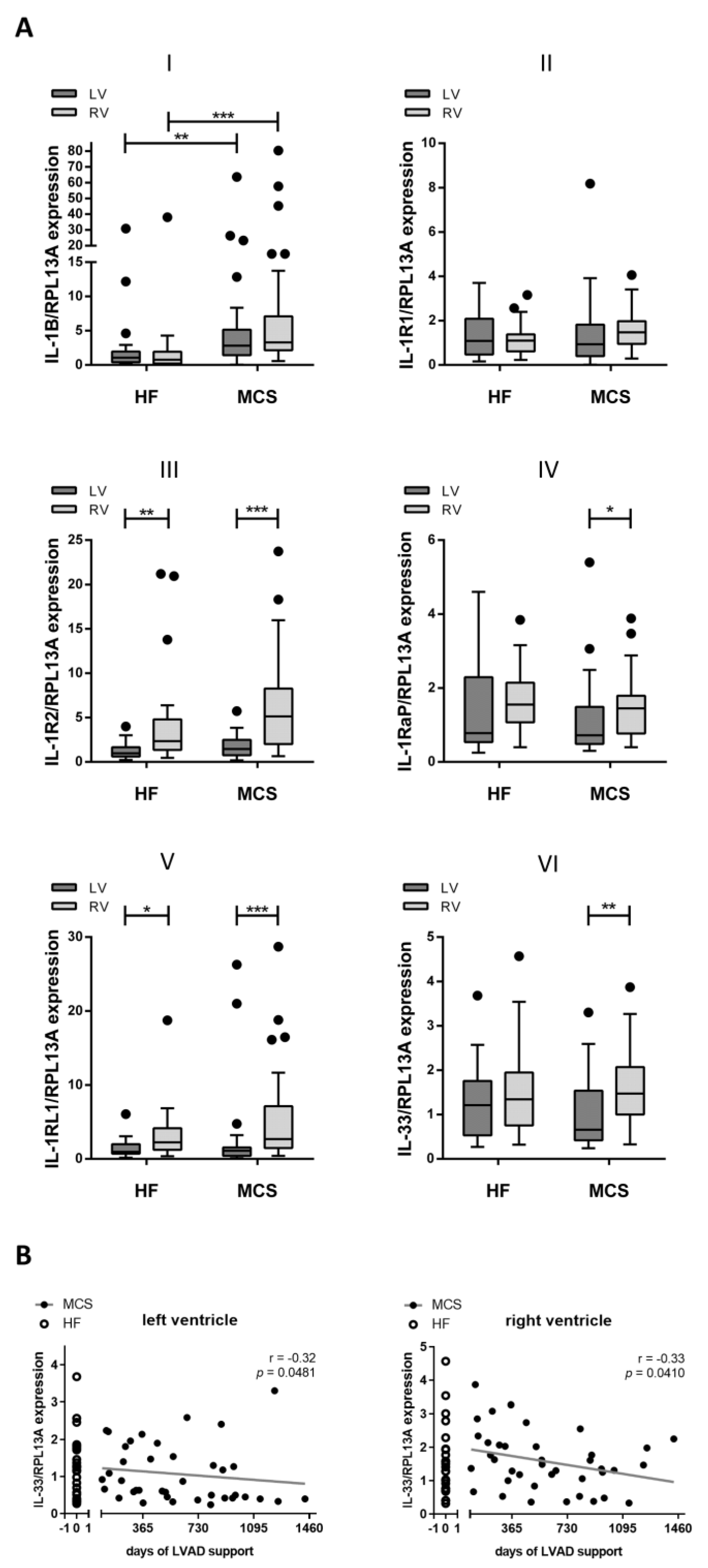
3.2. Increased Gene Expression of IL-1B in Patients with MCS
3.3. Correlation of IL-1 Receptor and IL-33 Expression
3.4. Altered Expression and Correlation Patterns in Right versus Left Ventricle
3.5. Correlation with CRP Plasma Levels and Leucocyte Count
3.6. Differences between Patients with ICM and DCM
4. Discussion
5. Strengths and Limitations of the Study
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Frigerio, M. Left Ventricular Assist Device: Indication, Timing, and Management. Heart Fail. Clin. 2021, 17, 619–634. [Google Scholar] [CrossRef] [PubMed]
- Grosman-Rimon, L.; Billia, F.; Fuks, A.; Jacobs, I.; McDonald, M.A.; Cherney, D.Z.; Rao, V.; Billia, F. New therapy, new challenges: The effects of long-term continuous flow left ventricular assist device on inflammation. Int. J. Cardiol. 2016, 215, 424–430. [Google Scholar] [CrossRef]
- Kawaguchi, M.; Takahashi, M.; Hata, T.; Kashima, Y.; Usui, F.; Morimoto, H.; Izawa, A.; Takahashi, Y.; Masumoto, J.; Koyama, J.; et al. Inflammasome Activation of Cardiac Fibroblasts Is Essential for Myocardial Ischemia/Reperfusion Injury. Circulation 2011, 123, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Bujak, M.; Dobaczewski, M.; Chatila, K.; Mendoza, L.H.; Li, N.; Reddy, A.; Frangogiannis, N. Interleukin-1 Receptor Type I Signaling Critically Regulates Infarct Healing and Cardiac Remodeling. Am. J. Pathol. 2008, 173, 57–67. [Google Scholar] [CrossRef]
- Eriksson, U.; Kurrer, M.O.; Sonderegger, I.; Iezzi, G.; Tafuri, A.; Hunziker, L.; Suzuki, S.; Bachmaier, K.; Bingisser, R.; Penninger, J.; et al. Activation of Dendritic Cells through the Interleukin 1 Receptor 1 Is Critical for the Induction of Autoimmune Myocarditis. J. Exp. Med. 2003, 197, 323–331. [Google Scholar] [CrossRef]
- Francis, S.; Holden, H.; Holt, C.M.; Duff, G.W. Interleukin-1 in Myocardium and Coronary Arteries of Patients with Dilated Cardiomyopathy. J. Mol. Cell. Cardiol. 1998, 30, 215–223. [Google Scholar] [CrossRef]
- Humeres, C.; Frangogiannis, N.G. Fibroblasts in the Infarcted, Remodeling, and Failing Heart. JACC Basic Transl. Sci. 2019, 4, 449–467. [Google Scholar] [CrossRef]
- Saxena, A.; Chen, W.; Su, Y.; Rai, V.; Uche, O.U.; Li, N.; Frangogiannis, N. IL-1 Induces Proinflammatory Leukocyte Infiltration and Regulates Fibroblast Phenotype in the Infarcted Myocardium. J. Immunol. 2013, 191, 4838–4848. [Google Scholar] [CrossRef]
- Segiet, O.A. The role of interleukins in heart failure with reduced ejection fraction. Anatol. J. Cardiol. 2019, 22, 287–299. [Google Scholar] [CrossRef]
- Barton, P.J.; Birks, E.J.; Felkin, L.; Cullen, E.M.; Koban, M.U.; Yacoub, M.H. Increased expression of extracellular matrix regulators TIMP1 and MMP1 in deteriorating heart failure. J. Heart Lung Transplant. 2003, 22, 738–744. [Google Scholar] [CrossRef]
- Bedi, M.S.; Alvarez, R.J., Jr.; Kubota, T.; Sheppard, R.; Kormos, R.L.; Siegenthaler, M.P.; Feldman, A.M.; McTiernan, C.F.; McNamara, D.M. Myocardial Fas and Cytokine Expression in End-Stage Heart Failure: Impact of LVAD Support. Clin. Transl. Sci. 2008, 1, 245–248. [Google Scholar] [CrossRef]
- Garlanda, C.; Dinarello, C.A.; Mantovani, A. The Interleukin-1 Family: Back to the Future. Immunity 2013, 39, 1003–1018. [Google Scholar] [CrossRef]
- Veeraveedu, P.T.; Sanada, S.; Okuda, K.; Fu, H.Y.; Matsuzaki, T.; Araki, R.; Yamato, M.; Yasuda, K.; Sakata, Y.; Yoshimoto, T.; et al. Ablation of IL-33 gene exacerbate myocardial remodeling in mice with heart failure induced by mechanical stress. Biochem. Pharmacol. 2017, 138, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Sanada, S.; Hakuno, D.; Higgins, L.J.; Schreiter, E.; McKenzie, A.N.; Lee, R.T. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J. Clin. Investig. 2007, 117, 1538–1549. [Google Scholar] [CrossRef]
- Abston, E.D.; Barin, J.G.; Cihakova, D.; Bucek, A.; Coronado, M.J.; Brandt, J.E.; Bedja, D.; Kim, J.B.; Georgakopoulos, D.; Gabrielson, K.L.; et al. IL-33 Independently Induces Eosinophilic Pericarditis and Cardiac Dilation. Circ. Heart Fail. 2012, 5, 366–375. [Google Scholar] [CrossRef]
- Chen, W.-Y.; Hong, J.; Gannon, J.; Kakkar, R.; Lee, R.T. Myocardial pressure overload induces systemic inflammation through endothelial cell IL-33. Proc. Natl. Acad. Sci. USA 2015, 112, 7249–7254. [Google Scholar] [CrossRef]
- Schmitz, J.; Owyang, A.; Oldham, E.; Song, Y.; Murphy, E.; McClanahan, T.K.; Zurawski, G.; Moshrefi, M.; Qin, J.; Li, X.; et al. IL-33, an Interleukin-1-like Cytokine that Signals via the IL-1 Receptor-Related Protein ST2 and Induces T Helper Type 2-Associated Cytokines. Immunity 2005, 23, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Díez, J.; Bayes-Genis, A. Compelling Benefit of Soluble Suppression of Tumorigenicity-2 in Post–Myocardial Infarction Estimation of Risk: The Time Is Right for Its Routine Use in the Clinic. J. Am. Heart Assoc. 2017, 6, 6. [Google Scholar] [CrossRef]
- Zhang, T.; Xu, C.; Zhao, R.; Cao, Z. Diagnostic Value of sST2 in Cardiovascular Diseases: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2021, 8, 697837. [Google Scholar] [CrossRef]
- Bi, J.; Garg, V.; Yates, A. Galectin-3 and sST2 as Prognosticators for Heart Failure Requiring Extracorporeal Life Support: Jack n’ Jill. Biomolecules 2021, 11, 166. [Google Scholar] [CrossRef]
- Tseng, C.C.S.; Huibers, M.M.H.; Van Kuik, J.; De Weger, R.A.; Vink, A.; De Jonge, N. The Interleukin-33/ST2 Pathway Is Expressed in the Failing Human Heart and Associated with Pro-fibrotic Remodeling of the Myocardium. J. Cardiovasc. Transl. Res. 2017, 11, 15–21. [Google Scholar] [CrossRef]
- Bartunek, J.; Delrue, L.; Van Durme, F.; Muller, O.; Casselman, F.; De Wiest, B.; Croes, R.; Verstreken, S.; Goethals, M.; de Raedt, H.; et al. Nonmyocardial Production of ST2 Protein in Human Hypertrophy and Failure Is Related to Diastolic Load. J. Am. Coll. Cardiol. 2008, 52, 2166–2174. [Google Scholar] [CrossRef] [PubMed]
- Demyanets, S.; Kaun, C.; Pentz, R.; Krychtiuk, K.; Rauscher, S.; Pfaffenberger, S.; Zuckermann, A.; Aliabadi, A.; Gröger, M.; Maurer, G.; et al. Components of the interleukin-33/ST2 system are differentially expressed and regulated in human cardiac cells and in cells of the cardiac vasculature. J. Mol. Cell. Cardiol. 2013, 60, 16–26. [Google Scholar] [CrossRef]
- Tseng, C.C.S.; Huibers, M.M.H.; Gaykema, L.H.; Koning, E.S.-D.; Ramjankhan, F.Z.; Maisel, A.S.; de Jonge, N. Soluble ST2 in end-stage heart failure, before and after support with a left ventricular assist device. Eur. J. Clin. Investig. 2018, 48, e12886. [Google Scholar] [CrossRef]
- Aleksova, A.; Beltrami, A.P.; Carriere, C.; Barbati, G.; Lesizza, P.; Perrieri-Montanino, M.; Isola, M.; Gentile, P.; Salvioni, E.; Not, T.; et al. Interleukin-1β levels predict long-term mortality and need for heart transplantation in ambulatory patients affected by idiopathic dilated cardiomyopathy. Oncotarget 2017, 8, 25131–25140. [Google Scholar] [CrossRef][Green Version]
- Szekely, Y.; Arbel, Y. A Review of Interleukin-1 in Heart Disease: Where Do We Stand Today? Cardiol. Ther. 2018, 7, 25–44. [Google Scholar] [CrossRef] [PubMed]
- Vieira, J.L.; Ventura, H.O.; Mehra, M.R. Mechanical circulatory support devices in advanced heart failure: 2020 and beyond. Prog. Cardiovasc. Dis. 2020, 63, 630–639. [Google Scholar] [CrossRef]
- Vanderheyden, M.; Paulus, W.; Voss, M.; Knuefermann, P.; Sivasubramanian, N.; Mann, D.; Baumgarten, G. Myocardial cytokine gene expression is higher in aortic stenosis than in idiopathic dilated cardiomyopathy. Heart 2005, 91, 926–931. [Google Scholar] [CrossRef]
- Birks, E.J.; Latif, N.; Owen, V.; Bowles, C.; Felkin, L.E.; Mullen, A.J.; Khaghani, A.; Barton, P.J.; Polak, J.M.; Pepper, J.R.; et al. Quantitative Myocardial Cytokine Expression and Activation of the Apoptotic Pathway in Patients Who Require Left Ventricular Assist Devices. Circulation 2001, 104, 233–240. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Küchler, A.M.; Pollheimer, J.; Balogh, J.; Sponheim, J.; Manley, L.; Sorensen, D.R.; De Angelis, P.M.; Scott, H.; Haraldsen, G. Nuclear Interleukin-33 Is Generally Expressed in Resting Endothelium but Rapidly Lost upon Angiogenic or Proinflammatory Activation. Am. J. Pathol. 2008, 173, 1229–1242. [Google Scholar] [CrossRef]
- Ho, J.E.; Chen, W.-Y.; Chen, M.-H.; Larson, M.G.; McCabe, E.L.; Cheng, S.; Ghorbani, A.; Coglianese, E.; Emilsson, V.; Johnson, A.D.; et al. Common genetic variation at the IL1RL1 locus regulates IL-33/ST2 signaling. J. Clin. Investig. 2013, 123, 4208–4218. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Liu, F.; Chen, F.; Jin, Y.; Chen, H.; Liu, D.; Cui, W. Amlodipine and atorvastatin improve ventricular hypertrophy and diastolic function via inhibiting TNF-α, IL-1β and NF-κB inflammatory cytokine networks in elderly spontaneously hypertensive rats. Biomed. Pharmacother. 2016, 83, 330–339. [Google Scholar] [CrossRef]
- Zahran, M.H.; Hussein, A.M.; Barakat, N.; Awadalla, A.; Khater, S.; Harraz, A.; Shokeir, A.A. Sildenafil activates antioxidant and antiapoptotic genes and inhibits proinflammatory cytokine genes in a rat model of renal ischemia/reperfusion injury. Int. Urol. Nephrol. 2015, 47, 1907–1915. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, M.; Chen, Y.; Wang, Y. Effects of atorvastatin and ticagrelor combination therapy on renal function and the levels of suppression of tumorigenicity 2 and interleukin-33 in patients with ST-segment elevation myocardial infarction. J. Int. Med Res. 2020, 48, 300060520959502. [Google Scholar] [CrossRef]
- Xia, J.; Qu, Y.; Yin, C.; Xu, D. Preliminary study of beta-blocker therapy on modulation of interleukin-33/ST2 signaling during ventricular remodeling after acute myocardial infarction. Cardiol. J. 2017, 24, 188–194. [Google Scholar] [CrossRef]
- Caselli, C.; D’Amico, A.; Ragusa, R.; Caruso, R.; Prescimone, T.; Cabiati, M.; Nonini, S.; Marraccini, P.; Del Ry, S.; Trivella, M.G.; et al. IL-33/ST2 Pathway and Classical Cytokines in End-Stage Heart Failure Patients Submitted to Left Ventricular Assist Device Support: A Paradoxic Role for Inflammatory Mediators? Mediat. Inflamm. 2013, 2013, 498703. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Más, J.; Lax, A.; Asensio-López, M.D.C.; Palacio, M.J.F.-D.; Caballero, L.; Santarelli, G.; Januzzi, J.L.; Figal, D.A.P. Modulation of IL-33/ST2 system in postinfarction heart failure: Correlation with cardiac remodelling markers. Eur. J. Clin. Investig. 2014, 44, 643–651. [Google Scholar] [CrossRef]
- Lax, A.M.; Sanchez-Mas, J.; Asensio-Lopez, M.C.; Palacio, M.J.F.-D.; Caballero, L.; Garrido, I.P.; Pastor-Perez, F.J.; Januzzi, J.L.; Pascual-Figal, D.A. Mineralocorticoid Receptor Antagonists Modulate Galectin-3 and Interleukin-33/ST2 Signaling in Left Ventricular Systolic Dysfunction After Acute Myocardial Infarction. JACC Heart Fail. 2015, 3, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Dewachter, L.; Dewachter, C. Inflammation in Right Ventricular Failure: Does It Matter? Front. Physiol. 2018, 9, 1056. [Google Scholar] [CrossRef] [PubMed]
- Begieneman, M.P.V.; van de Goot, F.R.W.; Bilt, I.A.C.V.D.; Noordegraaf, A.V.; Spreeuwenberg, M.D.; Paulus, W.J.; van Hinsbergh, V.W.M.; Visser, F.C.; Niessen, H.W.M. Pulmonary embolism causes endomyocarditis in the human heart. Heart 2007, 94, 450–456. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Frustaci, A.; Petrosillo, N.; Vizza, D.; Francone, M.; Badagliacca, R.; Verardo, R.; Fedele, F.; Ippolito, G.; Chimenti, C. Myocardial and microvascular inflammation/infection in patients with HIV-associated pulmonary artery hypertension. AIDS 2014, 28, 2541–2549. [Google Scholar] [CrossRef] [PubMed]
- Belhaj, A.; Dewachter, L.; Kerbaul, F.; Brimioulle, S.; Dewachter, C.; Naeije, R.; Rondelet, B. Heme Oxygenase-1 and Inflammation in Experimental Right Ventricular Failure on Prolonged Overcirculation-Induced Pulmonary Hypertension. PLoS ONE 2013, 8, e69470. [Google Scholar] [CrossRef] [PubMed]
- Dewachter, C.; Belhaj, A.; Rondelet, B.; Vercruyssen, M.; Schraufnagel, D.P.; Remmelink, M.; Brimioulle, S.; Kerbaul, F.; Naeije, R.; Dewachter, L. Myocardial inflammation in experimental acute right ventricular failure: Effects of prostacyclin therapy. J. Hear. Lung Transplant. 2015, 34, 1334–1345. [Google Scholar] [CrossRef] [PubMed]
- Segiet, O.A. The concentration of interleukin-33 in heart failure with reduced ejection fraction. Anatol. J. Cardiol. 2019, 21, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Pomerantz, B.J.; Reznikov, L.L.; Harken, A.H.; Dinarello, C.A. Inhibition of caspase 1 reduces human myocardial ischemic dysfunction via inhibition of IL-18 and IL-1. Proc. Natl. Acad. Sci. USA 2001, 98, 2871–2876. [Google Scholar] [CrossRef] [PubMed]
- Abbate, A.; Salloum, F.; Van Tassell, B.W.; Vecile, E.; Toldo, S.; Seropian, I.; Mezzaroma, E.; Dobrina, A. Alterations in the Interleukin-1/Interleukin-1 Receptor Antagonist Balance Modulate Cardiac Remodeling following Myocardial Infarction in the Mouse. PLoS ONE 2011, 6, e27923. [Google Scholar] [CrossRef] [PubMed]
- Sager, H.B.; Heidt, T.; Hulsmans, M.; Dutta, P.; Courties, G.; Sebas, M.; Wojtkiewicz, G.; Tricot, B.; Iwamoto, Y.; Sun, Y.; et al. Targeting Interleukin-1β Reduces Leukocyte Production After Acute Myocardial Infarction. Circulation 2015, 132, 1880–1890. [Google Scholar] [CrossRef] [PubMed]
- Panahi, M.; Papanikolaou, A.; Torabi, A.; Zhang, J.-G.; Khan, H.; Vazir, A.; Hasham, M.; Cleland, J.G.F.; Rosenthal, N.; Harding, S.; et al. Immunomodulatory interventions in myocardial infarction and heart failure: A systematic review of clinical trials and meta-analysis of IL-1 inhibition. Cardiovasc. Res. 2018, 114, 1445–1461. [Google Scholar] [CrossRef] [PubMed]
- Abbate, A.; Trankle, C.R.; Buckley, L.F.; Lipinski, M.J.; Appleton, D.; Kadariya, D.; Canada, J.M.; Carbone, S.; Roberts, C.S.; Abouzaki, N.; et al. Interleukin-1 Blockade Inhibits the Acute Inflammatory Response in Patients With ST-Segment–Elevation Myocardial Infarction. J. Am. Heart Assoc. 2020, 9, e014941. [Google Scholar] [CrossRef] [PubMed]
- Everett, B.M.; Cornel, J.; Lainscak, M.; Anker, S.D.; Abbate, A.; Thuren, T.; Libby, P.; Glynn, R.J.; Ridker, P.M. Anti-Inflammatory Therapy with Canakinumab for the Prevention of Hospitalization for Heart Failure. Circulation 2019, 139, 1289–1299. [Google Scholar] [CrossRef] [PubMed]
- Harouki, N.; Nicol, L.; Remy-Jouet, I.; Henry, J.-P.; Dumesnil, A.; Lejeune, A.; Renet, S.; Golding, F.; Djerada, Z.; Wecker, D.; et al. The IL-1β Antibody Gevokizumab Limits Cardiac Remodeling and Coronary Dysfunction in Rats with Heart Failure. JACC Basic Transl. Sci. 2017, 2, 418–430. [Google Scholar] [CrossRef] [PubMed]
- Van Tassell, B.W.; Abouzaki, N.A.; Erdle, C.O.; Carbone, S.; Trankle, C.R.; Melchior, R.D.; Turlington, J.S.; Thurber, C.J.; Christopher, S.; Dixon, D.L.; et al. Interleukin-1 Blockade in Acute Decompensated Heart Failure. J. Cardiovasc. Pharmacol. 2016, 67, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef] [PubMed]


| HF (n = 24) | MCS (n = 39) | p-Value | |
|---|---|---|---|
| Gender (male), n (%) | 17 (70.8%) | 33 (84.6%) | n.s. |
| Age (years) | 53 ± 2.2 | 55 ± 1.8 | n.s. |
| BMI | 24.58 ± 1.36 | 27.90 ± 0.92 | n.s. |
| NYHA Class IV | 0 (0%) | 4 (10.3%) | 0.001 |
| III | 2 (8.3%) | 18 (46.2%) | |
| II | 18 (75.0%) | 16 (41.0%) | |
| I | 4 (16.7%) | 1 (2.6%) | |
| LVEF % | 26 ± 2.1 | 22 ± 4.5 | n.s. |
| DCM, n (%) | 18 (75%) | 20 (51.3%) | n.s. |
| ICM, n (%) | 6 (25%) | 19 (48.7%) | n.s. |
| Comorbidities | |||
| Diabetes, n (%) | 8 (33.3%) | 13 (33.3%) | n.s. |
| Hypertension, n (%) | 9 (37.5%) | 21 (53.8%) | n.s. |
| Dyslipidemia, n (%) | 8 (33.3%) | 8 (20.5%) | n.s. |
| Kidney disease, n (%) | 14 (58.3%) | 22 (56.4%) | n.s. |
| History of smoking, n (%) | 2 (8.3%) | 3 (7.7%) | n.s. |
| Pre OP laboratory values | |||
| NT-proBNP (pg/mL) | 6674 ± 1580 | 2304 ± 883 | 0.002 |
| Bilirubin (mg/dL) | 1.2 ± 0.26 | 0.9 ± 0.12 | n.s. |
| Creatinine (mg/dL) | 1.7 ± 0.43 | 1.2 ± 0.08 | n.s. |
| Leucocytes (1000/µL) | 9.4 ± 0.84 | 8.2 ± 0.47 | n.s. |
| CRP (mg/dL) | 3.2 ± 1.6 | 2.1 ± 0.43 | n.s. |
| Treatments | |||
| ACE-I and/or ARB, n (%) | 17 (70.8%) | 18 (46.2%) | n.s. |
| Beta blockers, n (%) | 16 (66.7%) | 34 (87.2%) | n.s. |
| Statins, n (%) | 10 (41.6%) | 20 (51.3%) | n.s. |
| Antiplatelet agents, n (%) | 9 (37.5%) | 32 (82.1%) | 0.001 |
| Inotropic support, n (%) | 2 (8.3%) | 0 (0%) | n.s. |
| MR / Aldosterone antagonists, n (%) | 13 (54.2%) | 28 (71.8%) | n.s. |
| Other diuretics, n (%) | 19 (79.2%) | 27 (69.2%) | n.s. |
| Antiarrhythmic therapy, n (%) | 12 (50%) | 8 (20.5%) | 0.025 |
| Antidiabetic agents, n (%) | 2 (8.3%) | 7 (17.9%) | n.s. |
| Calcium antagonists, n (%) | 1 (4.2%) | 10 (25.6%) | 0.040 |
| PDE5i, n (%) | 4 (10.3%) | 22 (56.4%) | 0.003 |
| Allopurinol, n (%) | 6 (26.7%) | 7 (25%) | n.s. |
| r-Value | ||||||
| HF | LV | RV | ||||
| IL1RaP | IL1RL1 | IL-33 | IL1RaP | IL1RL1 | IL-33 | |
| IL1R1 | 0.73 | 0.48 | 0.93 | 0.79 | 0.75 | 0.78 |
| IL1RaP | 0.46 | 0.75 | 0.68 | 0.77 | ||
| IL1RL1 | 0.32 | 0.58 | ||||
| MCS | ||||||
| IL1R1 | 0.83 | 0.46 | 0.78 | 0.75 | 0.36 | 0.65 |
| IL1RaP | 0.29 | 0.74 | 0.29 | 0.57 | ||
| IL1RL1 | 0.22 | 0.02 | ||||
| p-Value | ||||||
| HF | LV | RV | ||||
| IL1RaP | IL1RL1 | IL-33 | IL1RaP | IL1RL1 | IL-33 | |
| IL1R1 | <0.0001 | 0.0163 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| IL1RaP | 0.0248 | <0.0001 | 0.0003 | <0.0001 | ||
| IL1RL1 | n.s. | 0.0031 | ||||
| MCS | ||||||
| IL1R1 | <0.0001 | 0.0028 | <0.0001 | <0.0001 | 0.0224 | <0.0001 |
| IL1RaP | n.s. (0.073) | <0.0001 | n.s. (0.078) | 0.0001 | ||
| IL1RL1 | n.s. | n.s. |
| r | HF | MCS | p | HF | MCS | ||||
| LV | RV | LV | RV | LV | RV | LV | RV | ||
| IL-1B | −0.15 | 0.05 | 0.00 | −0.06 | n.s. | n.s. | n.s. | n.s. | |
| IL-1R1 | 0.56 | 0.41 | 0.32 | −0.06 | 0.0067 | n.s. (0.059) | n.s. (0.053) | n.s. | |
| IL-1R2 | 0.10 | 0.11 | 0.32 | 0.08 | n.s. | n.s. | n.s. (0.061) | n.s. | |
| IL-1RaP | 0.27 | 0.18 | 0.15 | −0.18 | n.s. | n.s. | n.s. | n.s. | |
| IL-1RL1 | 0.23 | 0.44 | 0.34 | 0.17 | n.s. | 0.0421 | 0.0402 | n.s. | |
| IL-33 | 0.58 | 0.48 | 0.19 | −0.06 | 0.0041 | 0.0239 | n.s. | n.s. | |
| Serum leucocyte number | |||||||||
| r | HF | MCS | p | HF | MCS | ||||
| LV | RV | LV | RV | LV | RV | LV | RV | ||
| IL-1B | 0.01 | 0.15 | 0.19 | 0.05 | n.s. | n.s. | n.s. | n.s. | |
| IL-1R1 | 0.52 | 0.05 | 0.18 | 0.02 | 0.0097 | n.s. | n.s. | n.s. | |
| IL-1R2 | 0.11 | −0.20 | 0.17 | 0.10 | n.s. | n.s. | n.s. | n.s. | |
| IL-1RaP | 0.12 | −0.12 | −0.03 | 0.04 | n.s. | n.s. | n.s. | n.s. | |
| IL-1RL1 | 0.35 | 0.06 | 0.16 | 0.09 | n.s. (0.09) | n.s. | n.s. | n.s. | |
| IL-33 | 0.46 | 0.07 | −0.15 | 0.05 | 0.0242 | n.s. | n.s. | n.s. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niazy, N.; Mrozek, L.; Barth, M.; Immohr, M.B.; Kalampokas, N.; Saeed, D.; Aubin, H.; Sugimura, Y.; Westenfeld, R.; Boeken, U.; et al. Altered mRNA Expression of Interleukin-1 Receptors in Myocardial Tissue of Patients with Left Ventricular Assist Device Support. J. Clin. Med. 2021, 10, 4856. https://doi.org/10.3390/jcm10214856
Niazy N, Mrozek L, Barth M, Immohr MB, Kalampokas N, Saeed D, Aubin H, Sugimura Y, Westenfeld R, Boeken U, et al. Altered mRNA Expression of Interleukin-1 Receptors in Myocardial Tissue of Patients with Left Ventricular Assist Device Support. Journal of Clinical Medicine. 2021; 10(21):4856. https://doi.org/10.3390/jcm10214856
Chicago/Turabian StyleNiazy, Naima, Linus Mrozek, Mareike Barth, Moritz Benjamin Immohr, Nikolaos Kalampokas, Diyar Saeed, Hug Aubin, Yukiharu Sugimura, Ralf Westenfeld, Udo Boeken, and et al. 2021. "Altered mRNA Expression of Interleukin-1 Receptors in Myocardial Tissue of Patients with Left Ventricular Assist Device Support" Journal of Clinical Medicine 10, no. 21: 4856. https://doi.org/10.3390/jcm10214856
APA StyleNiazy, N., Mrozek, L., Barth, M., Immohr, M. B., Kalampokas, N., Saeed, D., Aubin, H., Sugimura, Y., Westenfeld, R., Boeken, U., Lichtenberg, A., & Akhyari, P. (2021). Altered mRNA Expression of Interleukin-1 Receptors in Myocardial Tissue of Patients with Left Ventricular Assist Device Support. Journal of Clinical Medicine, 10(21), 4856. https://doi.org/10.3390/jcm10214856








