Allografts for Skin Closure during In Utero Spina Bifida Repair in a Sheep Model
Abstract
1. Introduction
2. Methods
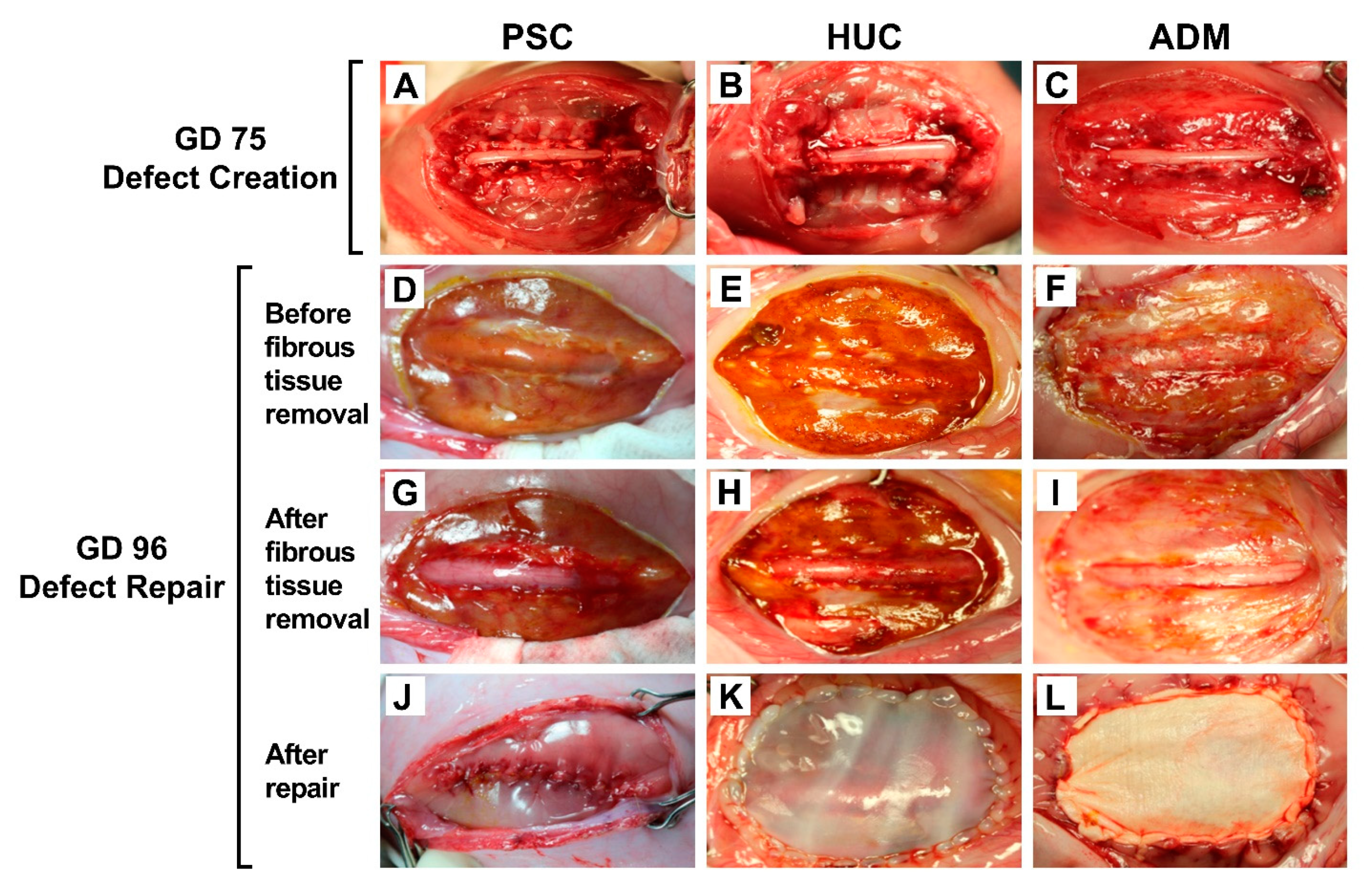
2.1. SB Model Creation
2.2. SB Defect Repair
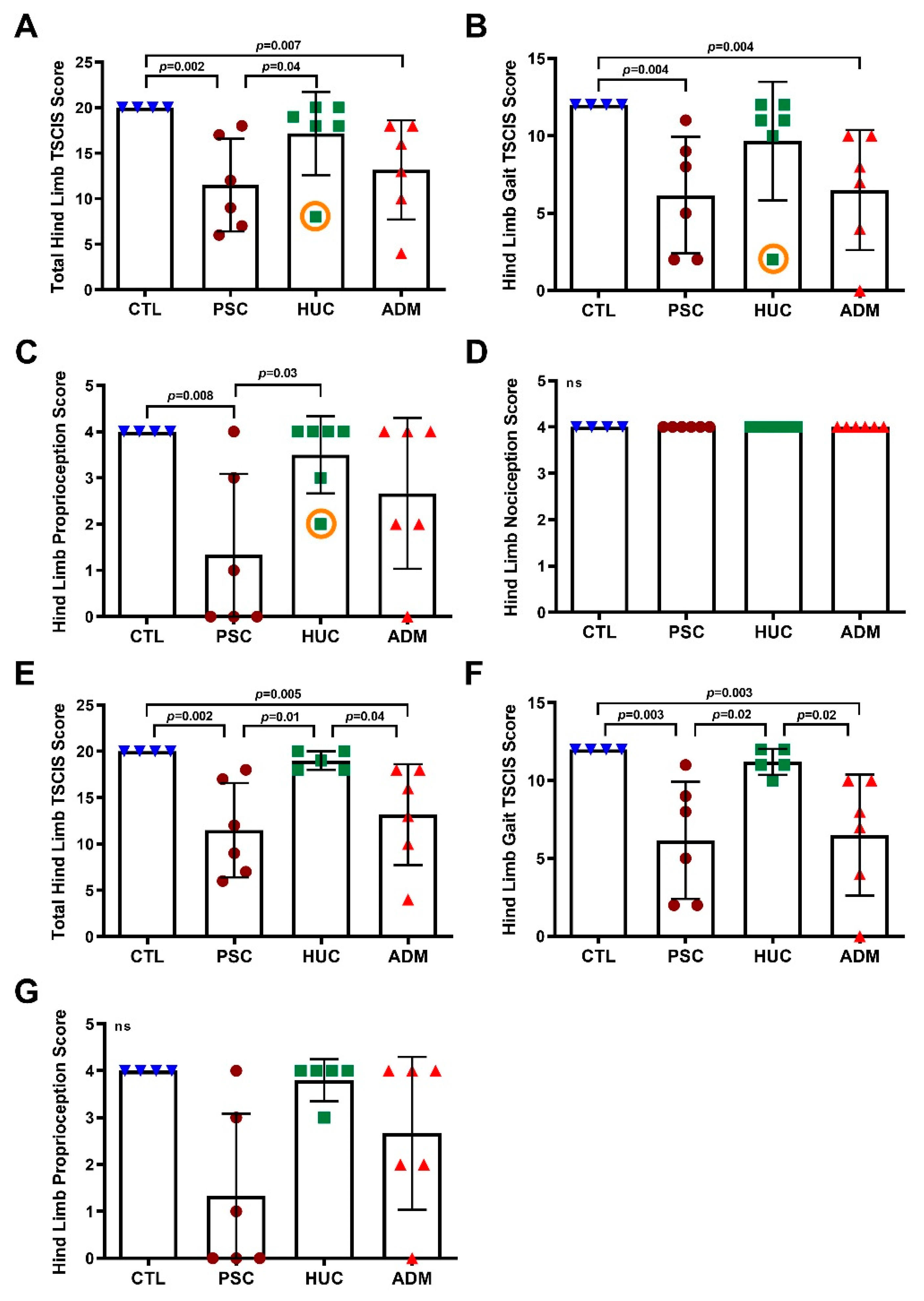
2.3. Delivery and Clinical Outcome Assessment
2.4. Magnetic Resonance Imaging and Analysis
2.5. Histology
2.6. Immunofluorescence
3. Statistics
4. Results
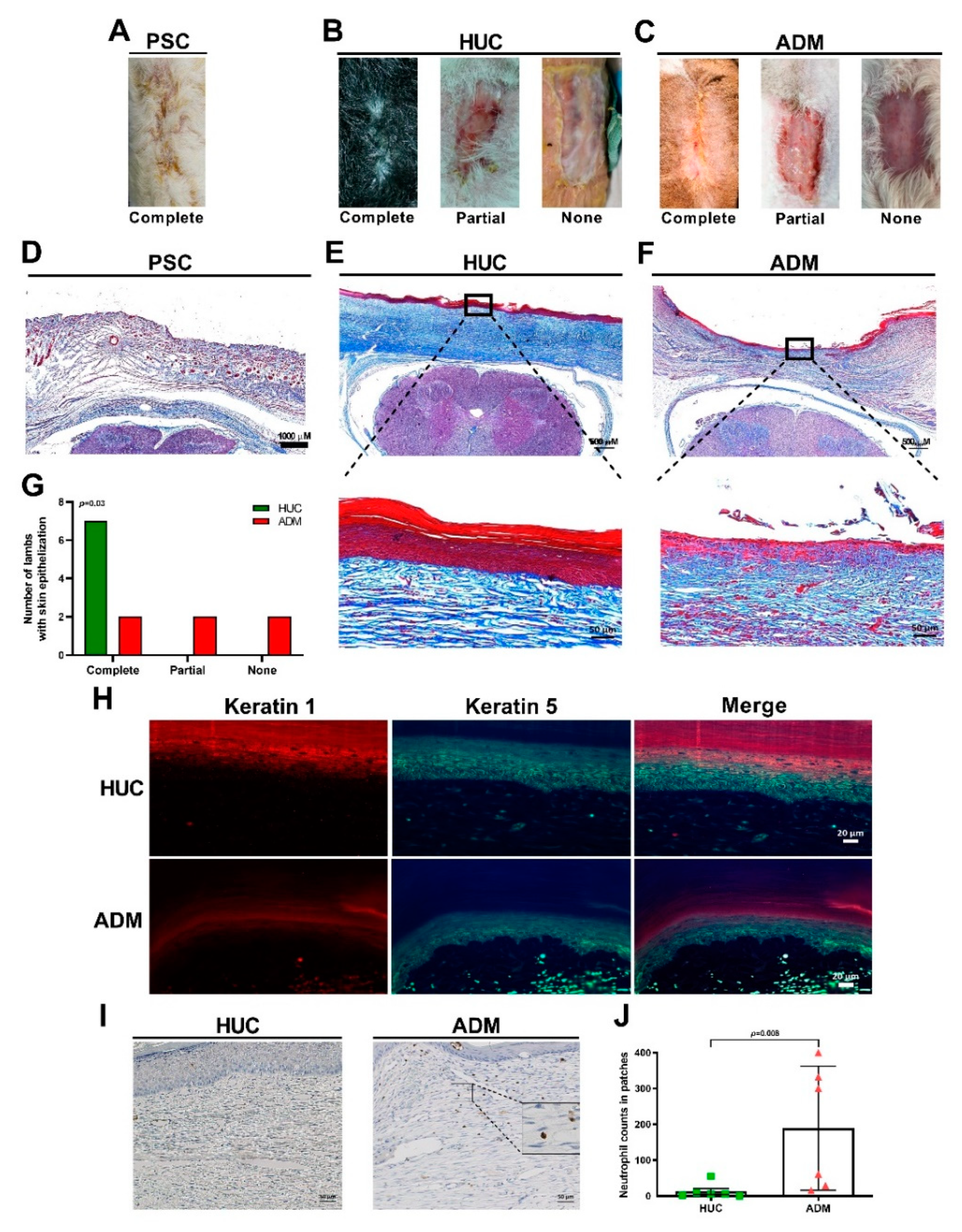
4.1. Delayed Epithelialization of the ADM Together with Neutrophil Accumulation
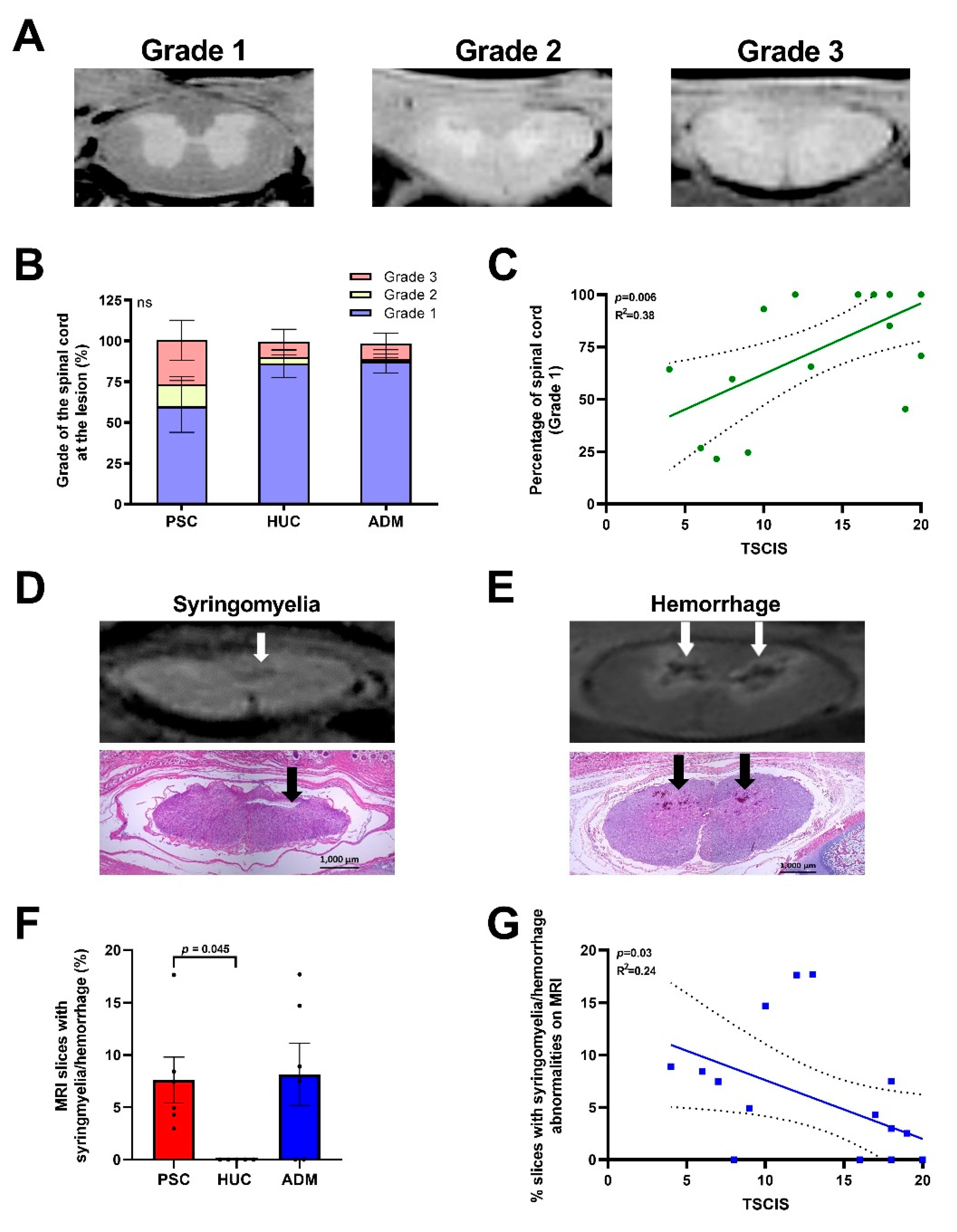
4.2. Radiological Evidence of Increased Spinal Cord Abnormalities in the PSC and ADM Repair Groups Compared to the HUC Repair Group
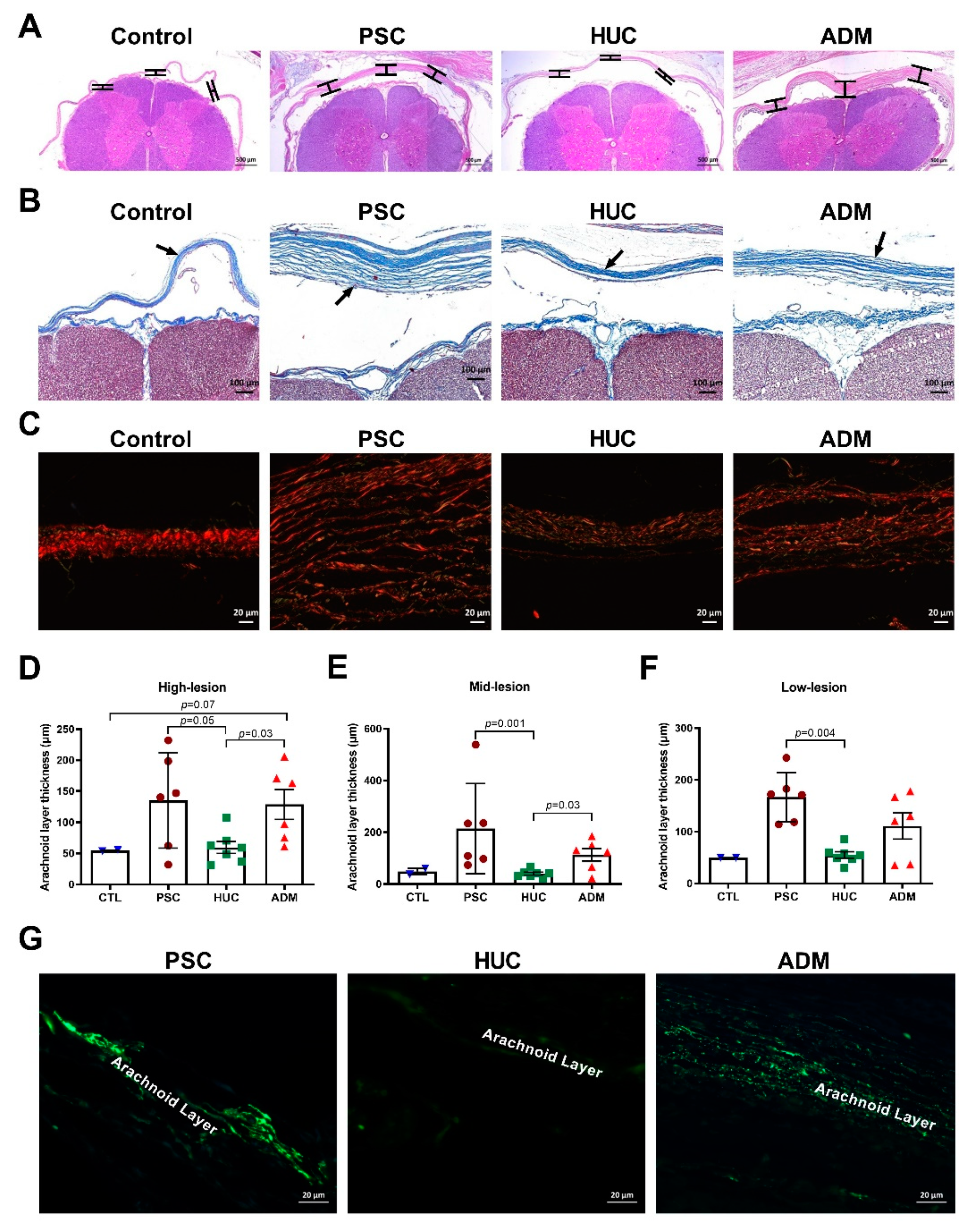
4.3. Meningeal Layer Thickness and Myofibroblasts in Outer Arachnoid Layer Differences
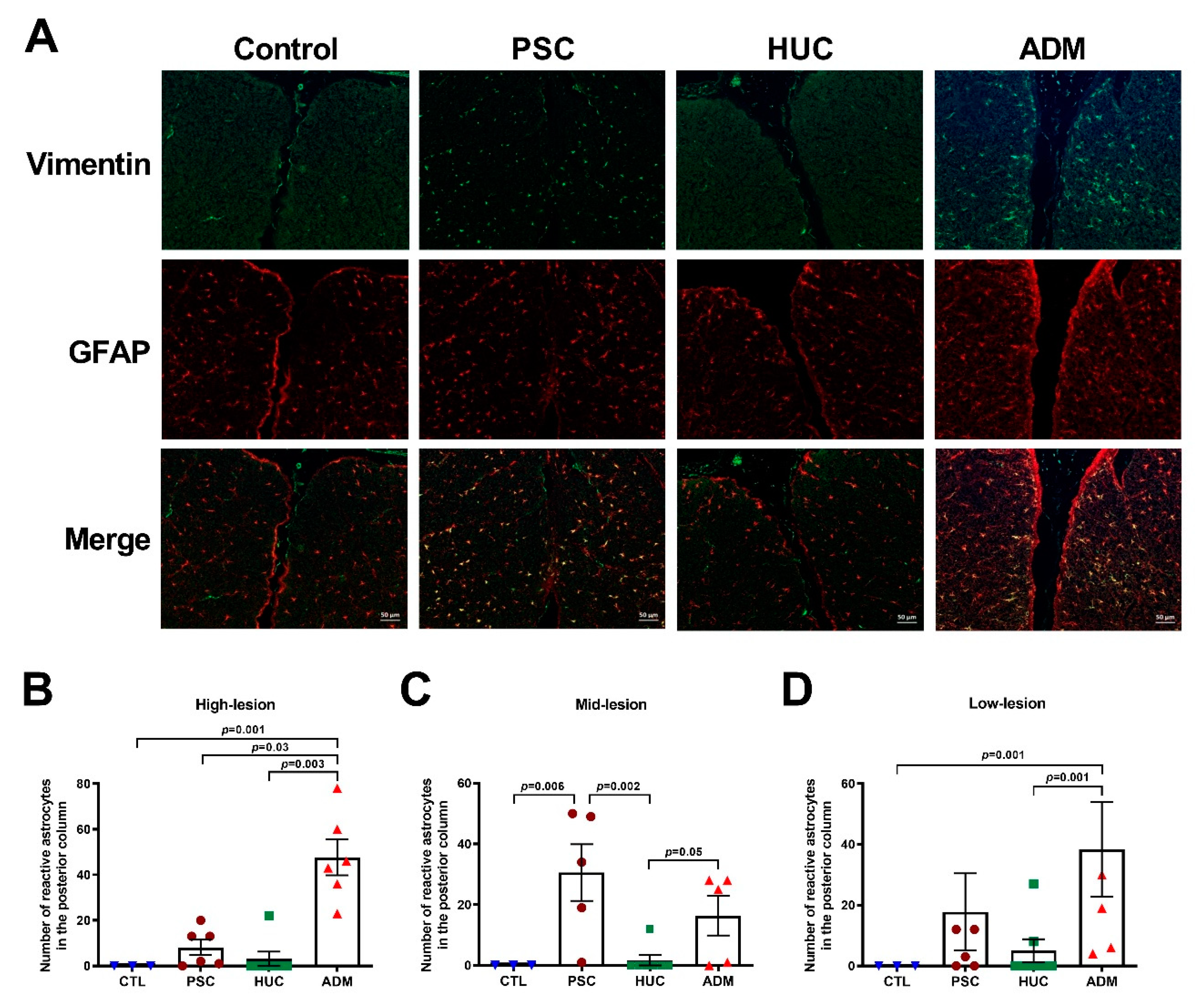
4.4. Reactive Astrocyte Enhancement in the PSC and ADM Repair Groups versus the HUC Repair Group
5. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Adzick, N.S.; Thom, E.A.; Spong, C.Y.; Brock, J.W.; Burrows, P.K.; Johnson, M.P.; Howell, L.J.; Farrell, J.A.; Dabrowiak, M.E.; Sutton, L.N.; et al. A randomized trial of prenatal versus postnatal repair of myelomeningocele. N. Engl. J. Med. 2011, 364, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Williams, H. A unifying hypothesis for hydrocephalus, Chiari malformation, syringomyelia, anencephaly and spina bifida. Cereb. Fluid Res. 2008, 5, 7. [Google Scholar] [CrossRef] [PubMed]
- Meuli, M.; Meuli-Simmen, C.; Hutchins, G.M.; Yingling, C.D.; Hoffman, K.M.; Harrison, M.R.; Adzick, N.S. In utero surgery rescues neurological function at birth in sheep with spina bifida. Nat. Med. 1995, 1, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, S.; Davey, M.G.; Rintoul, N.E.; Walsh, D.S.; Rorke, L.B.; Adzick, N.S. Correction of hindbrain herniation and anatomy of the vermis after in utero repair of myelomeningocele in sheep. J. Pediatric Surg. 2003, 38, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Houtrow, A.J.; Thom, E.A.; Fletcher, J.M.; Burrows, P.K.; Adzick, N.S.; Thomas, N.H.; Brock, J.W.; Cooper, T.; Lee, H.; Bilaniuk, L.; et al. Prenatal repair of myelomeningocele and school-age functional outcomes. Pediatrics 2020, 145, e20191544. [Google Scholar] [CrossRef]
- Farmer, D.L.; Thom, E.A.; Brock, J.W.; Burrows, P.K.; Johnson, M.P.; Howell, L.J.; Farrell, J.A.; Gupta, N.; Adzick, N.S. The management of myelomeningocele study: Full cohort 30-month pediatric outcomes. Am. J. Obstet. Gynecol. 2018, 218, 256.e1–256.e13. [Google Scholar] [CrossRef] [PubMed]
- Ben Miled, S.; Loeuillet, L.; Duong Van Huyen, J.P.; Bessieres, B.; Sekour, A.; Leroy, B.; Tantau, J.; Adle-Biassette, H.; Salhi, H.; Bonnière-Darcy, M.; et al. Severe and progressive neuronal loss in myelomeningocele begins before 16 weeks of pregnancy. Am. J. Obstet. Gynecol. 2020, 223, 256.e1–256.e9. [Google Scholar] [CrossRef]
- Mehta, V.A.; Bettegowda, C.; Ahmadi, S.A.; Berenberg, P.; Thomale, U.W.; Haberl, E.J.; Jallo, G.I.; Ahn, E.S. Spinal cord tethering following myelomeningocele repair. J. Neurosurg. Pediatrics 2010, 6, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Moldenhauer, J.S.; Soni, S.; Rintoul, N.E.; Spinner, S.S.; Khalek, N.; Martinez-Poyer, J.; Flake, A.W.; Hedrick, H.L.; Peranteau, W.H.; Rendon, N.; et al. Fetal myelomeningocele repair: The post-MOMS experience at the Children’s Hospital of Philadelphia. Fetal Diagn. Ther. 2015, 37, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Papanna, R.; Bahtiyar, O.; Bennett, K.A.; Emery, S.; Lillegard, J.B.; Goldstein, R.; Goodnight, W.; Jatres, J.; Lim, F.-Y.; McCullough, L.B.; et al. 229: Use of tissue grafts for in-utero spina bifida closure of large skin defects. Am. J. Obstet. Gynecol. 2019, 220 (Suppl. S1), S165–S166. [Google Scholar] [CrossRef]
- Liu, J.; Sheha, H.; Fu, Y.; Liang, L.; Tseng, S.C. Update on amniotic membrane transplantation. Expert Rev. Ophthalmol. 2010, 5, 645–661. [Google Scholar] [CrossRef] [PubMed]
- Acharya, G.; Pavlovic, M.; Ewing, L.; Nollmann, D.; Leshko, J.; Huhta, J.C. Comparison between pulsed-wave Doppler- and tissue Doppler-derived Tei indices in fetuses with and without congenital heart disease. Ultrasound Obstet. Gynecol. 2008, 31, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Cooke, M.; Tan, E.K.; Mandrycky, C.; He, H.; O’Connell, J.; Tseng, S.C. Comparison of cryopreserved amniotic membrane and umbilical cord tissue with dehydrated amniotic membrane/chorion tissue. J. Wound Care 2014, 23, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.K.; Cooke, M.; Mandrycky, C.; Mahabole, M.; He, H.; O’ Connell, J.; McDevitt, T.C.; Tseng, S.C.G. Structural and biological comparison of cryopreserved and fresh amniotic membrane tissues. J. Biomater. Tissue Eng. 2014, 4, 379–388. [Google Scholar] [CrossRef]
- He, H.; Li, W.; Tseng, D.Y.; Zhang, S.; Chen, S.Y.; Day, A.J.; Tseng, S.C. Biochemical characterization and function of complexes formed by hyaluronan and the heavy chains of inter-alpha-inhibitor (HC*HA) purified from extracts of human amniotic membrane. J. Biol. Chem. 2009, 284, 20136–20146. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; He, H.; Day, A.J.; Tseng, S.C. Constitutive expression of inter-alpha-inhibitor (IalphaI) family proteins and tumor necrosis factor-stimulated gene-6 (TSG-6) by human amniotic membrane epithelial and stromal cells supporting formation of the heavy chain-hyaluronan (HC-HA) complex. J. Biol. Chem. 2012, 287, 12433–12444. [Google Scholar]
- He, H.; Zhang, S.; Tighe, S.; Son, J.; Tseng, S.C. Immobilized heavy chain-hyaluronic acid polarizes lipopolysaccharide-activated macrophages toward M2 phenotype. J. Biol. Chem. 2013, 288, 25792–25803. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhu, Y.T.; Chen, S.Y.; He, H.; Tseng, S.C. Constitutive expression of pentraxin 3 (PTX3) protein by human amniotic membrane cells leads to formation of the heavy chain (HC)-hyaluronan (HA)-PTX3 complex. J. Biol. Chem. 2014, 289, 13531–13542. [Google Scholar] [CrossRef] [PubMed]
- Tseng, S.C.G.; Espana, E.M.; Kawakita, T.; Di Pascuale, M.A.; Li, W.; He, H.; Liu, T.-S.; Cho, T.-H.; Gao, Y.-Y.; Yeh, L.-K.; et al. How does amniotic membrane work? Ocul. Surf. 2004, 2, 177–187. [Google Scholar] [CrossRef]
- Li, W.; He, H.; Chen, Y.T.; Hayashida, Y.; Tseng, S.C. Reversal of myofibroblasts by amniotic membrane stromal extract. J. Cell. Physiol. 2008, 215, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Zhang, S.Z.; He, H.; Zhu, Y.T.; Tseng, S.C. PTX3 controls activation of matrix metalloproteinase 1 and apoptosis in conjunctivochalasis fibroblasts. Investig. Ophthalmol. Vis. Sci. 2012, 53, 3414–3423. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Han, B.; Zhu, Y.T.; Mahabole, M.; Huang, J.; Beebe, D.C.; Tseng, S.C.G. HC-HA/PTX3 purified from amniotic membrane promotes bmp signaling in limbal niche cells to maintain quiescence of limbal epithelial progenitor/stem cells. Stem Cells 2015, 33, 3341–3355. [Google Scholar] [CrossRef] [PubMed]
- Tseng, S.C. HC-HA/PTX3 purified from amniotic membrane as novel regenerative matrix: Insight into relationship between inflammation and regeneration. Investig. Ophthalmol. Vis. Sci. 2016, 57, ORSFh1–ORSFh8. [Google Scholar] [CrossRef] [PubMed]
- Dua, H.S.; Gomes, J.A.; King, A.J.; Maharajan, V.S. The amniotic membrane in ophthalmology. Surv. Ophthalmol. 2004, 49, 51–77. [Google Scholar] [CrossRef]
- Bouchard, C.S.; John, T. Amniotic membrane transplantation in the management of severe ocular surface disease: Indications and outcomes. Ocul. Surf. 2004, 2, 201–211. [Google Scholar] [CrossRef]
- He, H.; Tan, Y.; Duffort, S.; Perez, V.L.; Tseng, S.C. In vivo downregulation of innate and adaptive immune responses in corneal allograft rejection by HC-HA/PTX3 complex purified from amniotic membrane. Investig. Ophthalmol. Vis. Sci. 2014, 55, 1647–1656. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.T.; Li, F.; Zhang, Y.; Chen, S.Y.; Tighe, S.; Lin, S.Y.; Tseng, S.C.G. HC-HA/PTX3 purified from human amniotic membrane reverts human corneal fibroblasts and myofibroblasts to keratocytes by activating BMP signaling. Investig. Ophthalmol. Vis. Sci. 2020, 61, 62. [Google Scholar] [CrossRef] [PubMed]
- Papanna, R.; Moise Jr, K.J.; Mann, L.K.; Fletcher, S.; Schniederjan, R.; Bhattacharjee, M.B.; Stewart, R.J.; Kaur, S.; Prabhu, S.P.; Tseng, S.C.G. Cryopreserved human umbilical cord patch for in-utero spina bifida repair. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2016, 47, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Papanna, R.; Mann, L.K.; Snowise, S.; Morales, Y.; Prabhu, S.P.; Tseng, S.C.; Grill, R.; Fletcher, S.; Moise, K.J.; Papanna, R. Neurological outcomes after human umbilical cord patch for in utero spina bifida repair in a sheep model. AJP Rep. 2016, 6, e309–e317. [Google Scholar] [CrossRef] [PubMed]
- Mann, L.K.; Won, J.H.; Trenton, N.J.; Garnett, J.; Snowise, S.; Fletcher, S.A.; Tseng, S.C.G.; Diehl, M.R.; Papanna, R. Cryopreserved human umbilical cord versus acellular dermal matrix patches for in utero fetal spina bifida repair in a pregnant rat model. J. Neurosurg. Spine 2019, 32, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Papanna, R.; Fletcher, S.; Moise, K.J., Jr.; Mann, L.K.; Tseng, S.C. Cryopreserved human umbilical cord for in utero myeloschisis repair. Obstet. Gynecol. 2016, 128, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Joyeux, L.; Engels, A.C.; Van Der Merwe, J.; Aertsen, M.; Patel, P.A.; Deprez, M.; Khatoun, A.; Pranpanus, S.; Da Cunha, M.G.M.C.M.; De Vleeschauwer, S.; et al. Validation of the fetal lamb model of spina bifida. Sci. Rep. 2019, 9, 9327. [Google Scholar] [CrossRef]
- Brown, E.G.; Saadai, P.; Pivetti, C.D.; Beattie, M.S.; Bresnahan, J.C.; Wang, A.; Farmer, D.L. In utero repair of myelomeningocele with autologous amniotic membrane in the fetal lamb model. J. Pediatr. Surg. 2014, 49, 133–137. [Google Scholar] [CrossRef][Green Version]
- Levine, G.J.; Levine, J.M.; Budke, C.M.; Kerwin, S.C.; Au, J.; Vinayak, A.; Hettlich, B.; Slater, M.R. Description and repeatability of a newly developed spinal cord injury scale for dogs. Prev. Vet. Med. 2009, 89, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Olby, N.J.; De Risio, L.; Munana, K.R.; Wosar, M.A.; Skeen, T.M.; Sharp, N.J.; Keene, B.W. Development of a functional scoring system in dogs with acute spinal cord injuries. Am. J. Vet. Res. 2001, 62, 1624–1628. [Google Scholar] [CrossRef]
- Stokes, B.T.; Noyes, D.H.; Behrmann, D.L. An electromechanical spinal injury technique with dynamic sensitivity. J. Neurotrauma 1992, 9, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Levine, J.M.; Levine, G.J.; Kerwin, S.C.; Hettlich, B.F.; Fosgate, G.T. Association between various physical factors and acute thoracolumbar intervertebral disk extrusion or protrusion in Dachshunds. J. Am. Vet. Med Assoc. 2006, 229, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Mangena, M.; Gcebe, N.; Pierneef, R.; Thompson, P.N.; Adesiyun, A.A. Q Fever: Seroprevalence, risk factors in slaughter livestock and genotypes of coxiella burnetii in South Africa. Pathogens 2021, 10, 258. [Google Scholar] [CrossRef]
- Miller, K.L.; Stagg, C.J.; Douaud, G.; Jbabdi, S.; Smith, S.M.; Behrens, T.E.; Jenkinson, M.; Chance, S.A.; Esiri, M.M.; Voets, N.L.; et al. Diffusion imaging of whole, post-mortem human brains on a clinical MRI scanner. Neuroimage 2011, 57, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Alam, H.; Sehgal, L.; Kundu, S.T.; Dalal, S.N.; Vaidya, M.M. Novel function of keratins 5 and 14 in proliferation and differentiation of stratified epithelial cells. Mol. Biol. Cell. 2011, 22, 4068–4078. [Google Scholar] [CrossRef] [PubMed]
- Caplan, L.R.; Norohna, A.B.; Amico, L.L. Syringomyelia and arachnoiditis. J. Neurol. Neurosurg. Psychiatry 1990, 53, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.K.; Tator, C.H. Prevention of arachnoiditis and postoperative tethering of the spinal cord with Gore-Tex surgical membrane: An experimental study with rats. Neurosurgery 1998, 42, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Darby, I.A.; Zakuan, N.; Billet, F.; Desmouliere, A. The myofibroblast, a key cell in normal and pathological tissue repair. Cell. Mol. Life Sci. 2016, 73, 1145–1157. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Barres, B.A. Reactive astrocytes: Production, function, and therapeutic potential. Immunity 2017, 46, 957–967. [Google Scholar] [CrossRef]
- Caldarelli, M.; Boscarelli, A.; Massimi, L. Recurrent tethered cord: Radiological investigation and management. Child’s Nerv. Syst. ChNS Off. J. Int. Soc. Pediatric Neurosurg. 2013, 29, 1601–1609. [Google Scholar] [CrossRef] [PubMed]
- Heiss, J.D.; Snyder, K.; Peterson, M.M.; Patronas, N.J.; Butman, J.A.; Smith, R.K.; DeVroom, H.L.; Sansur, C.A.; Eskioglu, E.; Kammerer, W.A.; et al. Pathophysiology of primary spinal syringomyelia. J. Neurosurg. Spine 2012, 17, 367–380. [Google Scholar] [CrossRef]
- Heiss, J.D.; Patronas, N.; DeVroom, H.L.; Shawker, T.; Ennis, R.; Kammerer, W.; Eidsath, A.; Talbot, T.; Morris, J.; Eskioglu, E.; et al. Elucidating the pathophysiology of syringomyelia. J. Neurosurg. 1999, 91, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, E.H.; Muraszko, K.; Shawker, T.H.; Patronas, N.J. Pathophysiology of syringomyelia associated with Chiari I malformation of the cerebellar tonsils. Implications for diagnosis and treatment. J. Neurosurg. 1994, 80, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Galganski, L.A.; Kumar, P.; Vanover, M.A.; Pivetti, C.D.; Anderson, J.E.; Lankford, L.; Paxton, Z.J.; Chung, K.; Lee, C.; Hegazi, M.S.; et al. In utero treatment of myelomeningocele with placental mesenchymal stromal cells—Selection of an optimal cell line in preparation for clinical trials. J. Pediatric Surg. 2020, 55, 1941–1946. [Google Scholar] [CrossRef] [PubMed]
- Stavrinou, P.; Kunz, M.; Lehner, M.; Heger, A.; Muller-Felber, W.; Tonn, J.-C.; Peraud, A. Children with tethered cord syndrome of different etiology benefit from microsurgery-a single institution experience. Child’s Nerv. Syst. ChNS Off. J. Int. Soc. Pediatric Neurosurg. 2011, 27, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Brown, E.G.; Lankford, L.; Keller, B.A.; Pivetti, C.D.; Sitkin, N.A.; Beattie, M.S.; Bresnahan, J.C.; Farmer, D. Placental mesenchymal stromal cells rescue ambulation in ovine myelomeningocele. Stem Cells Transl. Med. 2015, 4, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Sanchez e Oliveira Rde, C.; Valente, P.R.; Abou-Jamra, R.C.; Araujo, A.; Saldiva, P.H.; Pedreira, D.A. Biosynthetic cellulose induces the formation of a neoduramater following pre-natal correction of meningomyelocele in fetal sheep. Acta Cir. Bras. 2007, 22, 174–181. [Google Scholar] [PubMed]
- Sayad, W.Y.; Harvey, S.C. The Regeneration of the meninges: The dura mater. Ann. Surg. 1923, 77, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Lear, M.; Harvey, S.C. The Regeneration of the meninges. Ann. Surg. 1924, 80, 536–544. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mann, L.K.; Won, J.H.; Patel, R.; Bergh, E.P.; Garnett, J.; Bhattacharjee, M.B.; Narayana, P.A.; Jain, R.; Fletcher, S.A.; Lai, D.; et al. Allografts for Skin Closure during In Utero Spina Bifida Repair in a Sheep Model. J. Clin. Med. 2021, 10, 4928. https://doi.org/10.3390/jcm10214928
Mann LK, Won JH, Patel R, Bergh EP, Garnett J, Bhattacharjee MB, Narayana PA, Jain R, Fletcher SA, Lai D, et al. Allografts for Skin Closure during In Utero Spina Bifida Repair in a Sheep Model. Journal of Clinical Medicine. 2021; 10(21):4928. https://doi.org/10.3390/jcm10214928
Chicago/Turabian StyleMann, Lovepreet K., Jong Hak Won, Rajan Patel, Eric P. Bergh, Jeannine Garnett, Meenakshi B. Bhattacharjee, Ponnada A. Narayana, Ranu Jain, Stephen A. Fletcher, Dejian Lai, and et al. 2021. "Allografts for Skin Closure during In Utero Spina Bifida Repair in a Sheep Model" Journal of Clinical Medicine 10, no. 21: 4928. https://doi.org/10.3390/jcm10214928
APA StyleMann, L. K., Won, J. H., Patel, R., Bergh, E. P., Garnett, J., Bhattacharjee, M. B., Narayana, P. A., Jain, R., Fletcher, S. A., Lai, D., & Papanna, R. (2021). Allografts for Skin Closure during In Utero Spina Bifida Repair in a Sheep Model. Journal of Clinical Medicine, 10(21), 4928. https://doi.org/10.3390/jcm10214928






