Blood Differential Gene Expression in Patients with Chronic Heart Failure and Systemic Iron Deficiency: Pathways Involved in Pathophysiology and Impact on Clinical Outcomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Study Population and Ethics
2.2. Selection Criteria and Definition of Study Cohorts
2.3. Baseline Clinical Assessment
2.4. Blood Sample Management and Laboratory Assessments
2.5. RNA Extraction and Blood Gene Expression Analyses
2.6. Follow Up and Major Heart Failure Events Ascertainment
2.7. Statistical Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bozkurt, B.; Coats, A.J.; Tsutsui, H.; Abdelhamid, M.; Adamopoulos, S.; Albert, N.; Anker, S.D.; Atherton, J.; Böhm, M.; Butler, J.; et al. Universal Definition and Classification of Heart Failure: A Report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. J. Card. Fail. 2021. [Google Scholar] [CrossRef]
- Maddox, T.M.; Januzzi, J.L., Jr.; Allen, L.A.; Breathett, K.; Butler, J.; Davis, L.L.; Fonarow, G.C.; Ibrahim, N.E.; Lindenfeld, J.; Masoudi, F.A.; et al. 2021 Update to the 2017 ACC Expert Consensus Decision Pathway for Optimization of Heart Failure Treatment: Answers to 10 Pivotal Issues About Heart Failure With Reduced Ejection Fraction: A Report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2021, 77, 772–810. [Google Scholar] [CrossRef]
- Farré, N.; Vela, E.; Clèries, M.; Bustins, M.; Cainzos-Achirica, M.; Enjuanes, C.; Moliner, P.; Ruiz, S.; Verdú-Rotellar, J.M.; Comín-Colet, J. Real world heart failure epidemiology and outcome: A population-based analysis of 88,195 patients. PLoS ONE 2017, 12, e0172745. [Google Scholar] [CrossRef]
- Klip, I.T.; Comin-Colet, J.; Voors, A.A.; Ponikowski, P.; Enjuanes, C.; Banasiak, W.; Lok, D.J.; Rosentryt, P.; Torrens, A.; Polonski, L.; et al. Iron deficiency in chronic heart failure: An international pooled analysis. Am. Heart J. 2013, 165, 575–582.e3. [Google Scholar] [CrossRef] [PubMed]
- Enjuanes, C.; Klip, I.T.; Bruguera, J.; Cladellas, M.; Ponikowski, P.; Banasiak, W.; van Veldhuisen, D.J.; van der Meer, P.; Jankowska, E.A.; Comín-Colet, J. Iron deficiency and health-related quality of life in chronic heart failure: Results from a multicenter European study. Int. J. Cardiol. 2014, 174, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Enjuanes, C.; Bruguera, J.; Grau, M.; Cladellas, M.; Gonzalez, G.; Meroño, O.; Moliner-Borja, P.; Verdú, J.M.; Farré, N.; Comín-Colet, J. Iron Status in Chronic Heart Failure: Impact on Symptoms, Functional Class and Submaximal Exercise Capacity. Rev. Esp. Cardiol. Engl. Ed. 2016, 69, 247–255. [Google Scholar] [CrossRef]
- von Haehling, S.; Ebner, N.; Evertz, R.; Ponikowski, P.; Anker, S.D. Iron Deficiency in Heart Failure: An Overview. JACC Heart Fail. 2019, 7, 36–46. [Google Scholar] [CrossRef] [PubMed]
- González-Costello, J.; Comín-Colet, J.; Lupón, J.; Enjuanes, C.; de Antonio, M.; Fuentes, L.; Moliner-Borja, P.; Farré, N.; Zamora, E.; Manito, N.; et al. Importance of iron deficiency in patients with chronic heart failure as a predictor of mortality and hospitalizations: Insights from an observational cohort study. BMC Cardiovasc. Disord. 2018, 18, 206. [Google Scholar] [CrossRef] [PubMed]
- Campodonico, J.; Nicoli, F.; Motta, I.; Migone De Amicis, M.; Bonomi, A.; Cappellini, M.; Agostoni, P. Prognostic role of transferrin saturation in heart failure patients. Eur. J. Prev. Cardiol. 2021, zwaa112. [Google Scholar] [CrossRef]
- Moliner, P.; Jankowska, E.A.; van Veldhuisen, D.J.; Farre, N.; Rozentryt, P.; Enjuanes, C.; Polonski, L.; Meroño, O.; Voors, A.A.; Ponikowski, P.; et al. Clinical correlates and prognostic impact of impaired iron storage versus impaired iron transport in an international cohort of 1821 patients with chronic heart failure. Int. J. Cardiol. 2017, 243, 360–366. [Google Scholar] [CrossRef]
- Anker, S.D.; Comin Colet, J.; Filippatos, G.; Willenheimer, R.; Dickstein, K.; Drexler, H.; Lüscher, T.F.; Bart, B.; Banasiak, W.; Niegowska, J.; et al. Ferric carboxymaltose in patients with heart failure and iron deficiency. N. Engl. J. Med. 2009, 361, 2436–2448. [Google Scholar] [CrossRef] [PubMed]
- Ponikowski, P.; van Veldhuisen, D.J.; Comin-Colet, J.; Ertl, G.; Komajda, M.; Mareev, V.; McDonagh, T.; Parkhomenko, A.; Tavazzi, L.; Levesque, V.; et al. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency†. Eur. Heart J. 2015, 36, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Ponikowski, P.; Kirwan, B.A.; Anker, S.D.; McDonagh, T.; Dorobantu, M.; Drozdz, J.; Fabien, V.; Filippatos, G.; Göhring, U.M.; Keren, A.; et al. Ferric carboxymaltose for iron deficiency at discharge after acute heart failure: A multicentre, double-blind, randomised, controlled trial. Lancet 2020, 396, 1895–1904. [Google Scholar] [CrossRef]
- Maeder, M.T.; Khammy, O.; dos Remedios, C.; Kaye, D.M. Myocardial and systemic iron depletion in heart failure implications for anemia accompanying heart failure. J. Am. Coll. Cardiol. 2011, 58, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Moliner, P.; Enjuanes, C.; Tajes, M.; Cainzos-Achirica, M.; Lupón, J.; Garay, A.; Jimenez-Marrero, S.; Yun, S.; Farré, N.; Cladellas, M.; et al. Association Between Norepinephrine Levels and Abnormal Iron Status in Patients With Chronic Heart Failure: Is Iron Deficiency More Than a Comorbidity? J. Am. Heart Assoc. 2019, 8, e010887. [Google Scholar] [CrossRef] [PubMed]
- Melenovsky, V.; Petrak, J.; Mracek, T.; Benes, J.; Borlaug, B.A.; Nuskova, H.; Pluhacek, T.; Spatenka, J.; Kovalcikova, J.; Drahota, Z.; et al. Myocardial iron content and mitochondrial function in human heart failure: A direct tissue analysis. Eur. J. Heart Fail. 2017, 19, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Haddad, S.; Wang, Y.; Galy, B.; Korf-Klingebiel, M.; Hirsch, V.; Baru, A.M.; Rostami, F.; Reboll, M.R.; Heineke, J.; Flögel, U.; et al. Iron-regulatory proteins secure iron availability in cardiomyocytes to prevent heart failure. Eur. Heart J. 2017, 38, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Hoes, M.F.; Grote Beverborg, N.; Kijlstra, J.D.; Kuipers, J.; Swinkels, D.W.; Giepmans, B.N.G.; Rodenburg, R.J.; van Veldhuisen, D.J.; de Boer, R.A.; van der Meer, P. Iron deficiency impairs contractility of human cardiomyocytes through decreased mitochondrial function. Eur. J. Heart Fail. 2018, 20, 910–919. [Google Scholar] [CrossRef] [PubMed]
- Tajes, M.; Díez-López, C.; Enjuanes, C.; Moliner, P.; Ferreiro, J.L.; Garay, A.; Jiménez-Marrero, S.; Yun, S.; Sosa, S.G.; Alcoberro, L.; et al. Neurohormonal activation induces intracellular iron deficiency and mitochondrial dysfunction in cardiac cells. Cell Biosci. 2021, 11, 89. [Google Scholar] [CrossRef]
- Bayes-Genis, A.; Liu, P.P.; Lanfear, D.E.; de Boer, R.A.; González, A.; Thum, T.; Emdin, M.; Januzzi, J.L. Omics phenotyping in heart failure: The next frontier. Eur. Heart J. 2020, 41, 3477–3484. [Google Scholar] [CrossRef]
- Gomes, C.P.C.; Schroen, B.; Kuster, G.M.; Robinson, E.L.; Ford, K.; Squire, I.B.; Heymans, S.; Martelli, F.; Emanueli, C.; Devaux, Y. EU-CardioRNA COST Action (CA17129). Regulatory RNAs in Heart Failure. Circulation 2020, 141, 313–328. [Google Scholar] [CrossRef]
- van der Wal, H.H.; Grote Beverborg, N.; Dickstein, K.; Anker, S.D.; Lang, C.C.; Ng, L.L.; van Veldhuisen, D.J.; Voors, A.A.; van der Meer, P. Iron deficiency in worsening heart failure is associated with reduced estimated protein intake, fluid retention, inflammation, and antiplatelet use. Eur. Heart J. 2019, 40, 3616–3625. [Google Scholar] [CrossRef] [PubMed]
- Calero-Molina, E.; Hidalgo, E.; Rosenfeld, L.; Verdú-Rotellar, J.M.; Verdú-Soriano, J.; Garay, A.; Alcoberro, L.; Jimenez-Marrero, S.; Garcimartin, P.; Yun, S.; et al. The relationship between self-care, long-term mortality, and heart failure hospitalization: Insights from a real-world cohort study. Eur. J. Cardiovasc. Nurs. 2021, zvab011. [Google Scholar] [CrossRef]
- Gavaldà-Manso, M.; Jimenez-Marrero, S.; Cainzos-Achirica, M.; Garay, A.; Enjuanes, C.; Yun, S.; Diez, C.; Gonzalez-Costello, J.; Tajes, M.; Farre, N.; et al. Reduced levels of vasopressin, an independent mechanism in the obesity paradox in patients with chronic heart failure: Insights from the DAMOCLES study. Int. J. Cardiol. 2019, 276, 171–176. [Google Scholar] [CrossRef]
- Alcaide-Aldeano, A.; Garay, A.; Alcoberro, L.; Jiménez-Marrero, S.; Yun, S.; Tajes, M.; García-Romero, E.; Díez-López, C.; González-Costello, J.; Mateus-Porta, G.; et al. Iron Deficiency: Impact on Functional Capacity and Quality of Life in Heart Failure with Preserved Ejection Fraction. J. Clin. Med. 2020, 9, 1199. [Google Scholar] [CrossRef] [PubMed]
- Coma, M.; González-Moneo, M.J.; Enjuanes, C.; Velázquez, P.P.; Espargaró, D.B.; Pérez, B.A.; Tajes, M.; Garcia-Elias, A.; Farré, N.; Sánchez-Benavides, G.; et al. Effect of Permanent Atrial Fibrillation on Cognitive Function in Patients With Chronic Heart Failure. Am. J. Cardiol. 2016, 117, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Comín-Colet, J.; Enjuanes, C.; González, G.; Torrens, A.; Cladellas, M.; Meroño, O.; Ribas, N.; Ruiz, S.; Gómez, M.; Verdú, J.M.; et al. Iron deficiency is a key determinant of health-related quality of life in patients with chronic heart failure regardless of anaemia status. Eur. J. Heart Fail. 2013, 15, 1164–1172. [Google Scholar] [CrossRef] [PubMed]
- González-Moneo, M.J.; Sánchez-Benavides, G.; Verdu-Rotellar, J.M.; Cladellas, M.; Bruguera, J.; Quiñones-Ubeda, S.; Enjuanes, C.; Peña-Casanova, J.; Comín-Colet, J. Ischemic aetiology, self-reported frailty, and gender with respect to cognitive impairment in chronic heart failure patients. BMC Cardiovasc. Disord. 2016, 16, 163. [Google Scholar] [CrossRef] [PubMed]
- Farré, N.; Aranyó, J.; Enjuanes, C.; Verdú-Rotellar, J.M.; Ruiz, S.; Gonzalez-Robledo, G.; Meroño, O.; de Ramon, M.; Moliner, P.; Bruguera, J.; et al. Differences in neurohormonal activity partially explain the obesity paradox in patients with heart failure: The role of sympathetic activation. Int. J. Cardiol. 2015, 181, 120–126. [Google Scholar] [CrossRef]
- García-Díez, I.; Hernández-Muñoz, I.; Hernández-Ruiz, E.; Nonell, L.; Puigdecanet, E.; Bódalo-Torruella, M.; Andrades, E.; Pujol, R.M.; Toll, A. Transcriptome and cytogenetic profiling analysis of matched in situ/invasive cutaneous squamous cell carcinomas from immunocompetent patients. Genes Chromosomes Cancer 2019, 58, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Irizarry, R.A.; Hobbs, B.; Collin, F.; Beazer-Barclay, Y.D.; Antonellis, K.J.; Scherf, U.; Speed, T.P. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 2003, 4, 249–264. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef] [PubMed]
- von Schnakenburg, C.; Strehlau, J.; Ehrich, J.H.; Melk, A. Quantitative gene expression of TGF-beta1, IL-10, TNF-alpha and Fas Ligand in renal cortex and medulla. Nephrol. Dial. Transplant. 2002, 17, 573–579. [Google Scholar] [CrossRef][Green Version]
- Wang, Z.; Meng, Q.; Zhu, X.; Sun, S.; Gao, S.; Gou, Y.; Liu, A. Evaluation and Validation of Reference Genes for Quantitative Real-Time PCR in Helopeltis theivora Waterhouse (Hemiptera: Miridae). Sci. Rep. 2019, 9, 13291. [Google Scholar] [CrossRef]
- Peng, Z.; Andersson, K.; Lindholm, J.; Dethlefsen, O.; Pramana, S.; Pawitan, Y.; Nistér, M.; Nilsson, S.; Li, C. Improving the Prediction of Prostate Cancer Overall Survival by Supplementing Readily Available Clinical Data with Gene Expression Levels of IGFBP3 and F3 in Formalin-Fixed Paraffin Embedded Core Needle Biopsy Material. PLoS ONE 2016, 11, e0145545. [Google Scholar] [CrossRef] [PubMed]
- Hartupee, J.; Mann, D.L. Neurohormonal activation in heart failure with reduced ejection fraction. Nat. Rev. Cardiol. 2017, 14, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, M.A.; Shah, A.M.; Borlaug, B.A. Heart Failure With Preserved Ejection Fraction In Perspective. Circ. Res. 2019, 124, 1598–1617. [Google Scholar] [CrossRef]
- Comin-Colet, J.; Lainscak, M.; Dickstein, K.; Filippatos, G.S.; Johnson, P.; Lüscher, T.F.; Mori, C.; Willenheimer, R.; Ponikowski, P.; Anker, S.D. The effect of intravenous ferric carboxymaltose on health-related quality of life in patients with chronic heart failure and iron deficiency: A subanalysis of the FAIR-HF study. Eur. Heart J. 2013, 34, 30–38. [Google Scholar] [CrossRef]
- Gonzalez-Costello, J.; Cainzos-Achirica, M.; Lupón, J.; Farré, N.; Moliner-Borja, P.; Enjuanes, C.; de Antonio, M.; Fuentes, L.; Díez-López, C.; Bayés-Genis, A.; et al. Use of intravenous iron in patients with iron deficiency and chronic heart failure: Real-world evidence. Eur. J. Intern. Med. 2020, 80, 91–98. [Google Scholar] [CrossRef]
- Klattenhoff, C.A.; Scheuermann, J.C.; Surface, L.E.; Bradley, R.K.; Fields, P.A.; Steinhauser, M.L.; Ding, H.; Butty, V.L.; Torrey, L.; Haas, S.; et al. Braveheart, a long noncoding RNA required for cardiovascular lineage commitment. Cell 2013, 152, 570–583. [Google Scholar] [CrossRef] [PubMed]
- Chistiakov, D.A.; Orekhov, A.N.; Bobryshev, Y.V. Cardiac-specific miRNA in cardiogenesis, heart function, and cardiac pathology (with focus on myocardial infarction). J. Mol. Cell. Cardiol. 2016, 94, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Duan, Q.; Yang, L.; Gong, W.; Chaugai, S.; Wang, F.; Chen, C.; Wang, P.; Zou, M.H.; Wang, D.W. MicroRNA-214 Is Upregulated in Heart Failure Patients and Suppresses XBP1-Mediated Endothelial Cells Angiogenesis. J. Cell. Physiol. 2015, 230, 1964–1973. [Google Scholar] [CrossRef]
- Qiang, L.; Hong, L.; Ningfu, W.; Huaihong, C.; Jing, W. Expression of miR-126 and miR-508-5p in endothelial progenitor cells is associated with the prognosis of chronic heart failure patients. Int. J. Cardiol. 2013, 168, 2082–2088. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.P.; Verdonschot, J.; Collier, T.; Wang, P.; Pizard, A.; Bär, C.; Björkman, J.; Boccanelli, A.; Butler, J.; Clark, A.; et al. Proteomic Bioprofiles and Mechanistic Pathways of Progression to Heart Failure. Circ. Heart Fail. 2019, 12, e005897. [Google Scholar] [CrossRef] [PubMed]
- Eulalio, A.; Mano, M.; Dal Ferro, M.; Zentilin, L.; Sinagra, G.; Zacchigna, S.; Giacca, M. Functional screening identifies miRNAs inducing cardiac regeneration. Nature 2012, 492, 376–381. [Google Scholar] [CrossRef]
- Brown, D.A.; Perry, J.B.; Allen, M.E.; Sabbah, H.N.; Stauffer, B.L.; Shaikh, S.R.; Cleland, J.G.; Colucci, W.S.; Butler, J.; Voors, A.A.; et al. Expert consensus document: Mitochondrial function as a therapeutic target in heart failure. Nat. Rev. Cardiol. 2017, 14, 238–250. [Google Scholar] [CrossRef]
- Sabbah, H.N. Targeting the Mitochondria in Heart Failure: A Translational Perspective. JACC Basic Transl. Sci. 2020, 27, 88–106. [Google Scholar] [CrossRef] [PubMed]
- Haigis, M.C.; Sinclair, D.A. Mammalian sirtuins: Biological insights and disease relevance. Annu. Rev. Pathol. 2010, 5, 253–295. [Google Scholar] [CrossRef]
- Vakhrusheva, O.; Smolka, C.; Gajawada, P.; Kostin, S.; Boettger, T.; Kubin, T.; Braun, T.; Bober, E. Sirt7 increases stress resistance of cardiomyocytes and prevents apoptosis and inflammatory cardiomyopathy in mice. Circ. Res. 2008, 102, 703–710. [Google Scholar] [CrossRef]
- Winnik, S.; Auwerx, J.; Sinclair, D.A.; Matter, C.M. Protective effects of sirtuins in cardiovascular diseases: From bench to bedside. Eur. Heart J. 2015, 36, 3404–3412. [Google Scholar] [CrossRef]
- Kane, A.E.; Sinclair, D.A. Sirtuins and NAD+ in the Development and Treatment of Metabolic and Cardiovascular Diseases. Circ. Res. 2018, 123, 868–885. [Google Scholar] [CrossRef] [PubMed]
- Dennerlein, S.; Oeljeklaus, S.; Jans, D.; Hellwig, C.; Bareth, B.; Jakobs, S.; Deckers, M.; Warscheid, B.; Rehling, P. MITRAC7 Acts as a COX1-Specific Chaperone and Reveals a Checkpoint during Cytochrome c Oxidase Assembly. Cell Rep. 2015, 12, 1644–1655. [Google Scholar] [CrossRef] [PubMed]
- Rocca, C.; Scavello, F.; Granieri, M.C.; Pasqua, T.; Amodio, N.; Imbrogno, S.; Gattuso, A.; Mazza, R.; Cerra, M.C.; Angelone, T. Phoenixin-14: Detection and novel physiological implications in cardiac modulation and cardioprotection. Cell. Mol. Life Sci. 2018, 75, 743–756. [Google Scholar] [CrossRef] [PubMed]
- Campanella, A.; Rovelli, E.; Santambrogio, P.; Cozzi, A.; Taroni, F.; Levi, S. Mitochondrial ferritin limits oxidative damage regulating mitochondrial iron availability: Hypothesis for a protective role in Friedreich ataxia. Hum. Mol. Genet. 2009, 18, 1–11. [Google Scholar] [CrossRef] [PubMed]
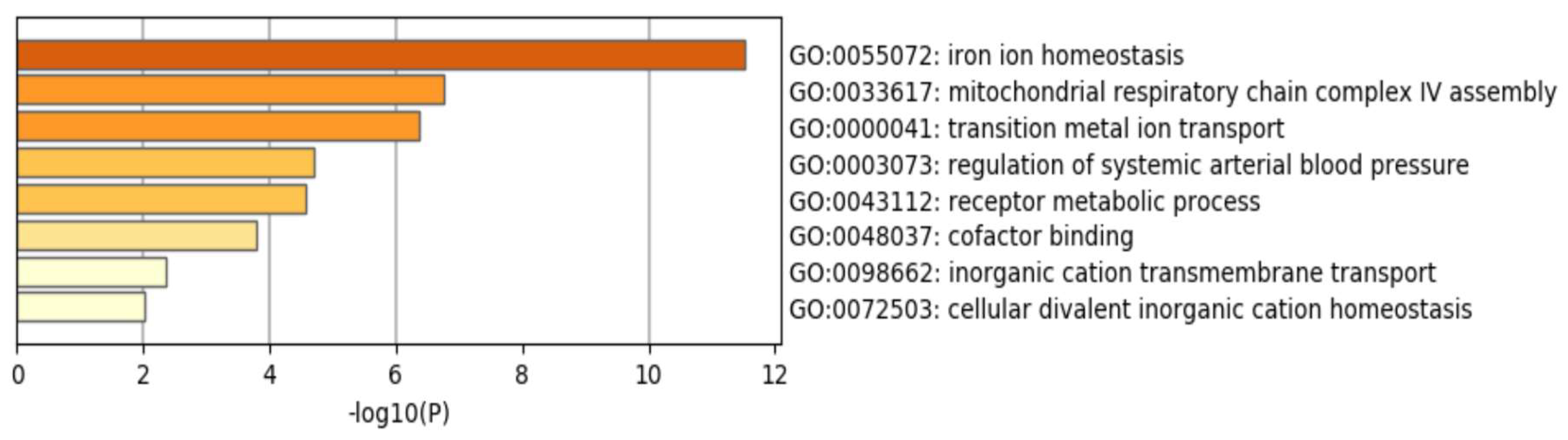

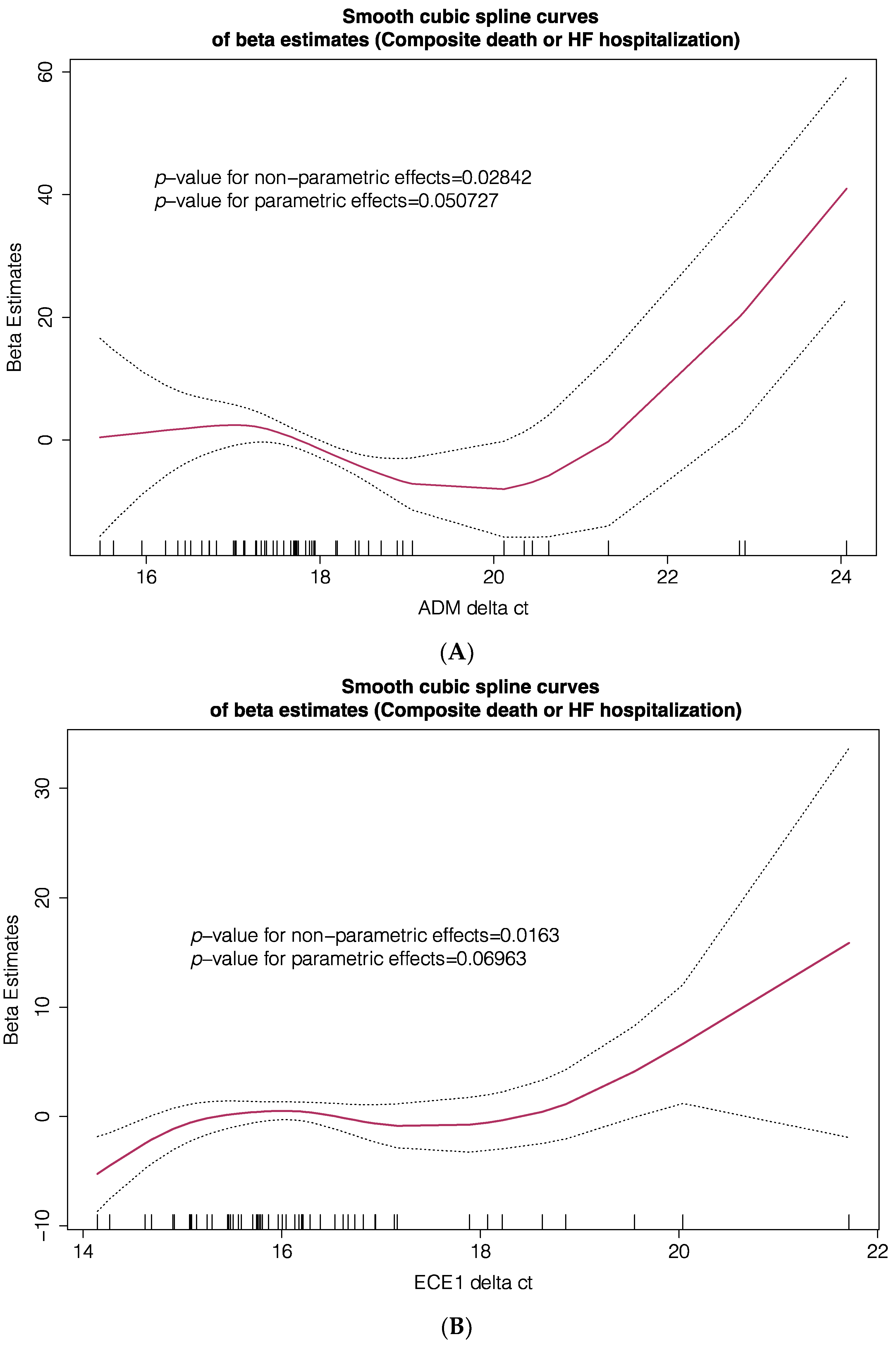
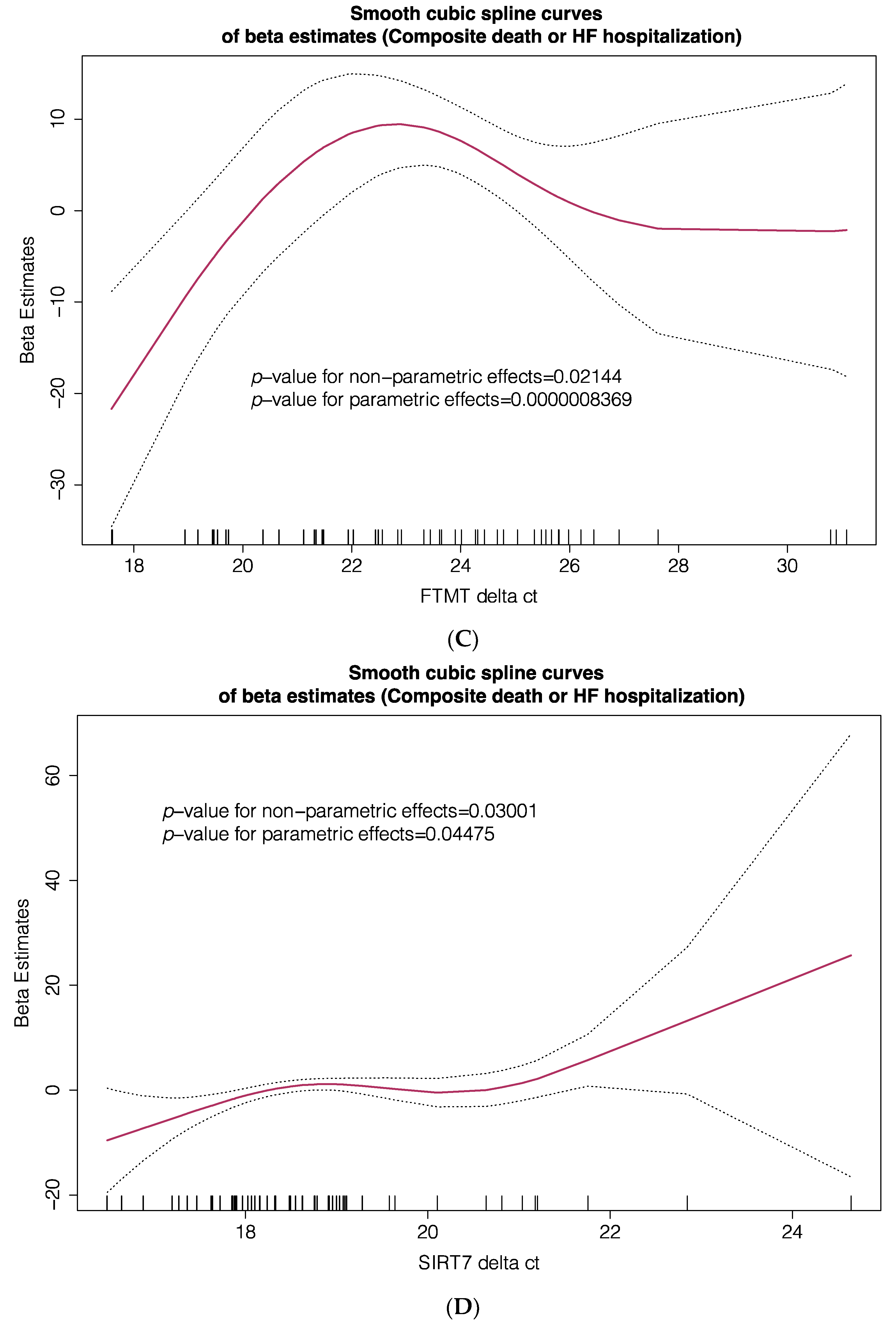
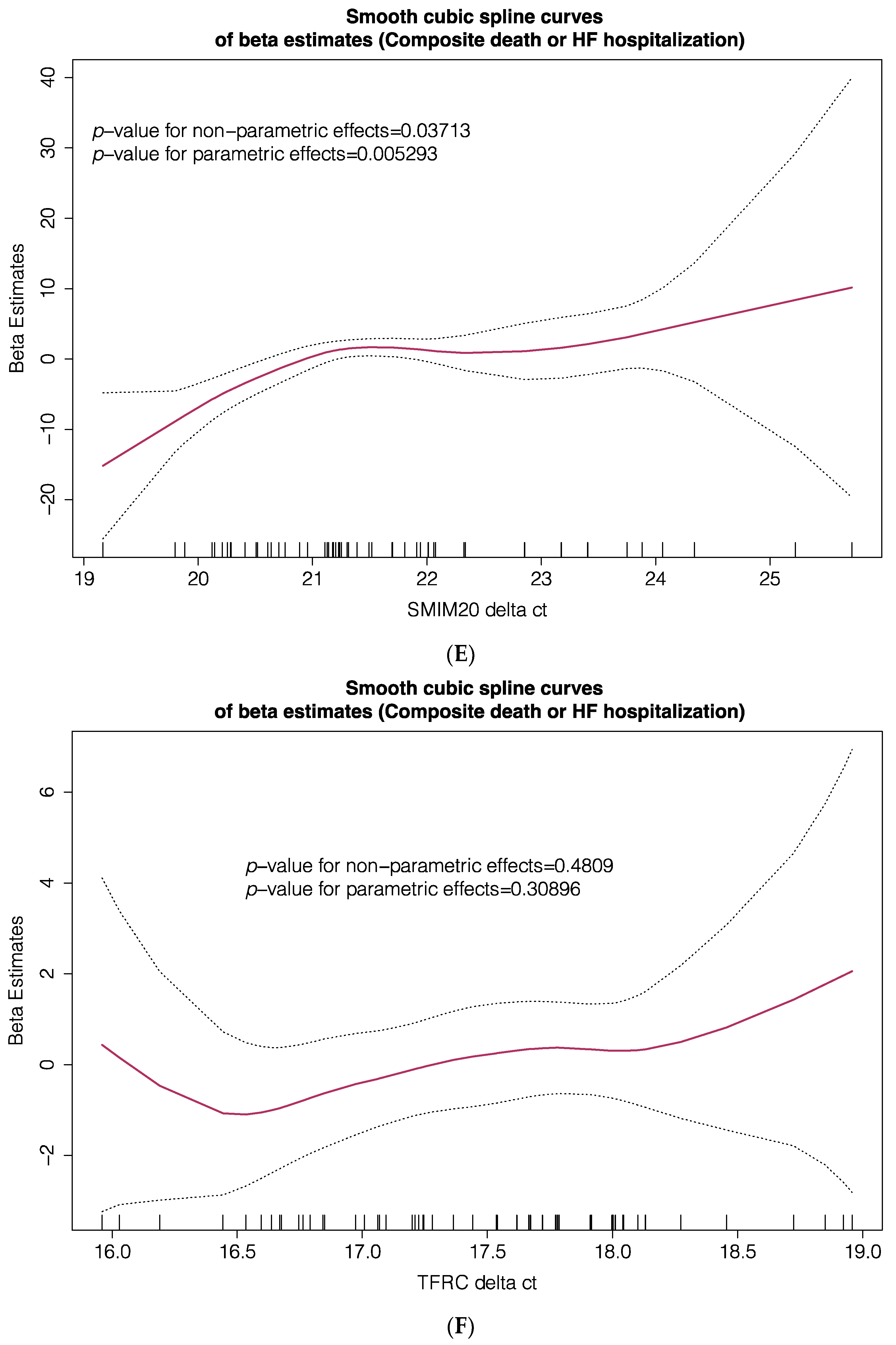
| Cohort #1 (Transcriptome) n = 14 | Cohort #2 (TLDA PCR) n = 71 | |||||
|---|---|---|---|---|---|---|
| ID | Non-ID | ID | Non-ID | |||
| N = 7 | N = 7 | P-Value | N = 35 | N = 36 | p-Value | |
| Demographic and Clinical Factors | ||||||
| Age, years | 70 (9) | 71 (10) | 0.762 | 70 (12) | 72 (11) | 0.472 |
| Sex (male), n (%) | 6 (86) | 6 (86) | 1.0 | 23 (64) | 22 (63) | 1.0 |
| Systolic blood pressure, mmHg | 135 (19) | 124 (22) | 0.309 | 123 (20) | 120 (17) | 0.495 |
| NYHA functional class | ||||||
| I-II | 5 (71) | 5 (71) | 1.0 | |||
| III-IV | 2 (29) | 2 (29) | ||||
| LVEF, % | 36 (5) | 35 (6) | 0.772 | 33 (7) | 34 (6) | 0.908 |
| Ischemic etiology of HF, n (%) | 5 (71) | 5 (71) | 1.0 | 22 (61) | 18 (51) | 0.477 |
| 6 mwt distance | 248 (138) | 287 (155) | 0.618 | 230 (141) | 257 (172) | 0.488 |
| Comorbidities | ||||||
| Hypertension, n (%) | 6 (86) | 6 (86) | 1.0 | 29 (81) | 27 (77) | 0.778 |
| Diabetes Mellitus, n (%) | 5 (71) | 2 (29) | 0.286 | 28 (78) | 18 (51) | 0.026 |
| Previous MI, n (%) | 2 (29) | 2 (29) | 1.0 | 11 (31) | 9 (26) | 0.793 |
| CKD a, n (%) | 2 (29) | 2 (29) | 1.0 | 16 (44) | 17 (49) | 0.814 |
| Anemia WHO, n (%) | 3 (43) | 2 (29) | 1.0 | 23 (64) | 21 (60) | 0.809 |
| Anemia, Hb < 12 g/dL, n (%) | 0 (0) | 0 (0) | 1.0 | 16 (44) | 13 (37) | 0.631 |
| Comorbidity index | 3 (1) | 3 (1) | 1.0 | 4 (1) | 3 (1) | 0.266 |
| Treatments (%) | ||||||
| ACEI or ARBs | 5 (71) | 5 (71) | 1.0 | 24 (67) | 27 (77) | 0.430 |
| Beta-blockers | 6 (86) | 5 (71) | 1.0 | 34 (94) | 32 (91) | 0.674 |
| Diuretics | 7 (100) | 6 (86) | 1.0 | 32 (89) | 31 (89) | 1.0 |
| Laboratory | ||||||
| Hemoglobin, g/dL | 13.6 (1.2) | 13.4 (0.6) | 0.645 | 11.9 (1.5) | 12.2 (1.3) | 0.321 |
| eGFR, ml/min/1.73 m2 | 65 (21) | 65 (19) | 0.975 | 67 (28) | 65 (33) | 0.839 |
| NT-proBNP, pg/mL | 1574 (497–1028) | 536 (481–1178) | 0.318 | 1892 (832–4776) | 1450 (536–5901) | 0.890 |
| hs-CRP | 0.4 (0.2–1.4) | 0.5 (0.2–6.2) | 0.628 | 0.6 (0.3–1.4) | 0.3 (0.2–0.9) | 0.086 |
| Iron status and hematinic | ||||||
| Serum iron | 50.9 (9.2) | 85.3 (29.3) | 0.012 | 45.8 (17.2) | 89.6 (32.8) | <0.001 |
| Serum ferritin | 57.3 (32.2) | 262.0 (132.8) | 0.002 | 45.4 (26.9) | 322.9 (238.8) | <0.001 |
| TSAT | 13.2 (2.0) | 25.4 (6.3) | <0.001 | 11.4 (3.6) | 27.1 (10.7) | <0.001 |
| sTfR | 2.1 (0.4) | 1.5 (0.8) | 0.095 | 2.0 (0.6) | 1.2 (0.4) | <0.001 |
| Gene Name | Symbol | Function |
|---|---|---|
| Aconitase 1 | ACO1 | Essential enzyme in the TCA cycle and interacts with mRNA to control de levels of iron inside cells |
| Adrenomedullin | ADM | Vasodilation, regulation of hormone secretion, promotion of angiogenesis and antimicrobial activity |
| ATPase H+ transporting accessory protein 2 | ATP6AP2 | Associated with ATPases (fundamental roles in energy conservation, secondary active transport…) |
| Cytochrome C oxidase assembly factor 3 | COA3 | Localized to mitochondria and essential for cytochrome c oxidase function. Component of MITRAC |
| Cytochrome C oxidase copper chaperone 17 | COX17 | Copper metallochaperone essential for the assembly of the mitochondrial respiratory chain complex IV (cytochrome c oxidase) |
| C-X-C motif chemokine ligand 8 | CXCL8 | Member of C-X-C chemokine family and is a major mediator of the inflammation response |
| Endothelin-converting enzyme 1 | ECE1 | Proteolytic processing of endothelin precursors to biologically active peptides |
| Mitochondrial Ferritin | FTMT | Stores iron in a soluble, non-toxic, readily available form (ferric iron binding) and ferroxidase activity |
| Hydroxyacyl-coA dehydrogenase trifunctional multienzyme complex subunit alpha | HADHA | Subunit of the mitochondrial protein which catalyzes the last 3 steps of mitochondrial beta-oxidation of long chain fatty acids |
| Hypoxia inducible domain family member 1A | HIGD1A | Subunit of cytochrome c oxidase, may play a role in the assembly of respiratory super complexes in mitochondria |
| Hemopexin | HPX | Plasma glycoprotein that binds heme with high affinity and may be involved in protecting cells from oxidative stress |
| Lipocalin-2 | LCN2 | Iron-trafficking protein involved in multiple processes (apoptosis, innate immunity and renal development) |
| DNA Ligase 3 | LIG3 | Member of the DNA ligase family, involved in excision repair and is in mitochondria and nucleus |
| Mitochondrial calcium uniporter regulator 1 | MCUR1 | Key regulator of MCU (Mitochondrial calcium uniporter) required for calcium entry into mitochondria |
| Metallothionein 2A | MT2A | Member of the metallothionein family, act as anti-oxidant, important in homeostatic control of metal in cell |
| Myosin heavy chain 7B | MYH7B | Encodes a heavy chain of myosin II, involved in muscle contraction |
| Sirtuin 7 | SIRT7 | NAD-dependent protein-lysine deacylase; functions of huma sirtuins have not yet been well determined |
| Small integral membrane protein 20 | SMIM20 | Component of MITRAC complex, that regulates cytochrome c oxidase assembly in mitochondria |
| STEAP3 metalloreductase | STEAP3 | Multipass membrane protein that functions as an iron transporter |
| Transferrin Receptor | TFRC | Cell surface receptor necessary for cellular iron uptake by receptor-mediated endocytosis |
| ATP-dependent Zinc metalloprotease | YME1L1 | ATP-dependent metalloprotease that catalyzes the degradation of folded and unfolded proteins in mitochondria |
| Zinc finger protein 260 | ZNF260 | Transcription factor that acts as a cardiac regulator and an effector of alpha1-adrenergic signaling |
| Symbol | ID vs. Ct (Fold Change) | p-Value |
|---|---|---|
| ACO1 | 0.76 | 0.18 |
| ADM | 0.62 | 0.03 |
| ATP6AP2 | 0.98 | 0.87 |
| COA3 | 0.85 | 0.42 |
| COX17 | 0.66 | 0.08 |
| CXCL8 | 1.18 | 0.53 |
| ECE1 | 0.70 | 0.03 |
| FTMT | 0.26 | 0.02 |
| HADHA | 0.95 | 0.74 |
| HIGD1A | 1.01 | 0.95 |
| HPX | 1.04 | 0.85 |
| LCN2 | 0.86 | 0.36 |
| LIG3 | 0.71 | 0.09 |
| MCUR1 | 0.76 | 0.15 |
| MT2A | 1.05 | 0.79 |
| MYH7B | 0.87 | 0.56 |
| SIRT7 | 0.63 | 0.02 |
| SMIM20 | 0.68 | 0.04 |
| STEAP3 | 1.02 | 0.92 |
| TFRC | 1.31 | 0.03 |
| YME1L1 | 0.82 | 0.28 |
| ZNF260 | 0.51 | 0.13 |
| Composite Primary Endpoint | |||
| HR | 95% CI | p-Value | |
| FTMT Δct, T2 vs. T1 + T3 | 2.396 | 1.043–5.502 | 0.039 |
| SIRT71 Δct, T2 + T3 vs. T1 | 5.495 | 1.784–16.923 | 0.003 |
| SMIM20 Δct, T2 + T3 vs. T1 | 9.511 | 2.698–33.530 | 0.000459 |
| TFRC Δct, T3 vs. T1 + T2 | 1.042 | 0.347–3.126 | 0.942 |
| ADM Δct, T2 + T3 vs. T1 | 1.296 | 0.456–3.683 | 0.626 |
| ECE1 Δct, T2 + T3 vs. T1 | 1.489 | 0.588–3.767 | 0.401 |
| All-Cause Death | |||
| HR | 95% CI | p-Value | |
| FTMT Δct, T2 vs. T1 + T3 | 4.448 | 1.497–13.216 | 0.007 |
| SIRT71 Δct, T2 + T3 vs. T1 | 7.122 | 1.848–27.438 | 0.004 |
| SMIM20 Δct, T2 + T3 vs. T1 | 7.44 | 1.882–27.930 | 0.03 |
| TFRC Δct, T3 vs. T1 + T2 | 2.056 | 0.700–6.036 | 0.19 |
| ADM Δct, T2 + T3 vs. T1 | 1.497 | 0.460–4.869 | 0.502 |
| ECE1 Δct, T2 + T3 vs. T1 | 1.083 | 0.310–3.783 | 0.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díez-López, C.; Tajes Orduña, M.; Enjuanes Grau, C.; Moliner Borja, P.; González-Costello, J.; García-Romero, E.; Francesch Manzano, J.; Yun Viladomat, S.; Jiménez-Marrero, S.; Ramos-Polo, R.; et al. Blood Differential Gene Expression in Patients with Chronic Heart Failure and Systemic Iron Deficiency: Pathways Involved in Pathophysiology and Impact on Clinical Outcomes. J. Clin. Med. 2021, 10, 4937. https://doi.org/10.3390/jcm10214937
Díez-López C, Tajes Orduña M, Enjuanes Grau C, Moliner Borja P, González-Costello J, García-Romero E, Francesch Manzano J, Yun Viladomat S, Jiménez-Marrero S, Ramos-Polo R, et al. Blood Differential Gene Expression in Patients with Chronic Heart Failure and Systemic Iron Deficiency: Pathways Involved in Pathophysiology and Impact on Clinical Outcomes. Journal of Clinical Medicine. 2021; 10(21):4937. https://doi.org/10.3390/jcm10214937
Chicago/Turabian StyleDíez-López, Carles, Marta Tajes Orduña, Cristina Enjuanes Grau, Pedro Moliner Borja, José González-Costello, Elena García-Romero, Josep Francesch Manzano, Sergi Yun Viladomat, Santiago Jiménez-Marrero, Raul Ramos-Polo, and et al. 2021. "Blood Differential Gene Expression in Patients with Chronic Heart Failure and Systemic Iron Deficiency: Pathways Involved in Pathophysiology and Impact on Clinical Outcomes" Journal of Clinical Medicine 10, no. 21: 4937. https://doi.org/10.3390/jcm10214937
APA StyleDíez-López, C., Tajes Orduña, M., Enjuanes Grau, C., Moliner Borja, P., González-Costello, J., García-Romero, E., Francesch Manzano, J., Yun Viladomat, S., Jiménez-Marrero, S., Ramos-Polo, R., Ras Jiménez, M. d. M., & Comín-Colet, J. (2021). Blood Differential Gene Expression in Patients with Chronic Heart Failure and Systemic Iron Deficiency: Pathways Involved in Pathophysiology and Impact on Clinical Outcomes. Journal of Clinical Medicine, 10(21), 4937. https://doi.org/10.3390/jcm10214937






