Longitudinal Morphological Changes in the Adenoids and Tonsils in Japanese School Children
Abstract
:1. Introduction
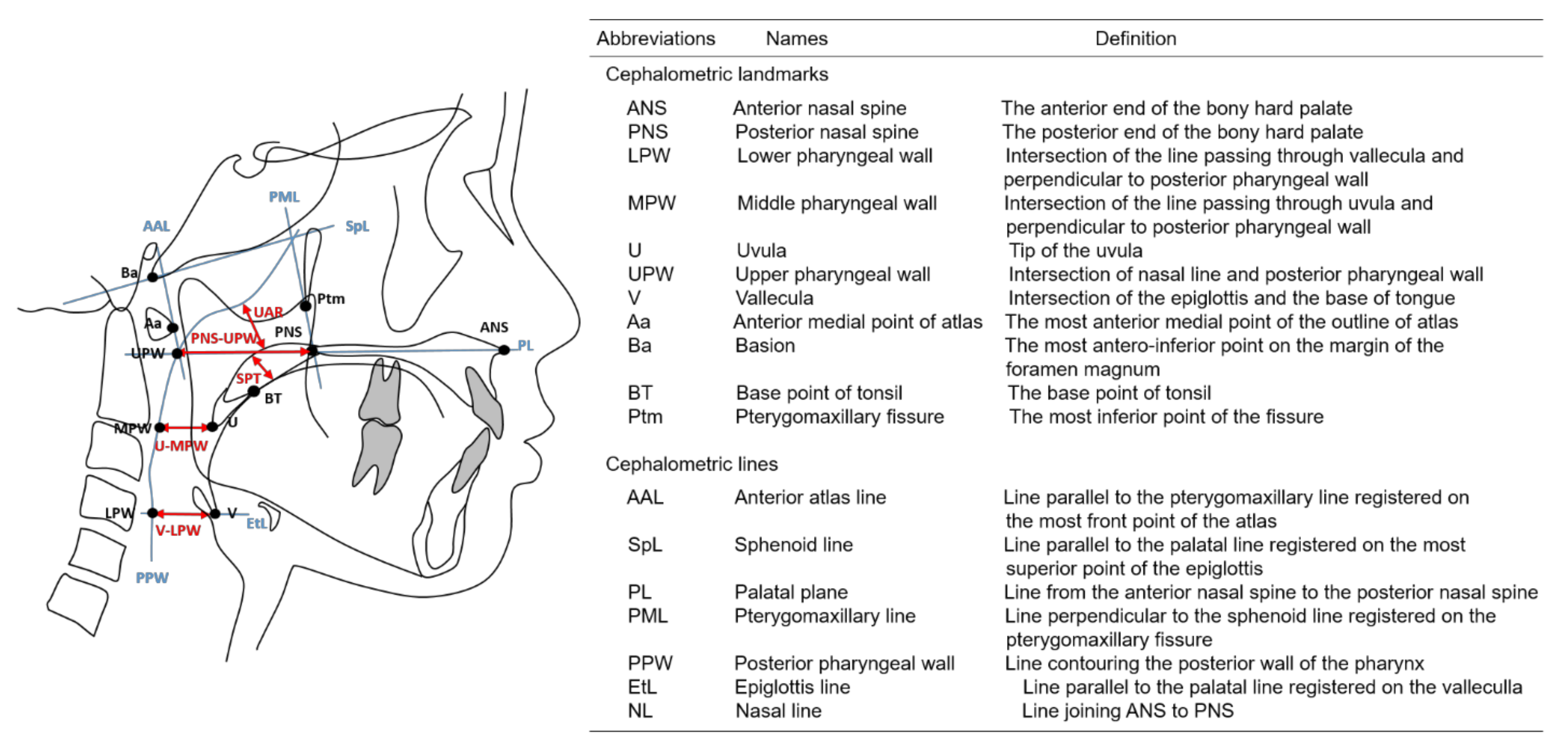
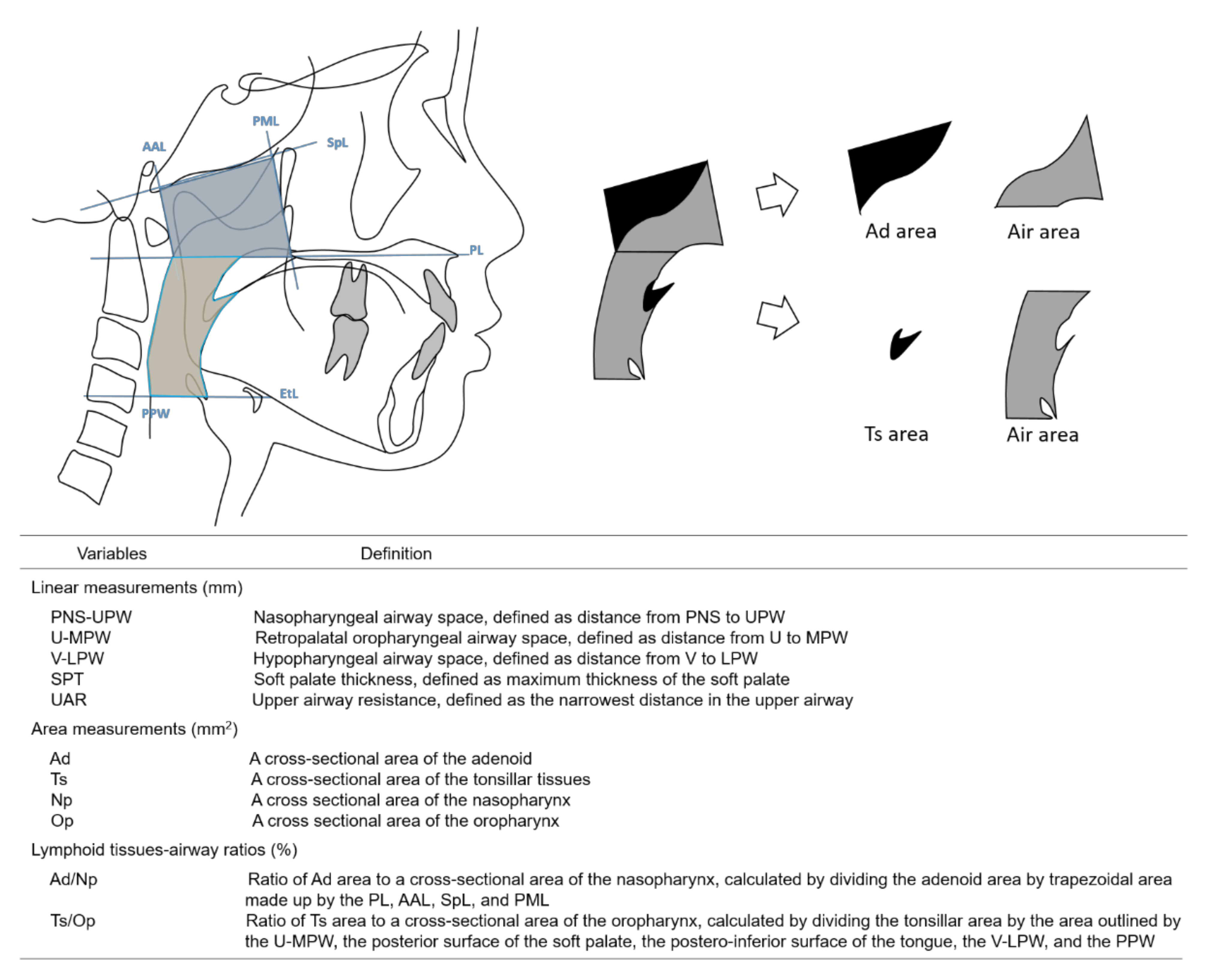
2. Materials and Methods
3. Results
3.1. Participants
3.2. Cephalometric Measurements of the Lymphoid Tissues and Upper Airway and Their Correlations
3.3. Correlations of Continuous Variables
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manabe, A.; Ishida, T.; Yoon, H.S.; Yang, S.S.; Kanda, E.; Ono, T. Differential changes in the adenoids and tonsils in Japanese children and teenagers: A cross-sectional study. Sci. Rep. 2017, 7, 9734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scammon, R.E. The first seriatim study of human growth. Am. J. Phys. Anthropol. 1927, 10, 329–336. [Google Scholar] [CrossRef]
- Ishida, T.; Manabe, A.; Yang, S.S.; Yoon, H.S.; Kanda, E.; Ono, T. Patterns of adenoid and tonsil growth in Japanese children and adolescents: A longitudinal study. Sci. Rep. 2018, 8, 17088. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Gray, S.; Bennani, H.; Antoun, J.S.; Farella, M. Morphometric growth changes of the nasopharyngeal space in subjects with different vertical craniofacial features. Am. J. Orthod. Dentofac. Orthop. 2016, 150, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Di Carlo, G.; Zara, F.; Rocchetti, M.; Venturini, M.; Oritiz-Ruiz, A.J.; Luzzi, V.; Catteneo, P.M.; Polimeni, A.; Vozza, J. Prevalence of sleep-disordered breathing in children referring for first dental examination. A multicenter cross-sectional study using pediatric sleep questinonnaire. Int. J. Environ. Res. Public Health 2020, 17, 8460. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, T.; Miyazaki, S.; Kadotani, H.; Suzuki, H.; Kanemura, T.; Komada, I.; Nishikawa, M.; Kobayashi, R.; Okawa, M. Prevalence of obstructive sleep apnea syndrome in Japanese elementary school children aged 6–8 years. Sleep Breath. 2014, 18, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Matsumoto, N.; Kawamoto, T. Study on the craniofacial growth for Japanese school children. J. Osaka Odontol. Soc. 2003, 66, 183–197. [Google Scholar]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Savoldi, F.; Xinyue, G.; McGrath, C.P.; Yang, Y.; Chow, S.C.; Tsoi, J.K.H.; Gu, M. Reliability of lateral cephalometric radiographs in the assessment of the upper airway in children: A retrospective study. Angle Orthod. 2020, 90, 47–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kula, K.; Jeong, A.E.; Halum, S.; Kendall, D.; Ghoneima, A. Three dimensional evaluation of upper airway volume in children with different dental and skeletal malocclusions. J. Biomed. Graph. Comput. 2013, 3, 116–126. [Google Scholar] [CrossRef]
- Cohen, O.; Betito, H.R.; Adi, M.; Shapira-Galitz, Y.; Halperin, D.; Lahav, Y.; Warman, M. Development of the nasopharynx: A radiological study of children. Clin. Anat. 2020, 33, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Grauer, D.; Cevidanes, L.S.; Styner, M.A.; Ackerman, J.L.; Proffit, W.R. Pharyngeal airway volume and shape from cone-beam computed tomography: Relationship to facial morphology. Am. J. Orthod. Dentofacial Orthop. 2009, 136, 805–814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pirilä-Parkkinen, K.; Löppönen, H.; Nieminen, P.; Tolonen, U.; Pääkkö, E.; Pirttiniemi, P. Validity of upper airway assessment in children: A clinical, cephalometric, and MRI study. Angle Orthod. 2011, 81, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Oz, U.; Orhan, K.; Rubenduz, M. Two-dimensional lateral cephalometric evaluation of varying types of Class II subgroups on posterior airway space in postadolescent girls: A pilot study. J. Orofac. Orthop. 2013, 74, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, E.; Tanaka, E.; Tabe, H.; Wakisaka, N.; Nakata, Y.; Ueda, H.; Hori, T.; Abe, T.; Tanne, K. Sleep architecture and the apnoea-hypopnoea index in children with obstructive-sleep apnoea syndrome. J. Oral Rehabil. 2007, 34, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Hoekema, A.; Hovinga, B.; Stegenga, B.; De Bont, L.G.M. Craniofacial morphology and obstructive sleep apnoea: A cephalometric analysis. J. Oral Rehabil. 2003, 30, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Lopatiene, K.; Sidlauskas, A.; Vasiliauskas, A.; Cecyte, L.; Svalkauskiene, V.; Sidlauskas, M. Relationship between malocclusion, soft tissue profile, and pharyngeal airways: A cephalometric study. Medicina 2016, 52, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Kuhle, S.; Hoffmann, D.U.; Mitra, S.; Urschitz, M.S. Anti-inflammatory medications for obstructive sleep apnoea in children. Cochrane Database Syst. Rev. 2020, 1, CD007074. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.R.; Weckx, L.L.; Ortolani, C.L.; Bakor, S.F. Study of craniofacial alterations and of the importance of the rapid maxillary expansion after tonsillectomy. Braz. J. Otorhinolanryngol. 2012, 78, 111–117. [Google Scholar] [CrossRef] [Green Version]
| Grade 2 | Grade 4 | Grade 6 | Interaction | Sex | Grade | ||
|---|---|---|---|---|---|---|---|
| Height (cm) | Boy | 115.14 ± 4.75 | 120.55 ± 4.95 | 126.37 ± 5.29 | 0.654 | 0.614 | <0.001 |
| Girl | 115.69 ± 4.71 | 121.14 ± 4.97 | 126.73 ± 5.28 | ||||
| Weight (cm) | Boy | 20.20 (18.25–21.90) | 22.40 (20.20–24.50) | 25.00 (22.70–27.70) | 0.539 | 0.282 | <0.001 |
| Girl | 19.55 (18.38–20.85) | 21.60 (20.16–22.85) | 24.10 (22.75–25.40) | ||||
| BMI (cm) | Boy | 15.09 (14.39–15.95) | 15.10 (14.57–16.16) | 15.68 (14.09–16.77) | 0.163 | 0.007 | <0.001 |
| Girl | 14.69 (14.22–15.38) | 14.53 (14.13–15.34) | 15.00 (14.48–15.59) |
| Grade 2 | Grade 4 | Grade 6 | p-Value | Grade 2 vs. 4 § | Grade 4 vs. 6 § | Grade 2 vs. 4 § | |
|---|---|---|---|---|---|---|---|
| PNS-UPW (mm) | 20.73 ± 4.17 | 22.88 ± 4.58 | 23.28 ± 4.80 | <0.001 | <0.001 | 0.828 | <0.001 |
| U-MPW (mm) | 11.19 ± 3.11 | 11.26 ± 3.80 | 11.75 ± 3.46 | 0.198 | <0.001 | 0.447 | 0.293 |
| V-LPW (mm) | 13.74 (11.38–15.69) | 14.31 (12.66–16.02) | 15.88 (13.39–18.51) | <0.001 | 0.767 | 0.001 | 0.001 |
| SPT (mm) | 7.84 ± 1.12 | 8.07 ± 1.08 | 8.24 ± 1.22 | 0.022 | 0.290 | 0.624 | 0.039 |
| UAR (mm) | 8.74 ± 2.89 | 10.56 ± 3.12 | 10.68 ± 3.70 | <0.001 | <0.001 | 1.000 | <0.001 |
| Ad (mm2) | 340.16 (293.89–396.83) | 333.77 (284.41–398.78) | 386.66 (334.27–467.43) | <0.001 | 0.767 | 0.001 | 0.001 |
| Ts (mm2) | 125.91 (105.31–172.75) | 134.96 (102.25–177.50) | 135.56 (108.17–179.34) | 0.399 | 0.900 | 1.000 | 0.714 |
| Nasopharynx (mm2) | 529.76 (480.20–596.26) | 580.50 (523.27–647.47) | 656.47 (592.98–735.16) | <0.001 | <0.001 | <0.001 | <0.001 |
| Oropharynx (mm2) | 524.58 (445.48–644.24) | 580.49 (508.00–692.72) | 674.03 (575.50–788.62) | <0.001 | 0.140 | 0.001 | 0.001 |
| ANB (o) | 5.18 ± 2.56 | 4.73 ± 2.55 | 4.74 ± 2.59 | 0.001 | 0.001 | 1.000 | 0.009 |
| Ad/Np (%) | 63.34 (58.57–71.53) | 56.99 (49.54–65.29) | 59.99 (52.67–67.57) | <0.001 | 0.001 | 0.032 | 0.001 |
| Ts/Op (%) | 24.41 (18.46–30.16) | 23.88 (17.78–29.95) | 19.76 (15.56–23.97) | <0.001 | 0.165 | 0.001 | 0.001 |
| Correlation Coefficient | U-MPW (mm) | V-LPW (mm) | SPT (mm) | UAR (mm) | Ad (mm2) | Ts (mm2) | Nasopharynx (mm2) | Oropharynx (mm2) | ANB (o) | Height (cm) | Weight (kg) | BMI (kg/m2) | Ad/Np (%) | Ts/Op (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PNS-UPW (mm) | 0.248 *** | 0.284 **** | −0.087 | 0.584 **** | −0.130* | 0.053 | 0.416 **** | 0.333 **** | 0.061 | 0.221 *** | 0.123 * | −0.055 | −0.669 **** | −0.201 *** |
| U-MPW (mm) | 0.529 **** | −0.113 | 0.193 ** | 0.272 **** | 0.460 **** | 0.324 **** | 0.742 **** | −0.071 | 0.080 | 0.089 | 0.067 | 0.020 | 0.007 | |
| V-LPW (mm) | −0.081 | 0.157 ** | 0.168 ** | 0.218 *** | 0.290 **** | 0.759 **** | −0.063 | 0.267 **** | 0.229 **** | 0.079 | −0.092 | −0.264 **** | ||
| SPT (mm) | −0.184 ** | 0.136 * | 0.051 | 0.058 | −0.083 | 0.009 | 0.104 | 0.130 * | 0.112 | 0.107 | 0.117 * | |||
| UAR (mm) | −0.396 **** | 0.032 | 0.161 ** | 0.294 **** | −0.153 ** | 0.220 *** | 0.106 | −0.085 | −0.778 **** | −0.171 ** | ||||
| Ad (mm2) | 0.301 **** | 0.742 **** | 0.248 **** | 0.053 | 0.329 **** | 0.368 **** | 0.252 **** | 0.605 **** | 0.148 * | |||||
| Ts (mm2) | 0.252 **** | 0.495 **** | 0.046 | 0.099 | 00.110 | 0.074 | 0.159 ** | 0.755 **** | ||||||
| Nasopharynx (mm2) | 0.358 **** | −0.007 | 0.483 **** | 0.437 **** | 0.186 ** | −0.069 | 0.015 | |||||||
| Oropharynx (mm2) | −0.090 | 0.391 **** | 0.358 **** | 0.162 ** | −0.063 | −0.137 * | ||||||||
| ANB (o) | −0.085 | −0.087 | −0.046 | 0.094 | 0.108 | |||||||||
| Height (cm) | 0.882 **** | 0.351 **** | −0.106 | −0.188 ** | ||||||||||
| Weight (kg) | 0.745 **** | −0.002 | −0.158 ** | |||||||||||
| BMI (kg/m2) | 0.123 | −0.063 | ||||||||||||
| Ad/Np (%) | 0.222 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamada, H.; Sawada, M.; Higashino, M.; Abe, S.; El-Bialy, T.; Tanaka, E. Longitudinal Morphological Changes in the Adenoids and Tonsils in Japanese School Children. J. Clin. Med. 2021, 10, 4956. https://doi.org/10.3390/jcm10214956
Yamada H, Sawada M, Higashino M, Abe S, El-Bialy T, Tanaka E. Longitudinal Morphological Changes in the Adenoids and Tonsils in Japanese School Children. Journal of Clinical Medicine. 2021; 10(21):4956. https://doi.org/10.3390/jcm10214956
Chicago/Turabian StyleYamada, Hiroshi, Masaki Sawada, Masaaki Higashino, Susumu Abe, Tarek El-Bialy, and Eiji Tanaka. 2021. "Longitudinal Morphological Changes in the Adenoids and Tonsils in Japanese School Children" Journal of Clinical Medicine 10, no. 21: 4956. https://doi.org/10.3390/jcm10214956
APA StyleYamada, H., Sawada, M., Higashino, M., Abe, S., El-Bialy, T., & Tanaka, E. (2021). Longitudinal Morphological Changes in the Adenoids and Tonsils in Japanese School Children. Journal of Clinical Medicine, 10(21), 4956. https://doi.org/10.3390/jcm10214956








