Incidence and Predictors of Thrombotic Complications in 4742 Patients with COVID-19 or Other Acute Infectious Respiratory Diseases: A Propensity Score-Matched Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Variables
2.3. Ethical Approval
2.4. Outcome Measures
2.5. Statistical Analysis
2.6. Propensity Score Matched Analysis
3. Results
3.1. Thrombotic Complications in COVID-19 and No-COVID-19 Group
3.2. Propensity Score Matched Analysis
3.3. Safety Endpoints
3.4. Clinical Predictors of Thrombotic Events in AIRD Patients
3.4.1. Cumulative Arterial and Venous Thrombosis
3.4.2. Total Thrombotic Events
3.4.3. Clinical Predictors of Thrombotic Events in COVID-19 and No-COVID-19 Groups
4. Discussion
5. Limitations of the Study
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hui, D.S.; Azhar, E.I.; Madani, T.A.; Ntoumi, F.; Kock, R.; Dar, O.; Ippolito, G.; Mchugh, T.D.; Memish, Z.A.; Drosten, C.; et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health—The latest 2019 novel coronavirus outbreak in Wuhan, China. Int. J. Infect. Dis. 2020, 91, 264–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Xia, J.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef] [Green Version]
- Covino, M.; Sandroni, C.; Santoro, M.; Sabia, L.; Simeoni, B.; Bocci, M.G.; Ojetti, V.; Candelli, M.; Antonelli, M.; Gasbarrini, A.; et al. Predicting intensive care unit admission and death for COVID-19 patients in the emergency department using early warning scores. Resuscitation 2020, 156, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Chen, S.; Li, X.; Liu, S.; Wang, F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J. Thromb. Haemost. 2020, 18, 1421–1424. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Li, D.; Wang, X.; Sun, Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020, 18, 844–847. [Google Scholar] [CrossRef] [Green Version]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Chen, T.; Wu, D.; Chen, H.; Yan, W.; Yang, D.; Chen, G.; Ma, K.; Xu, D.; Yu, H.; Wang, H.; et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: Retrospective study. BMJ 2020, 368, m1091. [Google Scholar] [CrossRef] [Green Version]
- Wichmann, D.; Sperhake, J.P.; Lütgehetmann, M.; Steurer, S.; Edler, C.; Heinemann, A.; Heinrich, F.; Mushumba, H.; Kniep, I.; Schröder, A.S.; et al. Autopsy Findings and Venous Thromboembolism in Patients With COVID-19: A Prospective Cohort Study. Ann. Intern. Med. 2020, 173, 268–277. [Google Scholar] [CrossRef]
- Dolhnikoff, M.; Duarte-Neto, A.N.; de Almeida Monteiro, R.A.; da Silva, L.F.F.; de Oliveira, E.P.; Saldiva, P.H.N.; Mauad, T.; Negri, E.M. Pathological evidence of pulmonary thrombotic phenomena in severe COVID-19. J. Thromb. Haemost. 2020, 18, 1517–1519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, B.; Zhao, W.; Feng, R.; Zhang, X.; Li, X.; Zhou, Y.; Peng, L.; Li, Y.; Zhang, J.; Luo, J.; et al. The pathological autopsy of coronavirus disease 2019 (COVID-2019) in China: A review. Pathog. Dis. 2020, 78, ftaa026. [Google Scholar] [CrossRef] [PubMed]
- Fraissé, M.; Logre, E.; Pajot, O.; Mentec, H.; Plantefève, G.; Contou, D. Thrombotic and hemorrhagic events in critically ill COVID-19 patients: A French monocenter retrospective study. Crit. Care 2020, 24, 275. [Google Scholar] [CrossRef]
- Helms, J.; Severac, F.; Merdji, H.; Schenck, M.; Clere-Jehl, R.; Baldacini, M.; Ohana, M.; Grunebaum, L.; Castelain, V.; Anglés-Cano, E.; et al. Higher anticoagulation targets and risk of thrombotic events in severe COVID-19 patients: Bi-center cohort study. Ann. Intensive Care 2021, 11, 14. [Google Scholar] [CrossRef] [PubMed]
- Talasaz, A.H.; Sadeghipour, P.; Kakavand, H.; Aghakouchakzadeh, M.; Kordzadeh-Kermani, E.; Van Tassell, B.W.; Gheymati, A.; Ariannejad, H.; Hosseini, S.H.; Jamalkhani, S.; et al. Recent Randomized Trials of Antithrombotic Therapy for Patients with COVID-19: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 77, 1903–1921. [Google Scholar] [CrossRef]
- Paranjpe, I.; Fuster, V.; Lala, A.; Russak, A.J.; Glicksberg, B.S.; Levin, M.A.; Charney, A.W.; Narula, J.; Fayad, Z.A.; Bagiella, E.; et al. Association of Treatment Dose Anticoagulation with In-Hospital Survival among Hospitalized Patients With COVID-19. J. Am. Coll. Cardiol. 2020, 76, 122–124. [Google Scholar] [CrossRef] [PubMed]
- Al-Samkari, H.; Gupta, S.; Leaf, R.K.; Wang, W.; Rosovsky, R.P.; Brenner, S.K.; Hayek, S.S.; Berlin, H.; Kapoor, R.; Shaefi, S.; et al. Thrombosis, Bleeding, and the Observational Effect of Early Therapeutic Anticoagulation on Survival in Critically Ill Patients With COVID-19. Ann. Intern. Med. 2021, 174, 622–632. [Google Scholar] [CrossRef]
- Lemos, A.C.B.; do Espírito Santo, D.A.; Salvetti, M.C.; Gilio, R.N.; Agra, L.B.; Pazin-Filho, A.; Miranda, C.H. Therapeutic versus prophylactic anticoagulation for severe COVID-19: A randomized phase II clinical trial (HESACOVID). Thromb. Res. 2020, 196, 359–366. [Google Scholar] [CrossRef]
- Sadeghipour, P.; Talasaz, A.H.; Rashidi, F.; Sharif-Kashani, B.; Beigmohammadi, M.T.; Farrokhpour, M.; Sezavar, S.H.; Payandemehr, P.; Dabbagh, A.; et al.; INSPIRATION Investigators Effect of Intermediate-Dose vs Standard-Dose Prophylactic Anticoagulation on Thrombotic Events, Extracorporeal Membrane Oxygenation Treatment, or Mortality Among Patients with COVID-19 Admitted to the Intensive Care Unit: The INSPIRATION Randomized Clinical Trial. JAMA 2021, 325, 1620–1630. [Google Scholar] [PubMed]
- Moores, L.K.; Tritschler, T.; Brosnahan, S.; Carrier, M.; Collen, J.F.; Doerschug, K.; Holley, A.B.; Jimenez, D.; Le Gal, G.; Rali, P.; et al. Prevention, Diagnosis, and Treatment of VTE in Patients With Coronavirus Disease 2019: CHEST Guideline and Expert Panel Report. Chest 2020, 158, 1143–1163. [Google Scholar] [CrossRef] [PubMed]
- Spyropoulos, A.C.; Levy, J.H.; Ageno, W.; Connors, J.M.; Hunt, B.J.; Iba, T.; Levi, M.; Samama, C.M.; Thachil, J.; Giannis, D.; et al. Scientific and Standardization Committee communication: Clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID-19. J. Thromb. Haemost. 2020, 18, 1859–1865. [Google Scholar] [CrossRef] [PubMed]
- Bikdeli, B.; Madhavan, M.V.; Jimenez, D.; Chuich, T.; Dreyfus, I.; Driggin, E.; Nigoghossian, C.; Ageno, W.; Madjid, M.; Guo, Y.; et al. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-Up: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 2950–2973. [Google Scholar] [CrossRef]
- Klok, F.A.; Kruip, M.J.H.A.; van der Meer, N.J.M.; Arbous, M.S.; Gommers, D.A.M.P.J.; Kant, K.M.; Kaptein, F.H.J.; van Paassen, J.; Stals, M.A.M.; Huisman, M.V.; et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020, 191, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Avruscio, G.; Camporese, G.; Campello, E.; Bernardi, E.; Persona, P.; Passarella, C.; Noventa, F.; Cola, M.; Navalesi, P.; Cattelan, A.; et al. COVID-19 and Venous Thromboembolism in Intensive Care or Medical Ward. Clin. Transl. Sci. 2020, 13, 1108–1114. [Google Scholar] [CrossRef] [PubMed]
- Woodhead, M.; Blasi, F.; Ewig, S.; Garau, J.; Huchon, G.; Ieven, M.; Ortqvist, A.; Schaberg, T.; Torres, A.; van der Heijden, G.; et al. Guidelines for the management of adult lower respiratory tract infections—Summary. Clin. Microbiol. Infect. 2011, 17 (Suppl. 6), 1–24. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Clinical Management of Severe Acute Respiratory Infection When Novel Coronavirus (nCoV) Infection is Suspected: Interim Guidance. Available online: WHO/2019-nCoV/Clinical/2020.1 (accessed on 2 October 2021).
- Barbar, S.; Noventa, F.; Rossetto, V.; Ferrari, A.; Brandolin, B.; Perlati, M.; De Bon, E.; Tormene, D.; Pagnan, A.; Prandoni, P. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: The Padua Prediction Score. J. Thromb. Haemost. 2010, 8, 2450–2457. [Google Scholar] [CrossRef]
- Cuker, A.; Tseng, E.K.; Nieuwlaat, R.; Angchaisuksiri, P.; Blair, C.; Dane, K.; Davila, J.; DeSancho, M.T.; Diuguid, D.; Griffin, D.O.; et al. American Society of Hematology 2021 guidelines on the use of anticoagulation for thromboprophylaxis in patients with COVID-19. Blood Adv. 2021, 5, 872–888. [Google Scholar] [CrossRef]
- Schulman, S.; Kearon, C.; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J. Thromb. Haemost. 2005, 3, 692–694. [Google Scholar] [CrossRef]
- Kaatz, S.; Ahmad, D.; Spyropoulos, A.C.; Schulman, S.; Subcommittee on Control of Anticoagulation. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: Communication from the SSC of the ISTH. J. Thromb. Haemost. 2015, 13, 2119–2126. [Google Scholar] [CrossRef]
- Wang, L.; He, W.; Yu, X.; Hu, D.; Bao, M.; Liu, H.; Zhou, J.; Jiang, H. Coronavirus disease 2019 in elderly patients: Characteristics and prognostic factors based on 4-week follow-up. J. Infect. 2020, 80, 639–645. [Google Scholar] [CrossRef]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J.; HLH Across Speciality Collaboration, UK. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Schulman, S. Coronavirus Disease 2019, Prothrombotic Factors, and Venous Thromboembolism. Semin. Thromb. Hemost. 2020, 46, 772–776. [Google Scholar] [CrossRef] [PubMed]
- Spiezia, L.; Boscolo, A.; Poletto, F.; Cerruti, L.; Tiberio, I.; Campello, E.; Navalesi, P.; Simioni, P. COVID-19-Related Severe Hypercoagulability in Patients Admitted to Intensive Care Unit for Acute Respiratory Failure. Thromb. Haemost. 2020, 120, 998–1000. [Google Scholar] [CrossRef]
- Abou-Ismail, M.Y.; Diamond, A.; Kapoor, S.; Arafah, Y.; Nayak, L. The hypercoagulable state in COVID-19: Incidence, pathophysiology, and management. Thromb. Res. 2020, 194, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Waite, A.A.C.; Hamilton, D.O.; Pizzi, R.; Ageno, W.; Welters, I.D. Hypercoagulopathy in Severe COVID-19: Implications for Acute Care. Thromb. Haemost. 2020, 120, 1654–1667. [Google Scholar]
- Denas, G.; Gennaro, N.; Ferroni, E.; Fedeli, U.; Lorenzoni, G.; Gregori, D.; Iliceto, S.; Pengo, V. Reduction in all-cause mortality in COVID-19 patients on chronic oral anticoagulation: A population-based propensity score matched study. Int. J. Cardiol. 2021, 329, 266–269. [Google Scholar] [CrossRef]
- Campello, E.; Bulato, C.; Spiezia, L.; Boscolo, A.; Poletto, F.; Cola, M.; Gavasso, S.; Simion, C.; Radu, C.M.; Cattelan, A.; et al. Thrombin generation in patients with COVID-19 with and without thromboprophylaxis. Clin. Chem. Lab Med. 2021, 59, 1323–1330. [Google Scholar] [CrossRef]
- Boscolo, A.; Spiezia, L.; Correale, C.; Sella, N.; Pesenti, E.; Beghetto, L.; Campello, E.; Poletto, F.; Cerruti, L.; Cola, M.; et al. Different Hypercoagulable Profiles in Patients with COVID-19 Admitted to the Internal Medicine Ward and the Intensive Care Unit. Thromb. Haemost. 2020, 120, 1474–1477. [Google Scholar]
- Lodigiani, C.; Iapichino, G.; Carenzo, L.; Cecconi, M.; Ferrazzi, P.; Sebastian, T.; Kucher, N.; Studt, J.D.; Sacco, C.; Bertuzzi, A.; et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb. Res. 2020, 191, 9–14. [Google Scholar] [CrossRef] [PubMed]
| COVID-19 Group (n = 2579) | no-COVID-19 Group (n = 2163) | p | |
|---|---|---|---|
| Age (years) | 69.0 ± 14.9 | 72.5 ± 15.6 | <0.001 |
| Male sex (%) | 1588 (61.6) | 1245 (57.6) | 0.005 |
| Hypertension (%) | 1001 (38.8) | 703 (32.5) | <0.001 |
| Diabetes mellitus (%) | 547 (21.2) | 412 (19.0) | 0.070 |
| Obesity (%) | 47 (1.8) | 56 (2.6) | 0.073 |
| Charlson Comorbidity Index (n) | 3.5 ± 2.2 | 4.6 ± 2.6 | <0.001 |
| Known ischemic heart disease (%) | 306 (11.9) | 300 (13.9) | 0.040 |
| Chronic heart failure (%) | 190 (7.4) | 386 (17.8) | <0.001 |
| Peripheral artery disease (%) | 58 (2.2) | 96 (4.4) | <0.001 |
| Previous TIA/stroke (%) | 84 (3.3) | 127 (5.9) | <0.001 |
| COPD (%) | 221 (8.6) | 657 (30.4) | <0.001 |
| Chronic kidney disease (%) | 219 (8.5) | 143 (6.6) | 0.016 |
| Chronic liver disease (%) | 36 (1.4) | 37 (1.7) | 0.41 |
| Solid tumor (%) | 92 (3.6) | 246 (11.4) | <0.001 |
| Leukemia/Lymphoma (%) | 39 (1.5) | 42 (1.9) | 0.26 |
| Previous DVT (%) | 22 (0.9) | 56 (2.6) | <0.001 |
| Platelet disorders (%) | 19 (0.7) | 21 (1.0) | 0.43 |
| Connective tissue disease (%) | 19 (0.7) | 40 (1.8) | 0.001 |
| Cognitive impairment (%) | 173 (6.7) | 428 (19.8) | <0.001 |
| HIV infection (%) | 14 (0.5) | 28 (1.3) | 0.008 |
| Pharmacological therapy on admission | |||
| Low-dose ASA (%) | 400 (15.5) | 520 (24.0) | <0.001 |
| P2Y12 inhibitors (%) | 119 (4.6) | 146 (6.7) | 0.001 |
| Vitamin K antagonists (%) | 56 (2.2) | 66 (3.1) | 0.065 |
| DOACs (%) | 150 (5.8) | 174 (8.0) | 0.003 |
| Heparins (%) | 346 (13.4) | 139 (6.4) | <0.001 |
| Statins (%) | 166 (6.4) | 214 (9.9) | <0.001 |
| COVID-19 Group (n = 2579) | no-COVID-19 Group (n = 2163) | p | p * | |
|---|---|---|---|---|
| Total thrombotic events | 121 (4.7) | 150 (6.9) | <0.001 | 0.11 |
| Arterial thrombosis | 63 (2.4) | 79 (3.7) | 0.016 | 0.26 |
| Acute myocardial infarction (%) | 23 (0.9) | 16 (0.7) | 0.63 | - |
| Ischemic stroke (%) | 37 (1.4) | 49 (2.3) | 0.038 | 0.088 |
| Peripheral artery thrombosis (%) | 4 (0.2) | 14 (0.6) | 0.008 | 0.96 |
| Venous thrombosis | 61 (2.4) | 71 (3.3) | 0.063 | 0.38 |
| Deep venous thrombosis (%) | 6 (0.2) | 19 (0.9) | 0.002 | 0.028 |
| Pulmonary embolism (%) | 51 (2.0) | 55 (2.5) | 0.20 | - |
| Others (%) | 5 (0.2) | 8 (0.4) | 0.28 | - |
| COVID-19 Group (n = 1518) | no-COVID-19 Group (n = 1518) | SMD | p | |
|---|---|---|---|---|
| Age (years) | 70.2 ± 16.6 | 69.9 ± 15.0 | 0.02 | 0.62 |
| Male sex (%) | 899 (59.2) | 909 (59.9) | 0.02 | 0.74 |
| Hypertension (%) | 478 (31.5) | 484 (31.9) | 0.01 | 0.84 |
| Diabetes mellitus (%) | 302 (19.9) | 273 (18.0) | 0.07 | 0.19 |
| Obesity (%) | 36 (2.4) | 31 (2.0) | 0.08 | 0.62 |
| Charlson Comorbidity Index (n) | 3.8 ± 2.3 | 3.8 ± 2.4 | 0.00 | 0.95 |
| Known ischemic heart disease (%) | 202 (13.3) | 173 (11.4) | 0.09 | 0.11 |
| Chronic heart failure (%) | 165 (10.9) | 143 (9.4) | 0.08 | 0.21 |
| Peripheral artery disease (%) | 45 (3.0) | 38 (2.5) | 0.08 | 0.50 |
| Previous TIA/stroke (%) | 71 (4.7) | 67 (4.4) | 0.03 | 0.79 |
| COPD (%) | 212 (14.0) | 197 (13.0) | 0.05 | 0.46 |
| Chronic kidney disease (%) | 96 (6.3) | 105 (6.9) | 0.05 | 0.56 |
| Chronic liver disease (%) | 30 (2.0) | 27 (1.8) | 0.06 | 0.69 |
| Solid tumor (%) | 91 (6.0) | 105 (6.9) | 0.08 | 0.30 |
| Leukemia/Lymphoma (%) | 29 (1.9) | 31 (2.0) | 0.04 | 0.90 |
| Previous DVT (%) | 22 (1.4) | 26 (1.7) | 0.09 | 0.66 |
| Platelet disorders (%) | 14 (0.9) | 11 (0.7) | 0.10 | 0.69 |
| Connective tissue disease (%) | 16 (1.1) | 20 (1.3) | 0.08 | 0.62 |
| Cognitive impairment (%) | 162 (10.7) | 167 (11.0) | 0.02 | 0.81 |
| HIV infection (%) | 13 (0.9) | 15 (1.0) | 0.08 | 0.70 |
| Pharmacological therapy on admission | ||||
| Low-dose ASA (%) | 322 (21.2) | 317 (209) | 0.01 | 0.86 |
| P2Y12 inhibitors (%) | 85 (5.6) | 92 (6.1) | 0.05 | 0.64 |
| Vitamin K antagonists (%) | 38 (2.5) | 32 (2.1) | 0.09 | 0.47 |
| DOACs (%) | 110 (7.2) | 95 (6.3) | 0.08 | 0.28 |
| Heparins (%) | 106 (7.0) | 116 (7.6) | 0.05 | 0.53 |
| Statins (%) | 128 (8.4) | 125 (8.2) | 0.01 | 0.90 |
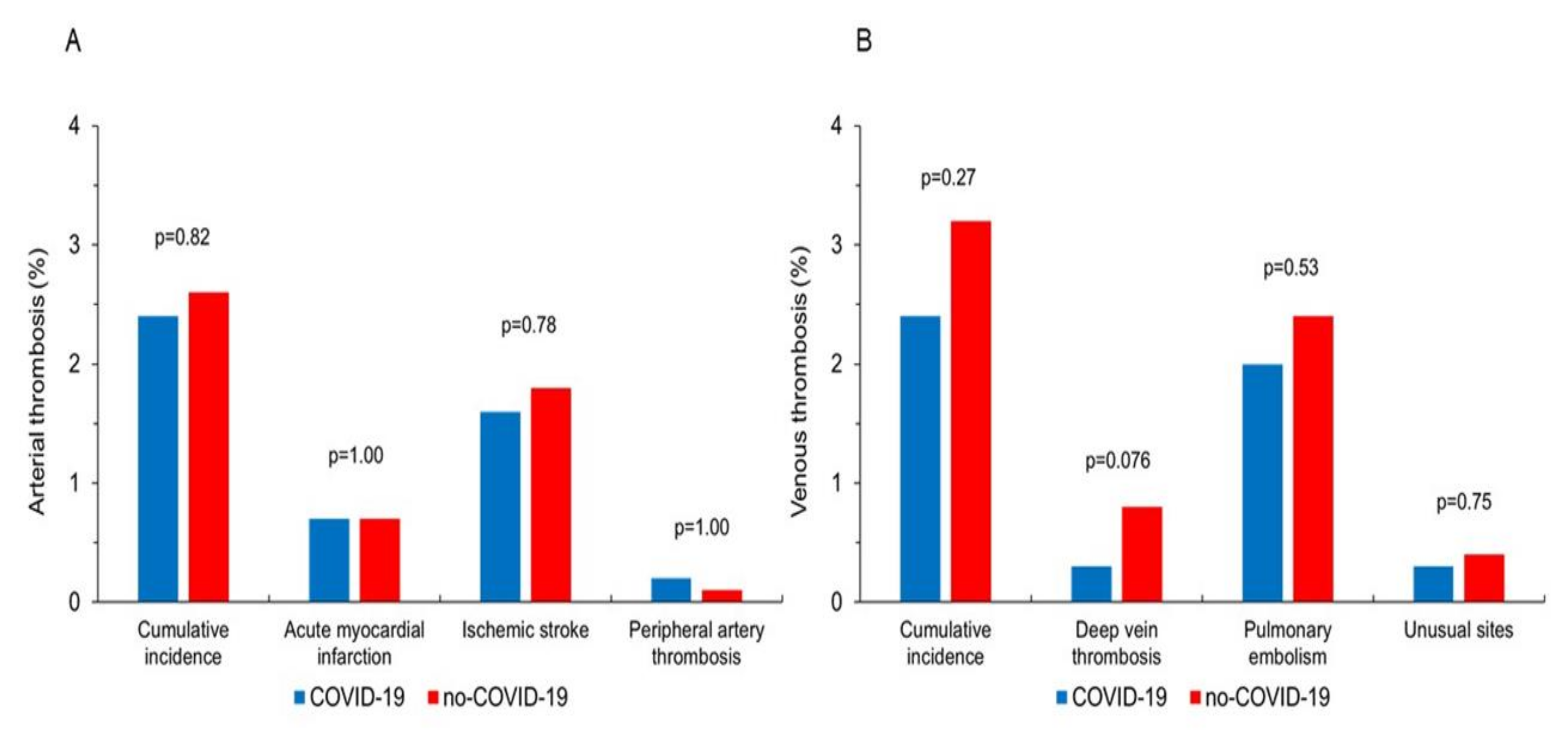
| COVID-19 Group (n = 1518) | no-COVID-19 Group (n = 1518) | p | |
|---|---|---|---|
| Total thrombotic events | 74 (4.9) | 88 (5.8) | 0.29 |
| Arterial thrombosis | 37 (2.4) | 40 (2.6) | 0.82 |
| Acute myocardial infarction (%) | 11 (0.7) | 11 (0.7) | 1.00 |
| Ischemic stroke (%) | 24 (1.6) | 27 (1.8) | 0.78 |
| Peripheral artery thrombosis (%) | 3 (0.2) | 2 (0.1) | 1.00 |
| Venous thrombosis | 37 (2.4) | 48 (3.2) | 0.27 |
| Deep venous thrombosis (%) | 4 (0.3) | 12 (0.8) | 0.076 |
| Pulmonary embolism (%) | 30 (2.0) | 36 (2.4) | 0.53 |
| Others (%) | 4 (0.3) | 6 (0.4) | 0.75 |
| YES (n = 142) | NO (n = 4600) | p univariate | OR | 95% C.I. | p multivariate | |
|---|---|---|---|---|---|---|
| COVID-19 diagnosis (%) | 63 (44.4) | 2516 (54.7) | 0.016 | |||
| Age (years) | 76.8 ± 11.0 | 70.4 ± 15.4 | <0.001 | 1.02 | 1.01–1.03 | 0.006 |
| Male sex (%) | 90 (63.4) | 2743 (59.6) | 0.37 | |||
| Hypertension (%) | 58 (40.8) | 1646 (35.8) | 0.22 | |||
| Diabetes mellitus (%) | 46 (32.4) | 913 (19.8) | <0.001 | 1.50 | 1.03–2.19 | 0.034 |
| Obesity (%) | 5 (3.5) | 98 (2.1) | 0.27 | |||
| Charlson Comorbidity Index (n) | 5.2 ± 2.0 | 3.9 ± 2.5 | <0.001 | |||
| Known ischemic heart disease (%) | 28 (19.7) | 578 (12.6) | 0.013 | |||
| Chronic heart failure (%) | 21 (14.8) | 555 (12.1) | 0.33 | |||
| Peripheral artery disease (%) | 27 (19.0) | 127 (2.8) | <0.001 | 5.45 | 3.34–8.88 | <0.001 |
| Previous TIA/stroke (%) | 37 (26.1) | 174 (3.8) | <0.001 | 6.34 | 4.12–9.76 | <0.001 |
| COPD (%) | 40 (28.2) | 838 (18.2) | 0.003 | |||
| Chronic kidney disease (%) | 9 (6.3) | 353 (7.7) | 0.56 | |||
| Chronic liver disease (%) | 2 (1.4) | 71 (1.5) | 0.90 | |||
| Solid tumor (%) | 7 (4.9) | 331 (7.2) | 0.30 | |||
| Leukemia/Lymphoma (%) | 1 (0.7) | 80 (1.7) | 0.36 | |||
| Previous DVT (%) | 2 (1.4) | 76 (1.7) | 0.82 | |||
| Platelet disorders (%) | 0 (0.0) | 40 (0.9) | 1.00 | |||
| Connective tissue disease (%) | 0 (0.0) | 59 (1.3) | 1.00 | |||
| Cognitive impairment (%) | 20 (14.1) | 581 (12.6) | 0.61 | |||
| HIV infection (%) | 0 (0.0) | 42 (0.9) | 1.00 | |||
| Pharmacological therapy on admission | ||||||
| Low-dose ASA (%) | 38 (26.8) | 882 (19.2) | 0.025 | |||
| P2Y12 inhibitors (%) | 16 (11.3) | 246 (5.4) | 0.004 | |||
| Vitamin K antagonists (%) | 6 (4.2) | 116 (2.5) | 0.21 | |||
| DOACs (%) | 16 (11.3) | 308 (6.7) | 0.036 | |||
| Heparins (%) | 11 (7.7) | 474 (10.3) | 0.32 | |||
| Statins (%) | 18 (12.7) | 362 (7.9) | 0.040 |
| YES (n = 132) | NO (n = 4610) | p univariate | OR | 95% C.I. | p multivariate | |
|---|---|---|---|---|---|---|
| COVID-19 diagnosis (%) | 61 (46.2) | 2518 (54.6) | 0.057 | |||
| Age (years) | 71.8 ± 13.6 | 70.5 ± 15.3 | 0.37 | |||
| Male sex (%) | 86 (65.2) | 2747 (59.6) | 0.20 | |||
| Hypertension (%) | 50 (37.9) | 1654 (35.9) | 0.64 | |||
| Diabetes mellitus (%) | 21 (15.9) | 938 (20.3) | 0.21 | |||
| Obesity (%) | 2 (1.5) | 101 (2.2) | 0.60 | |||
| Charlson Comorbidity Index (n) | 4.2 ± 2.4 | 4.0 ± 2.5 | 0.23 | |||
| Known ischemic heart disease (%) | 15 (11.4) | 591 (12.8) | 0.62 | |||
| Chronic heart failure (%) | 13 (9.8) | 563 (12.2) | 0.41 | |||
| Peripheral artery disease (%) | 2 (1.5) | 152 (3.3) | 0.27 | |||
| Previous TIA/stroke (%) | 2 (1.5) | 209 (4.5) | 0.11 | |||
| COPD (%) | 26 (19.7) | 852 (18.5) | 0.72 | |||
| Chronic kidney disease (%) | 7 (5.3) | 355 (7.7) | 0.31 | |||
| Chronic liver disease (%) | 3 (2.3) | 70 (1.5) | 0.49 | |||
| Solid tumor (%) | 20 (15.2) | 318 (6.9) | <0.001 | 12.86 | 7.42–22.3 | <0.001 |
| Leukemia/Lymphoma (%) | 1 (0.8) | 80 (1.7) | 0.41 | |||
| Previous DVT (%) | 20 (15.2) | 58 (1.3) | <0.001 | 2.02 | 1.21–3.38 | 0.007 |
| Platelet disorders (%) | 3 (2.3) | 37 (0.8) | 0.082 | |||
| Connective tissue disease (%) | 1 (0.8) | 58 (1.3) | 0.61 | |||
| Cognitive impairment (%) | 12 (9.1) | 589 (12.8) | 0.21 | |||
| HIV infection (%) | 1 (0.8) | 41 (0.9) | 0.87 | |||
| Pharmacological therapy on admission | ||||||
| Low-dose ASA (%) | 22 (16.7) | 898 (19.5) | 0.42 | |||
| P2Y12 inhibitors (%) | 8 (6.1) | 257 (5.6) | 0.81 | |||
| Vitamin K antagonists (%) | 0 (0.0) | 122 (2.6) | 1.00 | |||
| DOACs (%) | 5 (3.8) | 318 (6.9) | 0.17 | |||
| Heparins (%) | 11 (8.3) | 474 (10.3) | 0.47 | |||
| Statins (%) | 6 (4.5) | 374 (8.1) | 0.14 |
| COVID-19 | no-COVID-19 | |||||
|---|---|---|---|---|---|---|
| Odds Ratio | 95% C.I. | p | Odds Ratio | 95% C.I. | p | |
| Total thrombotic events | ||||||
| Age | 1.01 | 1.00–1.03 | 0.030 | 1.01 | 1.00–1.03 | 0.029 |
| Previous DVT | 4.53 | 1.48–13.8 | 0.008 | 9.98 | 5.27–18.9 | <0.001 |
| Previous TIA/stroke | 4.36 | 2.39–7.95 | <0.001 | 3.28 | 1.96–5.51 | <0.001 |
| Peripheral artery disease | - | - | - | 4.55 | 2.66–7.78 | <0.001 |
| Heparin | - | - | - | 0.38 | 0.16–0.89 | 0.025 |
| Mild cognitive impairment | - | - | - | 0.49 | 0.30–0.80 | 0.005 |
| Arterial thrombosis | ||||||
| Age | 1.02 | 1.00–1.04 | 0.050 | 1.03 | 1.01–1.05 | 0.012 |
| Peripheral artery disease | 3.22 | 1.25–8.30 | 0.016 | 8.28 | 4.57–15.0 | <0.001 |
| Previous TIA/stroke | 7.06 | 3.58–13.9 | <0.001 | 6.10 | 3.47–10.7 | <0.001 |
| Mild cognitive impairment | - | - | - | 0.51 | 0.27–0.96 | 0.038 |
| Venous thrombosis | ||||||
| Previous DVT | 8.82 | 2.84–27.4 | <0.001 | 16.63 | 8.66–32.0 | <0.001 |
| Solid tumor | 3.11 | 1.28–7.55 | 0.012 | - | - | - |
| COPD | 2.21 | 1.09–4.45 | 0.027 | - | - | - |
| DOACs | - | - | - | 0.12 | 0.02–0.88 | 0.038 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Vita, A.; De Matteis, G.; d’Aiello, A.; Ravenna, S.E.; Liuzzo, G.; Lanza, G.A.; Massetti, M.; Crea, F.; Gasbarrini, A.; Franceschi, F.; et al. Incidence and Predictors of Thrombotic Complications in 4742 Patients with COVID-19 or Other Acute Infectious Respiratory Diseases: A Propensity Score-Matched Study. J. Clin. Med. 2021, 10, 4973. https://doi.org/10.3390/jcm10214973
De Vita A, De Matteis G, d’Aiello A, Ravenna SE, Liuzzo G, Lanza GA, Massetti M, Crea F, Gasbarrini A, Franceschi F, et al. Incidence and Predictors of Thrombotic Complications in 4742 Patients with COVID-19 or Other Acute Infectious Respiratory Diseases: A Propensity Score-Matched Study. Journal of Clinical Medicine. 2021; 10(21):4973. https://doi.org/10.3390/jcm10214973
Chicago/Turabian StyleDe Vita, Antonio, Giuseppe De Matteis, Alessia d’Aiello, Salvatore Emanuele Ravenna, Giovanna Liuzzo, Gaetano Antonio Lanza, Massimo Massetti, Filippo Crea, Antonio Gasbarrini, Francesco Franceschi, and et al. 2021. "Incidence and Predictors of Thrombotic Complications in 4742 Patients with COVID-19 or Other Acute Infectious Respiratory Diseases: A Propensity Score-Matched Study" Journal of Clinical Medicine 10, no. 21: 4973. https://doi.org/10.3390/jcm10214973







