Macular Structure–Function Relationships of All Retinal Layers in Primary Open-Angle Glaucoma Assessed by Microperimetry and 8 × 8 Posterior Pole Analysis of OCT
Abstract
:1. Introduction
2. Materials and Methods
- Caucasian race and age between 40 and 90 years;
- Diagnosis of primary open-angle glaucoma (POAG) with threat to fixation according to our clinical records;
- Refractive error of 3 dioptres or less of spherical equivalent and;
- Best corrected visual acuity of 0.5 or better in the Snellen scale.
- Intraocular pressure > 21 mmHg in at least three different days;
- Glaucomatous optic disc changes and/or characteristic glaucomatous SAP abnormalities, as judged by a glaucoma specialist (J.J.G.M.), and;
- Open anterior chamber angle in gonioscopy.
- Previous intraocular or refractive surgery, or laser procedure in the six months before the recruitment;
- History of ocular trauma;
- Use of ocular or systemic medications that could affect the VF;
- Presence of other ophthalmic or systemic significant diseases (eyelid, corneal, lens or retinal disease, diabetes) that could influence microperimetry or OCT results.
- Autorefractometry;
- Best Corrected Visual Acuity (BCVA), using decimal scale;
- Intraocular pressure estimation using applanation tonometry.
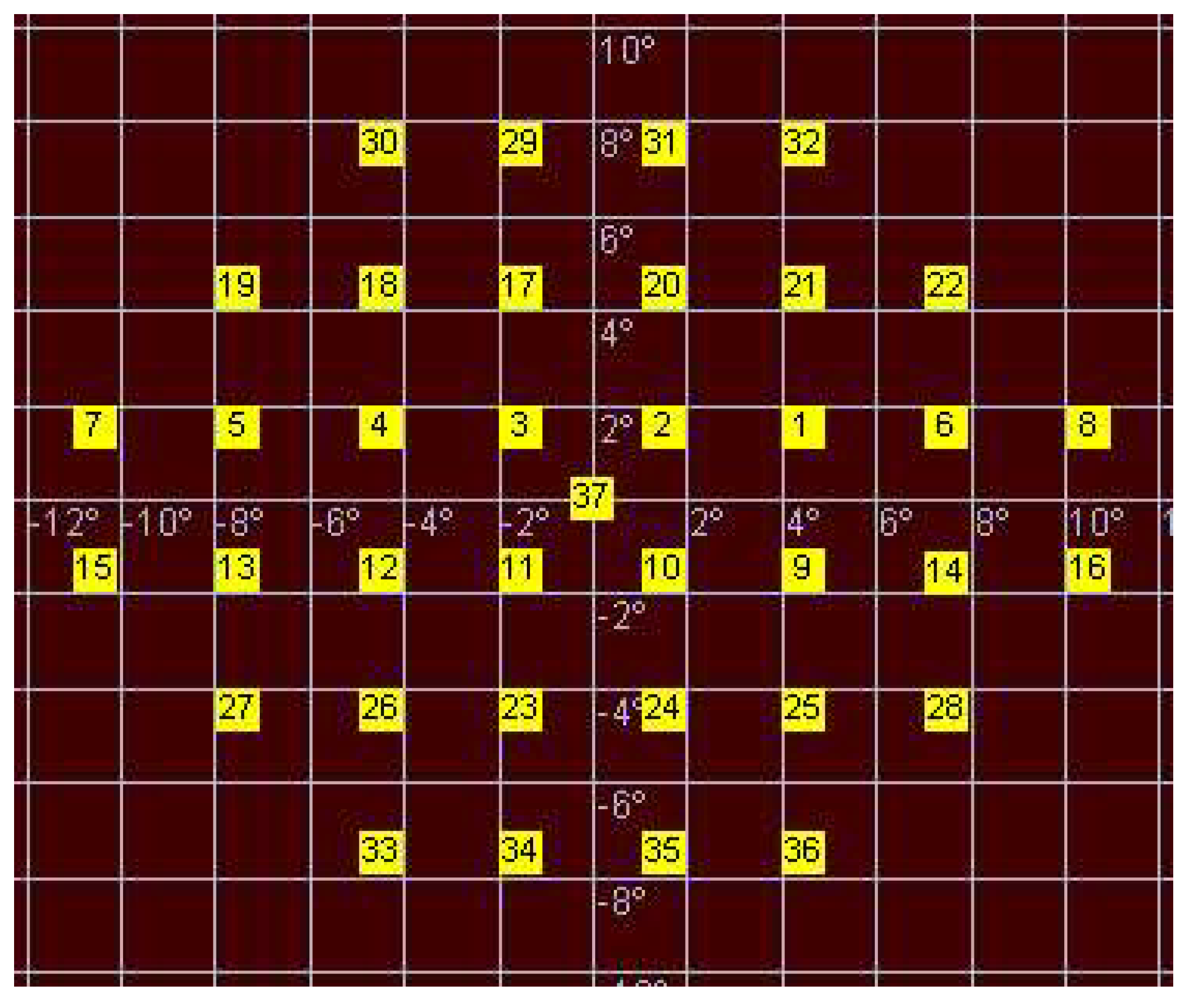
- The nearest stimuli from vertical and horizontal main axes were located at 1.5 degrees from these axes;
- The stimuli were separated 3 degrees from each.
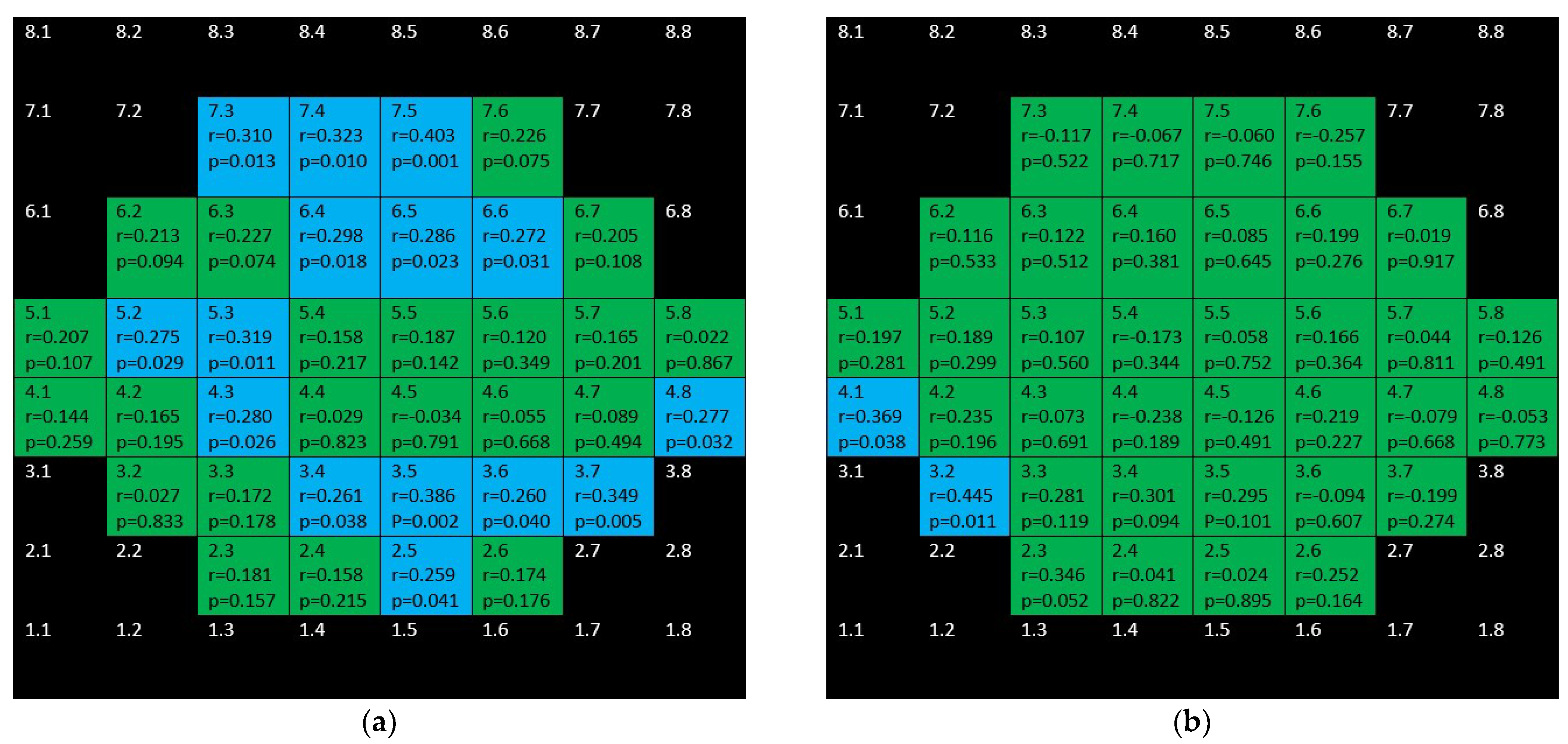
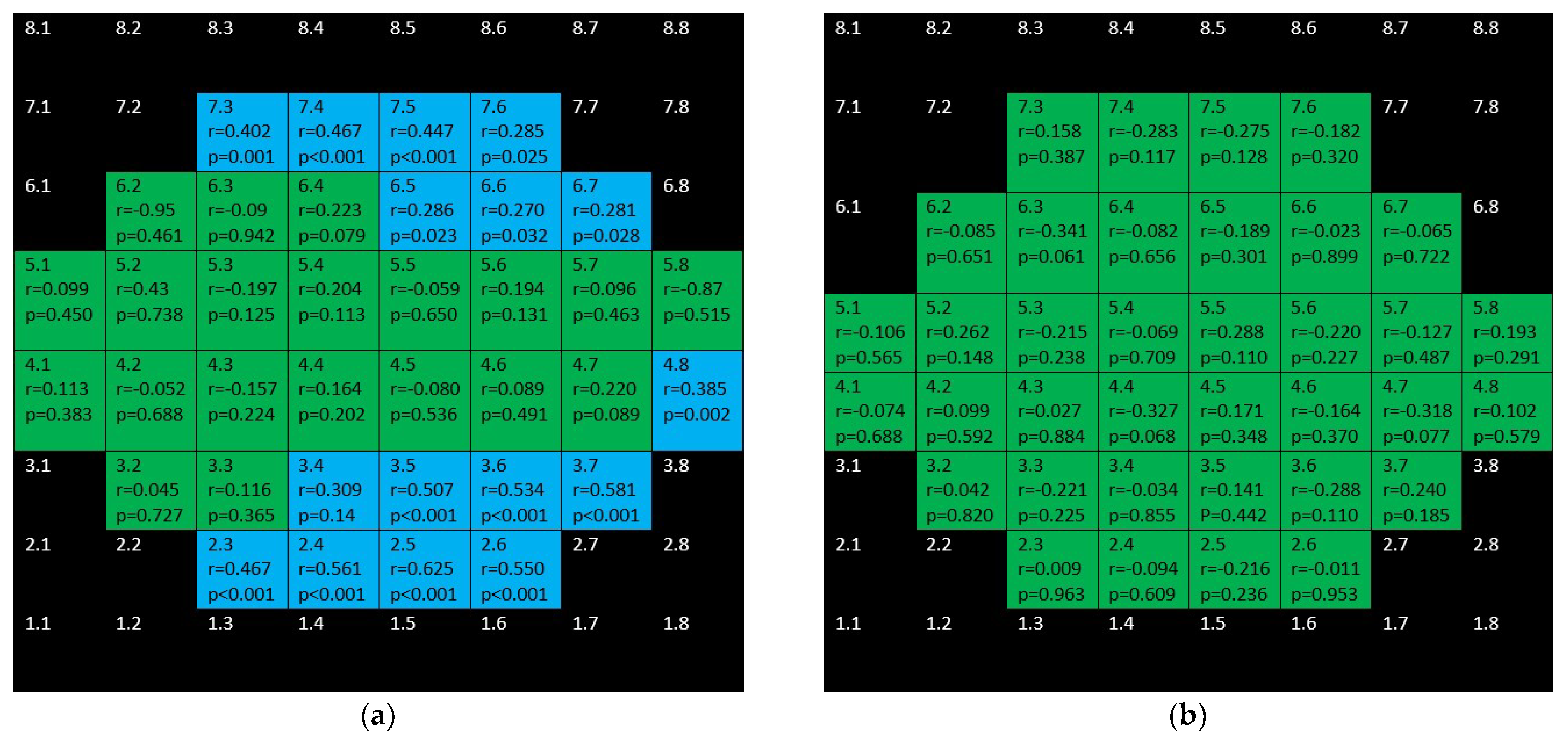
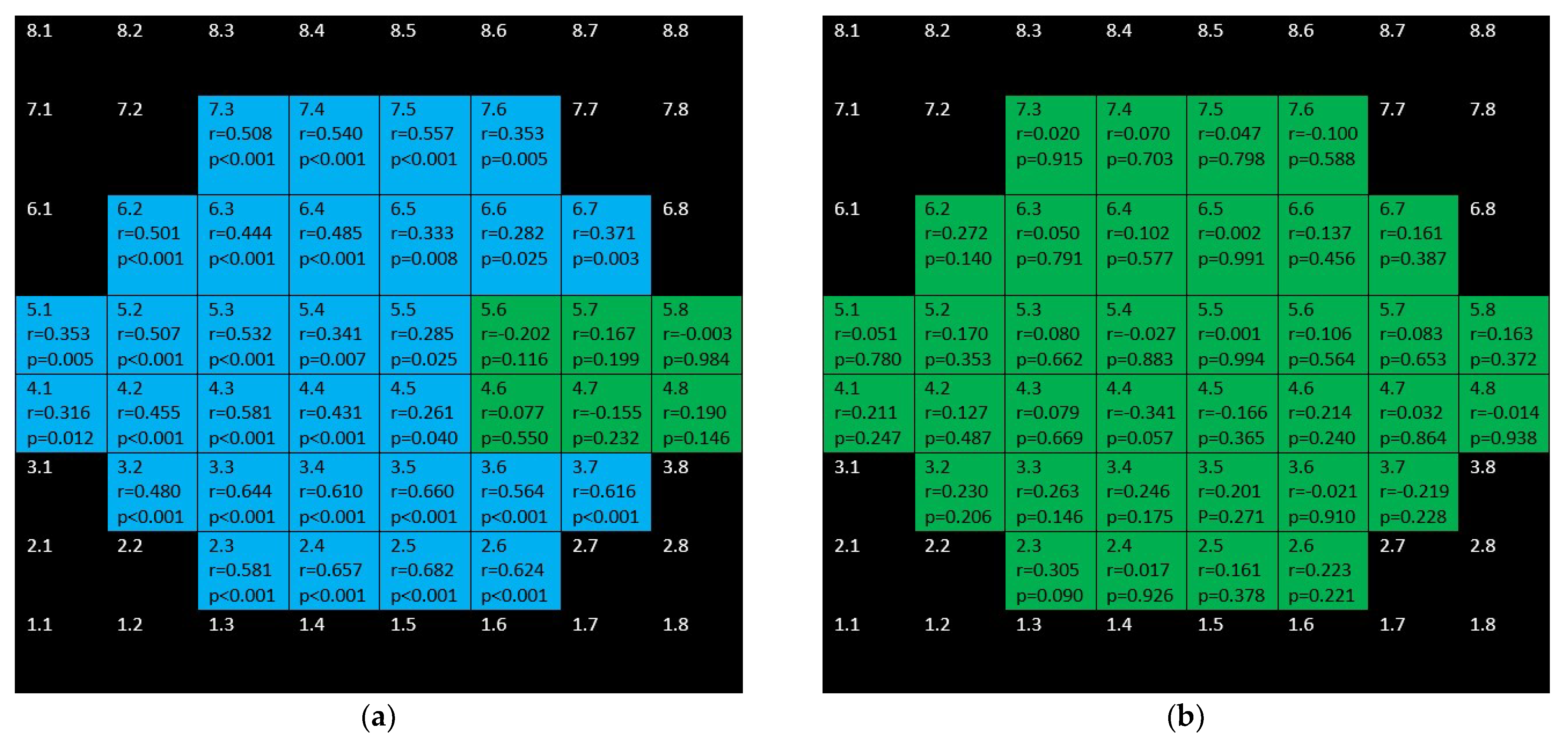
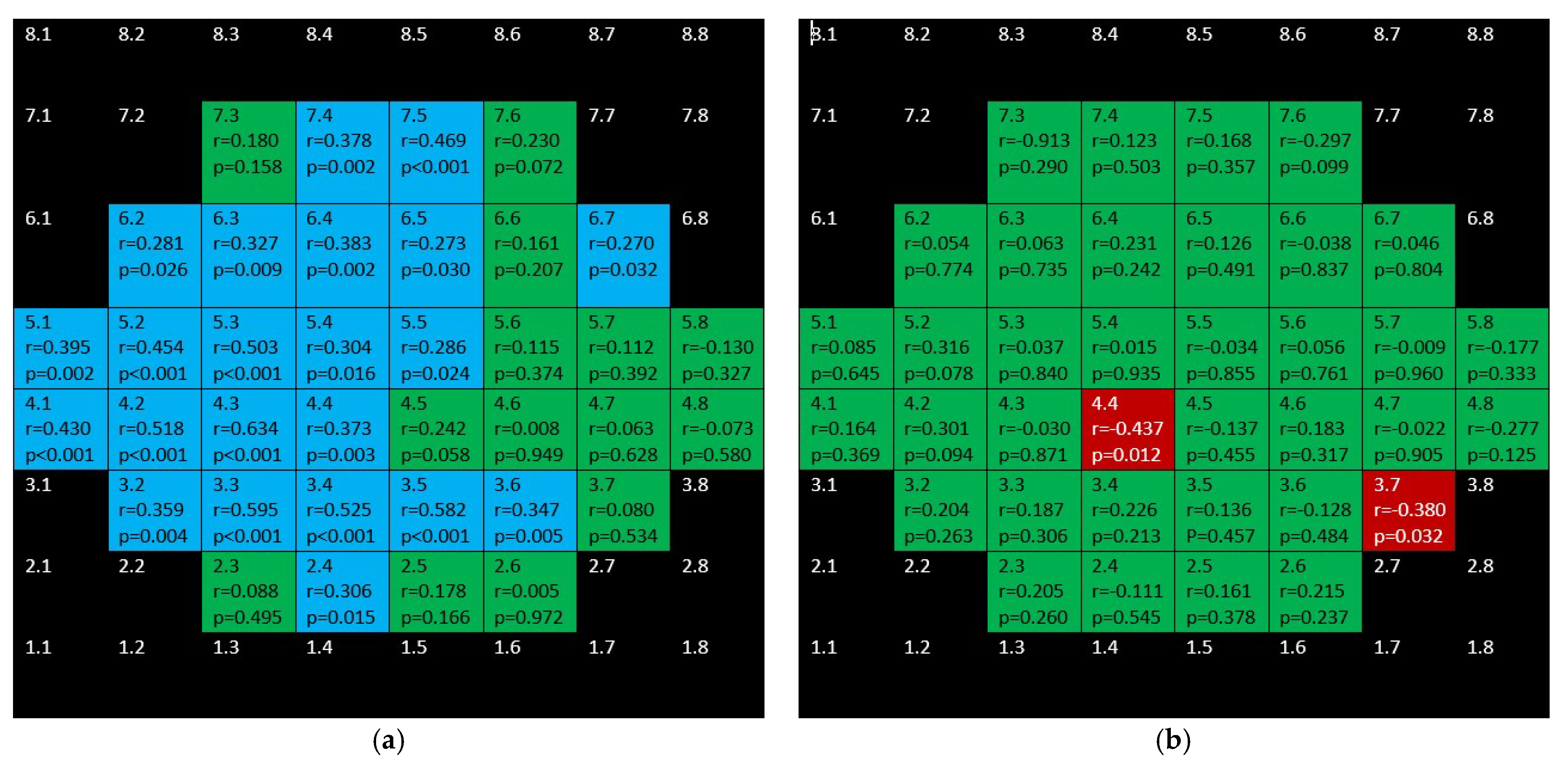
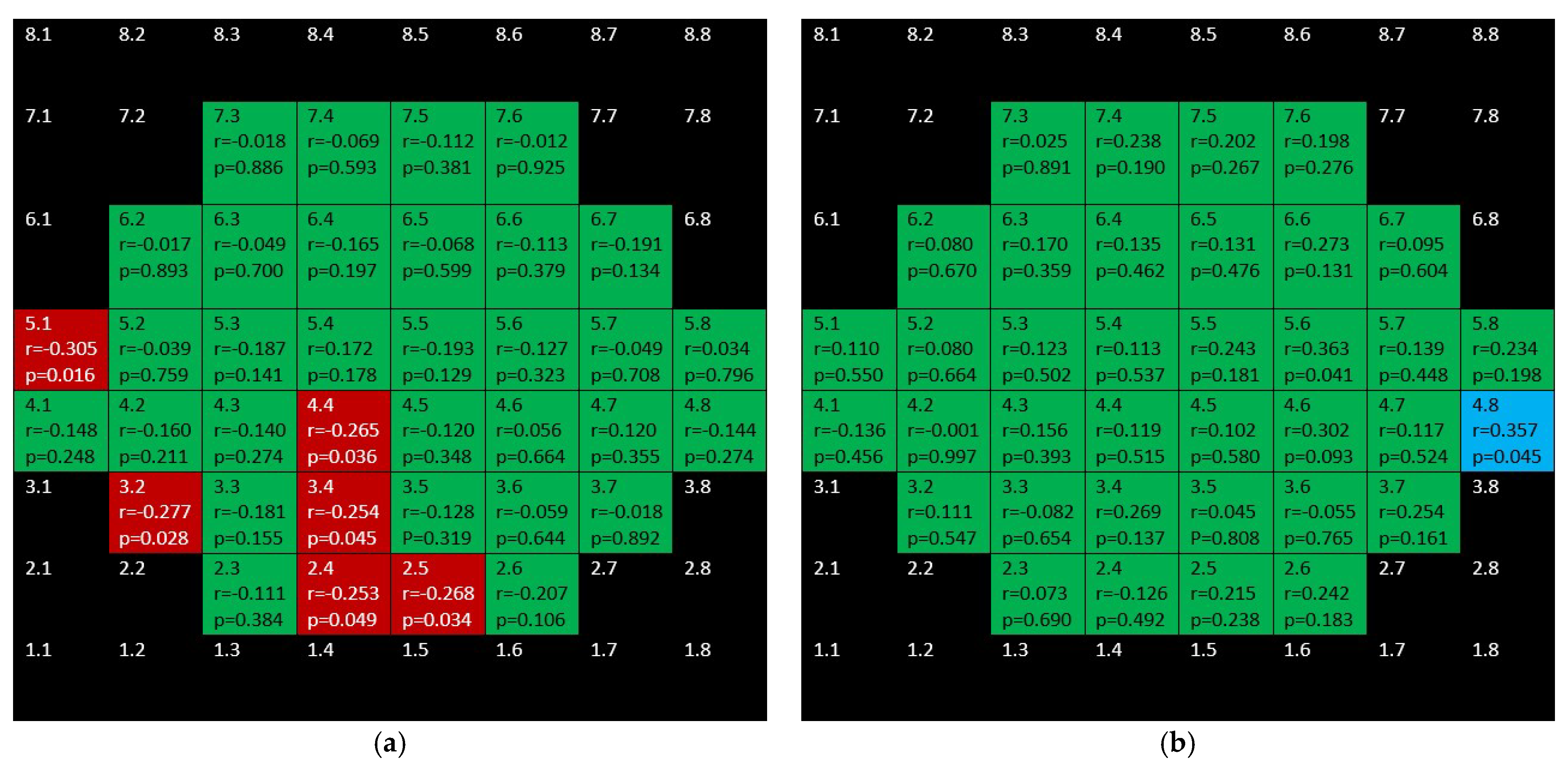
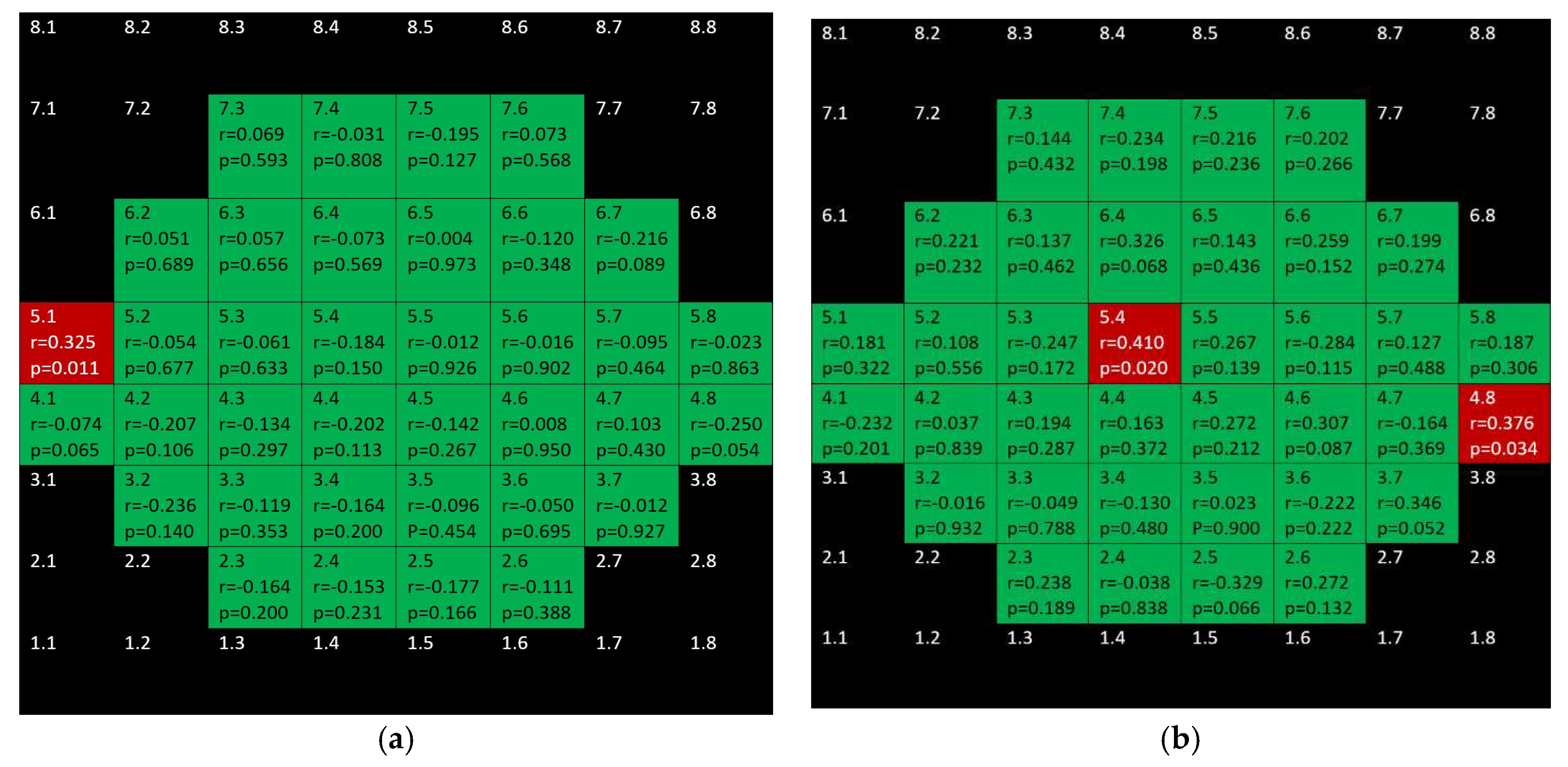
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The Pathophysiology and Treatment of Glaucoma. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Medina, J.; Del-Rio-Vellosillo, M.; Palazón-Cabanes, A.; Tudela-Molino, M.; Gómez-Molina, C.; Guardiola-Fernández, A.; Villegas-Pérez, M. Mapeo de los cambios de grosor en el glaucoma de las capas retinianas maculares segmentadas usando el programa de polo posterior de la tomografía de coherencia óptica de dominio espectral. Archivos de la Sociedad Española de Oftalmología 2018, 93, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Medina, J.J.; Del-Rio-Vellosillo, M.; Palazon-Cabanes, A.; Pinazo-Duran, M.D.; Zanon-Moreno, V.; Villegas-Perez, M.P. Glaucomatous Maculopathy: Thickness Differences on Inner and Outer Macular Layers between Ocular Hypertension and Early Primary Open-Angle Glaucoma Using 8 × 8 Posterior Pole Algorithm of SD-OCT. J. Clin. Med. 2020, 9, 1503. [Google Scholar] [CrossRef]
- Kitazawa, Y.; Yamamoto, T. Glaucomatous visual field defects: Their characteristics and how to detect them. Clin. Neurosci. (New York N.Y.) 1997, 4, 279–283. [Google Scholar]
- Torres, L.A.; Hatanaka, M. Correlating Structural and Functional Damage in Glaucoma. J. Glaucoma 2019, 28, 1079–1085. [Google Scholar] [CrossRef] [PubMed]
- Talib, M.; Jolly, J.; Boon, C.J.F. Measuring Central Retinal Sensitivity Using Microperimetry. Methods Mol. Biol. 2018, 1715, 339–349. [Google Scholar] [PubMed]
- Sato, S.; Hirooka, K.; Baba, T.; Tenkumo, K.; Nitta, E.; Shiraga, F. Correlation Between the Ganglion Cell-Inner Plexiform Layer Thickness Measured With Cirrus HD-OCT and Macular Visual Field Sensitivity Measured With Microperimetry. Investig. Opthalmol. Vis. Sci. 2013, 54, 3046–3051. [Google Scholar] [CrossRef] [Green Version]
- Rao, H.L.; Januwada, M.; Hussain, R.S.M.; Pillutla, L.N.; Begum, V.U.; Chaitanya, A.; Senthil, S.; Garudadri, C.S. Comparing the Structure–Function Relationship at the Macula With Standard Automated Perimetry and Microperimetry. Investig. Opthalmol. Vis. Sci. 2015, 56, 8063–8068. [Google Scholar] [CrossRef] [Green Version]
- Akar, S.; Tekeli, O.; Ozturker, Z.K. Macular Integrity Assessment Microperimeter, Humphrey Field Analyzer and Optical Coherence Tomography in Glaucoma Practice: A Correlation Study. Can. J. Ophthalmol. 2021, S0008418221002556. [Google Scholar] [CrossRef]
- Zabel, K.; Zabel, P.; Kaluzna, M.; Lamkowski, A.; Jaworski, D.; Wietlicka-Piszcz, M.; Kaluzny, J.J. Correlation of retinal sensitivity in microperimetry with vascular density in optical coherence tomography angiography in primary open-angle glaucoma. PLoS ONE 2020, 15, e0235571. [Google Scholar] [CrossRef]
- Zhang, L.; Drance, S.M.; Douglas, G.R. Automated perimetry in detecting threats to fixation. Ophthalmology 1997, 104, 1918–1920. [Google Scholar] [CrossRef]
- Lujan, B.J.; Roorda, A.; Croskrey, J.A.; Dubis, A.M.; Cooper, R.F.; Bayabo, J.K.; Duncan, J.L.; Antony, B.J.; Carroll, J. Directional optical coherence tomography provides accurate outer nuclear layer and henle fibre layer measurements. Retina 2015, 35, 1511–1520. [Google Scholar] [CrossRef] [Green Version]
- Orzalesi, N.; Miglior, S.; Lonati, C.; Rosetti, L. Microperimetry of localized retinal nerve fiber layer defects. Vis. Res. 1998, 38, 763–771. [Google Scholar] [CrossRef] [Green Version]
- Lima, V.C.; Prata, T.; De Moraes, C.G.V.; Kim, J.; Seiple, W.; Rosen, R.B.; Liebmann, J.M.; Ritch, R. A comparison between microperimetry and standard achromatic perimetry of the central visual field in eyes with glaucomatous paracentral visual-field defects. Br. J. Ophthalmol. 2009, 94, 64–67. [Google Scholar] [CrossRef]
- Vidal-Sanz, M.; Valiente-Soriano, F.J.; Ortín-Martínez, A.; Nadal-Nicolás, F.M.; Jiménez-López, M.; Salinas-Navarro, M.; Alarcón-Martínez, L.; García-Ayuso, D.; Avilés-Trigueros, M.; Agudo-Barriuso, M.; et al. Retinal neurodegeneration in experimental glaucoma. Prog. Brain Res. 2015, 220, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Ramakrishnan, H.; Viswanathan, S.; Akopian, A.; Bloomfield, S.A. Neuroprotection of the Inner Retina Also Prevents Secondary Outer Retinal Pathology in a Mouse Model of Glaucoma. Investig. Opthalmol. Vis. Sci. 2021, 62, 35. [Google Scholar] [CrossRef] [PubMed]
- Membrey, W.L.; Poinoosawmy, D.P.; Bunce, C.; Fitzke, F.W.; A Hitchings, R. Comparison of visual field progression in patients with normal pressure glaucoma between eyes with and without visual field loss that threatens fixation. Br. J. Ophthalmol. 2000, 84, 1154–1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKean-Cowdin, R.; Wang, Y.; Wu, J.; Azen, S.P.; Varma, R. Impact of Visual Field Loss on Health-Related Quality of Life in Glaucoma: The Los Angeles Latino Eye Study. Ophthalmology 2008, 115, 941–948.e1. [Google Scholar] [CrossRef] [Green Version]
- Sawada, H.; Fukuchi, T.; Abe, H. Evaluation of the relationship between quality of vision and the visual function index in Japanese glaucoma patients. Graefe’s Arch. Clin. Exp. Ophthalmol. 2011, 249, 1721–1727. [Google Scholar] [CrossRef]
- Peters, D.; Bengtsson, B.; Heijl, A. Threat to Fixation at Diagnosis and Lifetime Risk of Visual Impairment in Open-Angle Glaucoma. Ophthalmology 2015, 122, 1034–1039. [Google Scholar] [CrossRef] [PubMed]
- Swanson, W.H. Stimulus Size for Perimetry in Patients With Glaucoma. Investig. Opthalmol. Vis. Sci. 2013, 54, 3984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, L.; Wu, Z.; Guymer, R.H.; Anderson, A.J. Investigating the discrepancy between MAIA and MP-1 microperimetry results. Ophthalmic Physiol. Opt. 2021, 41, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- Bland, J.M.; Altman, D. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 327, 307–310. [Google Scholar] [CrossRef]
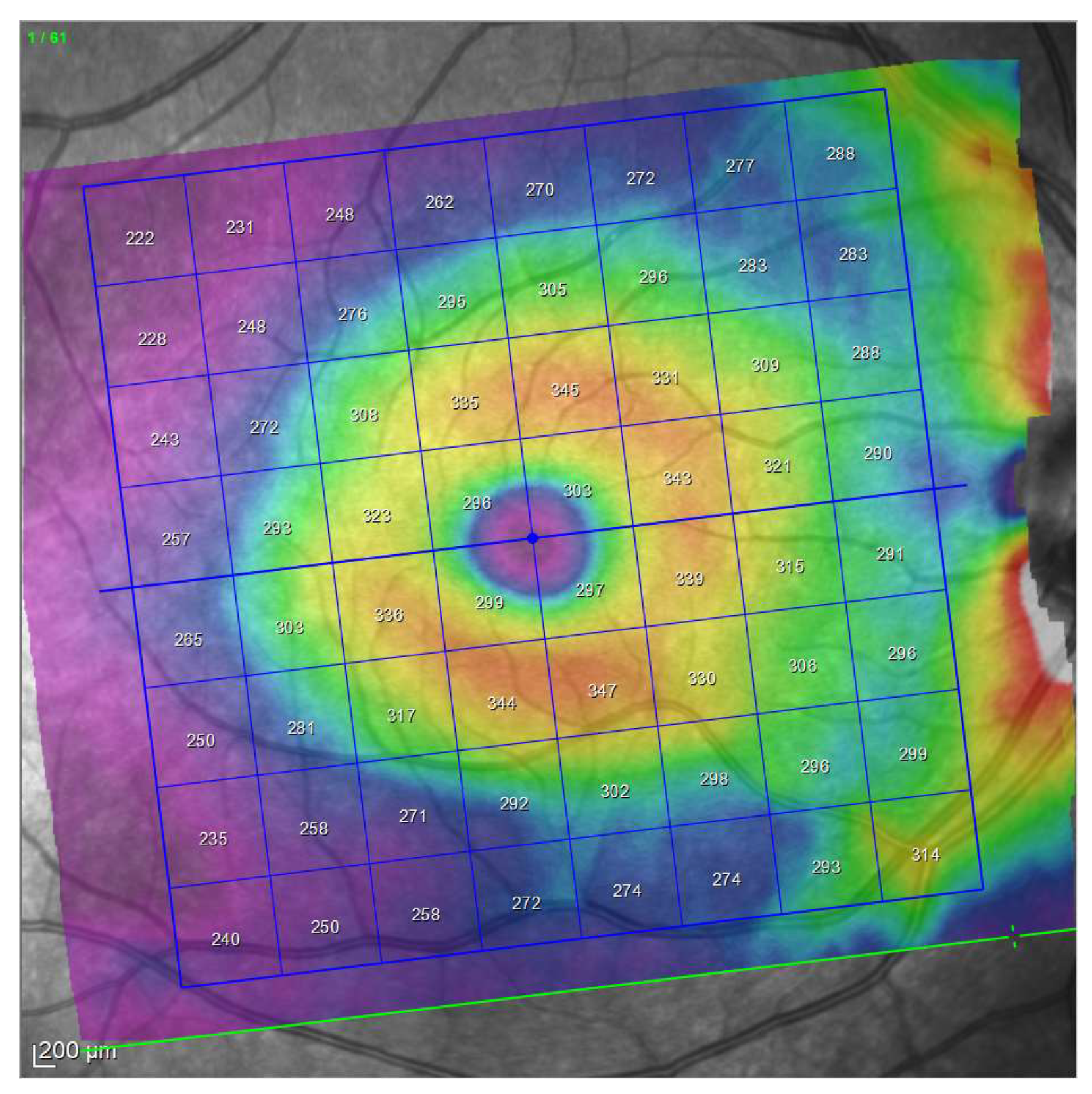
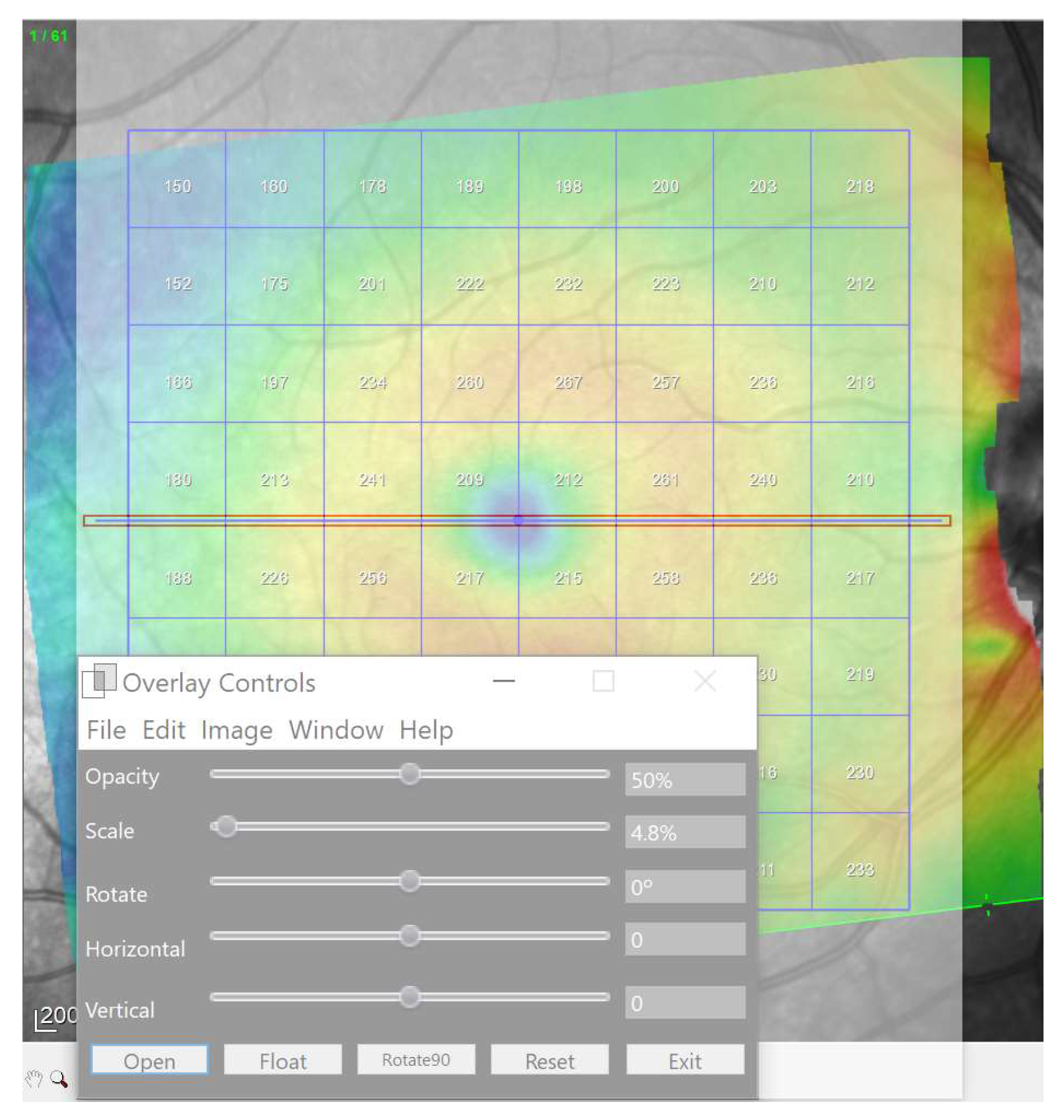
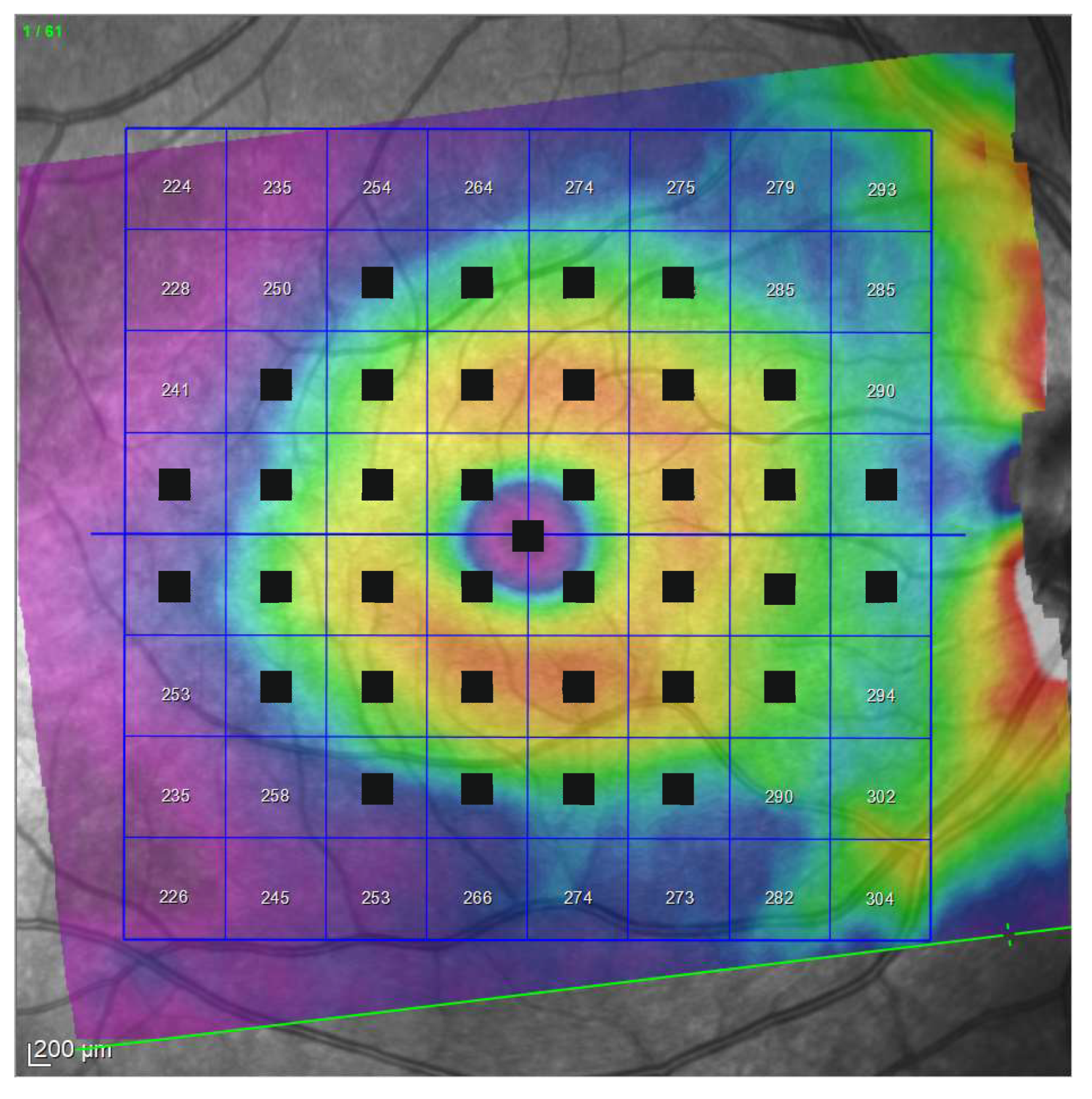
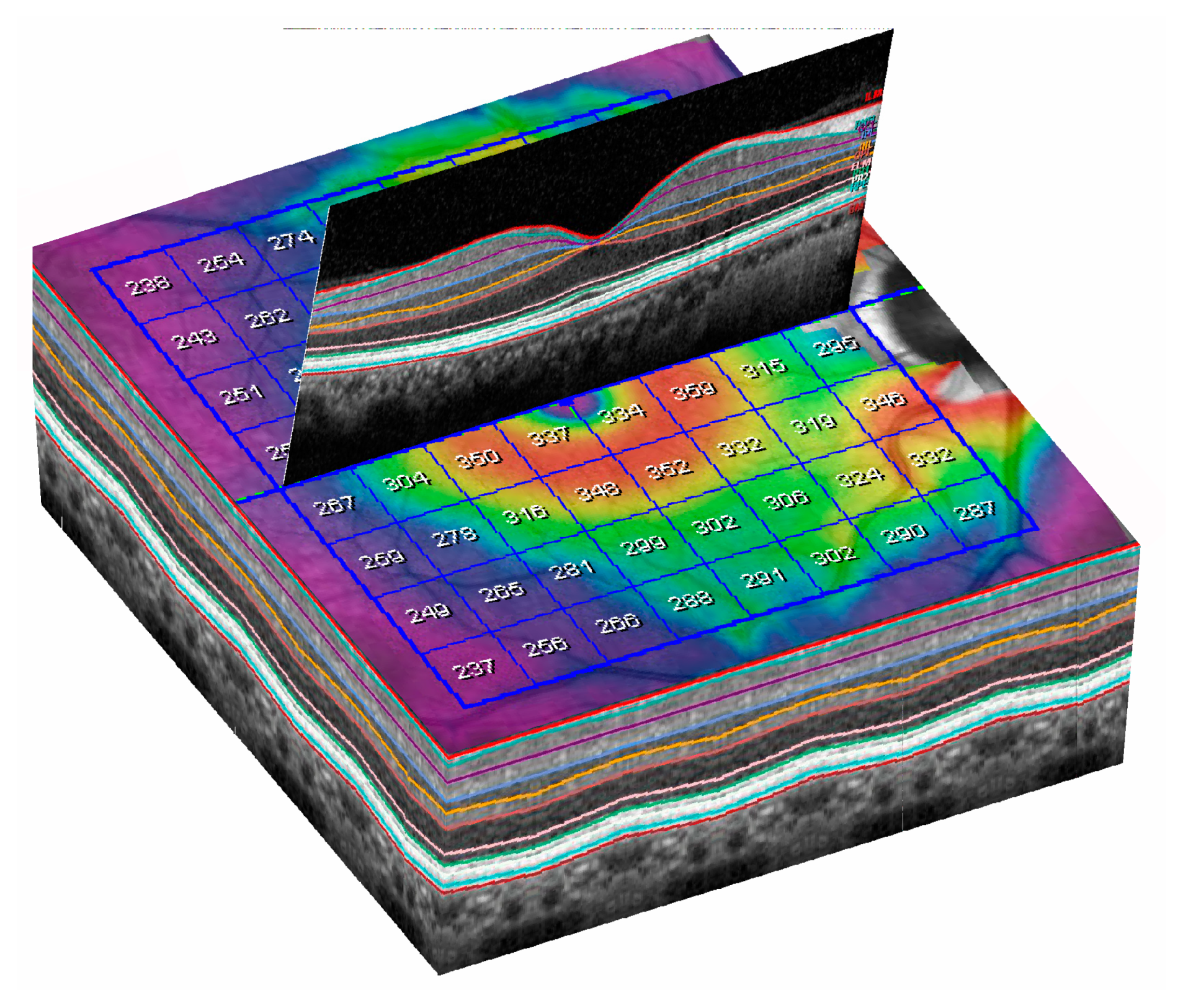



| Demographic and Clinical Data | Glaucoma Group | Control Group | Significance (Test) |
|---|---|---|---|
| Eyes and laterality | n = 60 Right eyes = 30 Left eyes = 30 | n = 31 Right eyes = 15 Left eyes = 16 | 1 (Fisher’s test) |
| Age (years) | 73 (15) | 67 (31) | 0.07 (Mann–Whitney test) |
| Sex | 29 men 32 women | 9 men 22 women | 0.11 (Fisher’s test) |
| BCVA | 0.9 (0.3) | 1 (1.9) | 0.07 (Mann–Whitney test) |
| IOP (mmHg) | 20 (7) | 17 (4) | 0.004 (Mann–Whitney test) |
| Spherical equivalent | 0.25 (4) | 1 (2) | 0.04 (Mann–Whitney test) |
| Vertical cupping | 0.8 (0.3) | 0.4 (0.2) | <0.001 (Mann–Whitney test) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-Medina, J.J.; Rotolo, M.; Rubio-Velazquez, E.; Pinazo-Duran, M.D.; del-Rio-Vellosillo, M. Macular Structure–Function Relationships of All Retinal Layers in Primary Open-Angle Glaucoma Assessed by Microperimetry and 8 × 8 Posterior Pole Analysis of OCT. J. Clin. Med. 2021, 10, 5009. https://doi.org/10.3390/jcm10215009
Garcia-Medina JJ, Rotolo M, Rubio-Velazquez E, Pinazo-Duran MD, del-Rio-Vellosillo M. Macular Structure–Function Relationships of All Retinal Layers in Primary Open-Angle Glaucoma Assessed by Microperimetry and 8 × 8 Posterior Pole Analysis of OCT. Journal of Clinical Medicine. 2021; 10(21):5009. https://doi.org/10.3390/jcm10215009
Chicago/Turabian StyleGarcia-Medina, Jose Javier, Maurilia Rotolo, Elena Rubio-Velazquez, Maria Dolores Pinazo-Duran, and Monica del-Rio-Vellosillo. 2021. "Macular Structure–Function Relationships of All Retinal Layers in Primary Open-Angle Glaucoma Assessed by Microperimetry and 8 × 8 Posterior Pole Analysis of OCT" Journal of Clinical Medicine 10, no. 21: 5009. https://doi.org/10.3390/jcm10215009







