Feasibility and Accuracy of the Automated Software for Dynamic Quantification of Left Ventricular and Atrial Volumes and Function in a Large Unselected Population
Abstract
:1. Introduction
2. Materials and Methods
2.1. Two-Dimensional Echocardiography
Automated Three-Dimensional Echocardiography
2.2. CMR Protocol
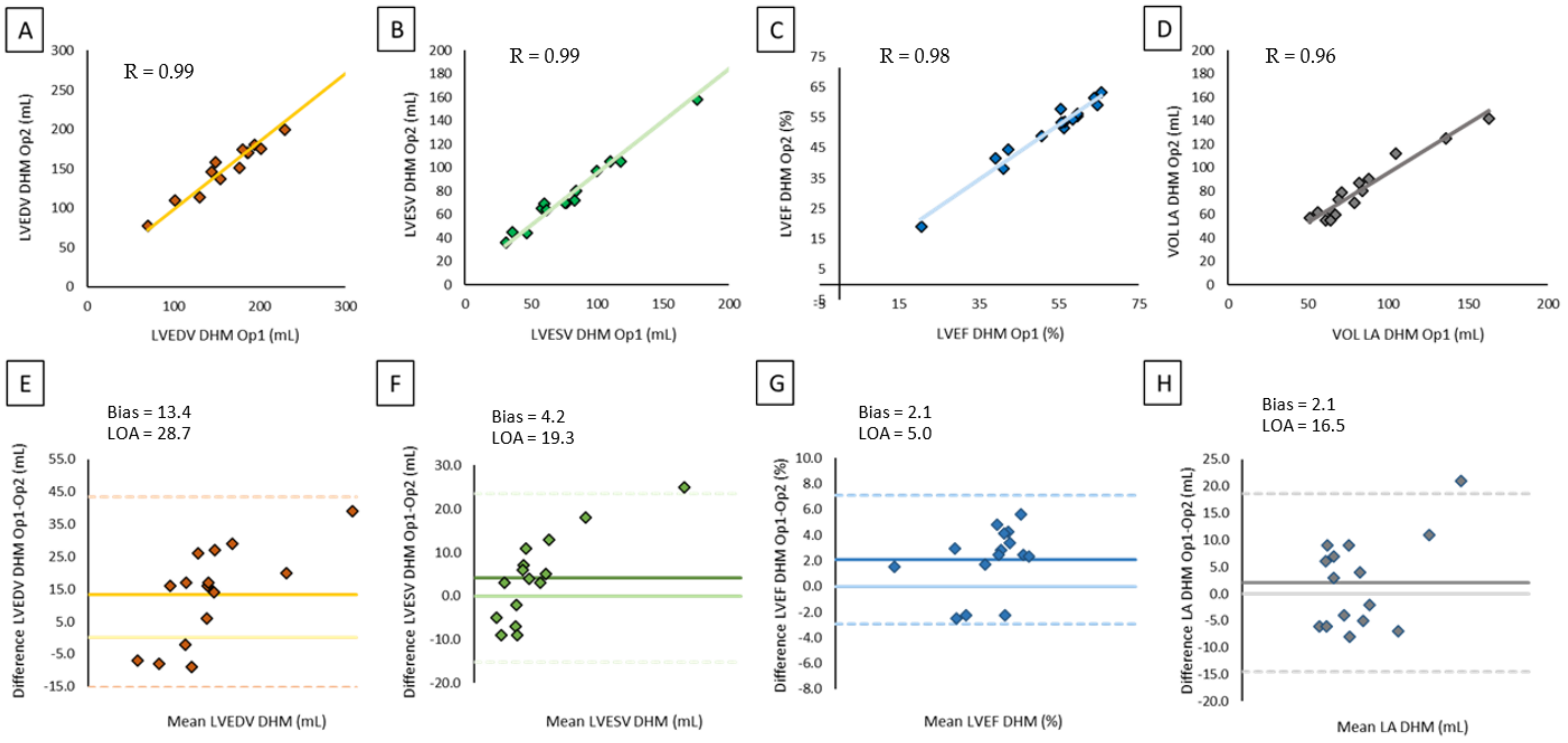
2.3. Reproducibility
2.4. Statistical Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsang, W.; Salgo, I.S.; Medvedofsky, D.; Takeuchi, M.; Prater, D.; Weinert, L.; Yamat, M.; Mor-Avi, V.; Patel, A.R.; Lang, R.M. Transthoracic 3D echocardiographic left heart chamber quantification using an automated adaptive analytics algorithm. JACC Cardiovasc. Imaging 2016, 9, 769–782. [Google Scholar] [CrossRef]
- Medvedofsky, D.; Mor-Avi, V.; Amzulescu, M.; Fernandez-Golfin, C.; Hinojar, R.; Monaghan, M.J.; Otani, K.; Reiken, J.; Takeuchi, M.; Tsang, W.; et al. Three-dimensional echocardiographic quantification of the left-heart chambers using an automated adaptive analytics algorithm: Multicenter validation study. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Narang, A.; Mor-Avi, V.; Prado, A.; Volpato, V.; Prater, D.; Tamborini, G.; Fusini, L.; Pepi, M.; Goyal, N.; Addetia, K.; et al. Machine learning based automated dynamic quantification of left heart chamber volumes. Eur. Heart J. Cardiovasc. Imaging 2018, 20, 541–549. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39. [Google Scholar] [CrossRef] [Green Version]
- Volpato, V.; Mor-Avi, V.; Narang, A.; Prater, D.; Gonçalves, A.; Tamborini, G.; Fusini, L.; Pepi, M.; Patel, A.R.; Lang, R.M. Automated, machine learning-based, 3D echocardiographic quantification of left ventricular mass. Echocardiography 2019, 36, 312–319. [Google Scholar] [CrossRef]
- Schulz-Menger, J.; Bluemke, D.A.; Bremerich, J.; Flamm, S.D.; Fogel, M.A.; Friedrich, M.G.; Kim, R.J.; Von Knobelsdorff-Brenkenhoff, F.; Kramer, C.M.; Pennell, D.J.; et al. Standardized image interpretation and post-processing in cardiovascular magnetic resonance—2020 update. J. Cardiovasc. Magn. Reson. 2020, 22, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Tamborini, G.; Piazzese, C.; Lang, R.M.; Muratori, M.; Chiorino, E.; Mapelli, M.; Fusini, L.; Ali, S.G.; Gripari, P.; Pontone, G.; et al. Feasibility and Accuracy of Automated Software for Transthoracic Three-Dimensional Left Ventricular Volume and Function Analysis: Comparisons with Two-Dimensional Echocardiography, Three-Dimensional Transthoracic Manual Method, and Cardiac Magnetic Resonance Imaging. J. Am. Soc. Echocardiogr. 2017, 30, 1049–1058. [Google Scholar] [CrossRef]
- Otani, K.; Nakazono, A.; Salgo, I.S.; Lang, R.M.; Takeuchi, M. Three-Dimensional Echocardiographic Assessment of Left Heart Chamber Size and Function with Fully Automated Quantification Software in Patients with Atrial Fibrillation. J. Am. Soc. Echocardiogr. 2016, 29, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-A.; Lee, S.-C.; Kim, E.-Y.; Hahm, S.-H.; Jang, S.Y.; Park, S.-J.; Choi, J.-O.; Park, S.W.; Choe, Y.H.; Oh, J.K. Feasibility of Single-Beat Full-Volume Capture Real-Time Three-Dimensional Echocardiography and Auto-Contouring Algorithm for Quantification of Left Ventricular Volume: Validation with Cardiac Magnetic Resonance Imaging. J. Am. Soc. Echocardiogr. 2011, 24, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Feng, H.; Ni, L.; Wang, H.; Gao, D. Realization of fully automated quantification of left ventricular volumes and systolic function using transthoracic 3D echocardiography. Cardiovasc. Ultrasound 2018, 16, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asch, F.M.; Poilvert, N.; Abraham, T.; Jankowski, M.; Cleve, J.; Adams, M.; Romano, N.; Hong, H.; Mor-Avi, V.; Martin, R.P.; et al. Automated Echocardiographic Quantification of Left Ventricular Ejection Fraction Without Volume Measurements Using a Machine Learning Algorithm Mimicking a Human Expert. Circ. Cardiovasc. Imaging 2019, 12, e009303. [Google Scholar] [CrossRef]
- Zeidan, Z.; Erbel, R.; Barkhausen, J.; Hunold, P.; Bartel, T.; Buck, T. Analysis of global systolic and diastolic left ventricular performance using volume-time curves by real-time three-dimensional echocardiography. J. Am. Soc. Echocardiogr. 2003, 16, 29–37. [Google Scholar] [CrossRef]
- Levy, F.; Schouver, E.D.; Iacuzio, L.; Civaia, F.; Rusek, S.; Dommerc, C.; Marechaux, S.; Dor, V.; Tribouilloy, C.; Dreyfus, G. Performance of new automated transthoracic three-dimensional echocardiographic software for left ventricular volumes and function assessment in routine clinical practice: Comparison with 3 Tesla cardiac magnetic resonance. Arch. Cardiovasc. Dis. 2017, 110, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Mor-Avi, V.; Jenkins, C.; Kühl, H.P.; Nesser, H.-J.; Marwick, T.; Franke, A.; Ebner, C.; Freed, B.H.; Steringer-Mascherbauer, R.; Pollard, H.; et al. Real-Time 3-Dimensional Echocardiographic Quantification of Left Ventricular Volumes: Multicenter Study for Validation With Magnetic Resonance Imaging and Investigation of Sources of Error. JACC Cardiovasc. Imaging 2008, 1, 413–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorosz, J.L.; Lezotte, D.C.; Weitzenkamp, D.A.; Allen, L.A.; Salcedo, E.E. Performance of 3-dimensional echocardiography in measuring left ventricular volumes and ejection fraction: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2012, 59, 1799–1808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkins, C.; Moir, S.; Chan, J.; Rakhit, D.; Haluska, B.; Marwick, T.H. Left ventricular volume measurement with echocardiography: A comparison of left ventricular opacification, three-dimensional echocardiography, or both with magnetic resonance imaging. Eur. Heart J. 2008, 30, 98–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabharwal, N.; Cemin, R.; Rajan, K.; Hickman, M.; Lahiri, A.; Senior, R. Usefulness of left atrial volume as a predictor of mortality in patients with ischemic cardiomyopathy. Am. J. Cardiol. 2004, 94, 760–763. [Google Scholar] [CrossRef] [PubMed]
- Ristow, B.; Ali, S.; Whooley, M.A.; Schiller, N.B. Usefulness of Left Atrial Volume Index to Predict Heart Failure Hospitalization and Mortality in Ambulatory Patients With Coronary Heart Disease and Comparison to Left Ventricular Ejection Fraction (from the Heart and Soul Study). Am. J. Cardiol. 2008, 102, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, T.; Dwivedi, G.; Hayat, S.A.; Majumdar, S.; Senior, R. Independent value of left atrial volume index for the prediction of mortality in patients with suspected heart failure referred from the community. Heart 2009, 95, 1172–1178. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.-I.; Shim, C.Y.; Kim, Y.J.; Kim, S.-A.; Rhee, S.J.; Choi, E.-Y.; Choi, D.; Jang, Y.; Chung, N.; Cho, S.-Y.; et al. Left Atrial Volume Index: A Predictor of Adverse Outcome in Patients With Hypertrophic Cardiomyopathy. J. Am. Soc. Echocardiogr. 2009, 22, 1338–1343. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.A.; Lavie, C.J.; Milani, R.V.; Ventura, H.O. Left Atrial Volume Index Predictive of Mortality Independent of Left Ventricular Geometry in a Large Clinical Cohort With Preserved Ejection Fraction. Mayo Clin. Proc. 2011, 86, 730–737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mor-Avi, V.; Yodwut, C.; Jenkins, C.; Kühl, H.; Nesser, H.J.; Marwick, T.H.; Franke, A.; Weinert, L.; Niel, J.; Steringer-Mascherbauer, R.; et al. Real-time 3D echocardiographic quantification of left atrial volume: Multicenter study for validation with CMR. JACC Cardiovasc. Imaging 2012, 5, 769–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkins, C.; Bricknell, K.; Hanekom, L.; Marwick, T.H. Reproducibility and accuracy of echocardiographic measurements of left ventricular parameters using real-time three-dimensional echocardiography. J. Am. Coll. Cardiol. 2004, 44, 878–886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobs, L.D.; Salgo, I.S.; Goonewardena, S.; Weinert, L.; Coon, P.; Bardo, D.; Gerard, O.; Allain, P.; Zamorano, J.L.; de Isla, L.P.; et al. Rapid online quantification of left ventricular volume from real-time three-dimensional echocardiographic data. Eur. Heart J. 2006, 27, 460–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
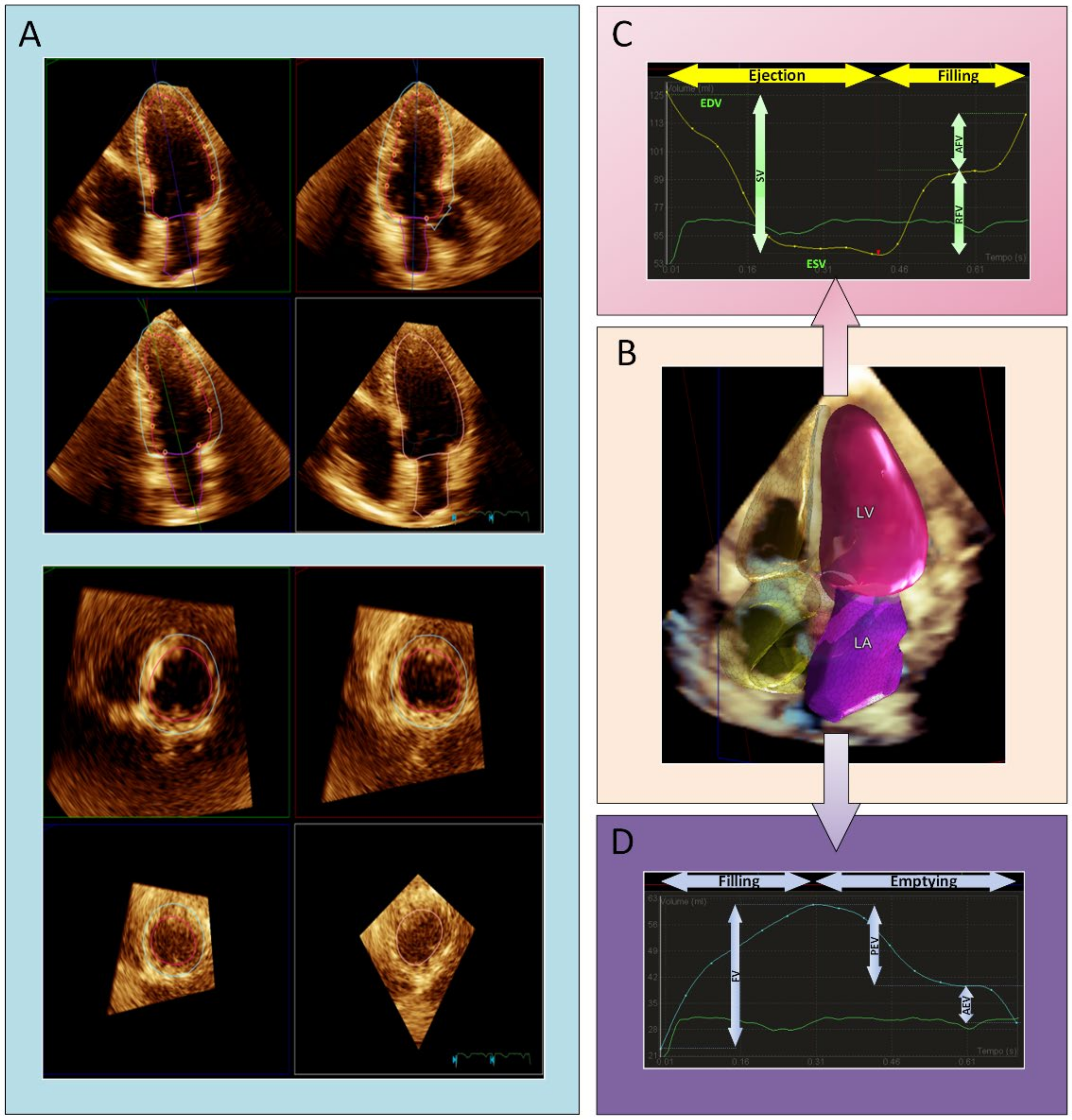
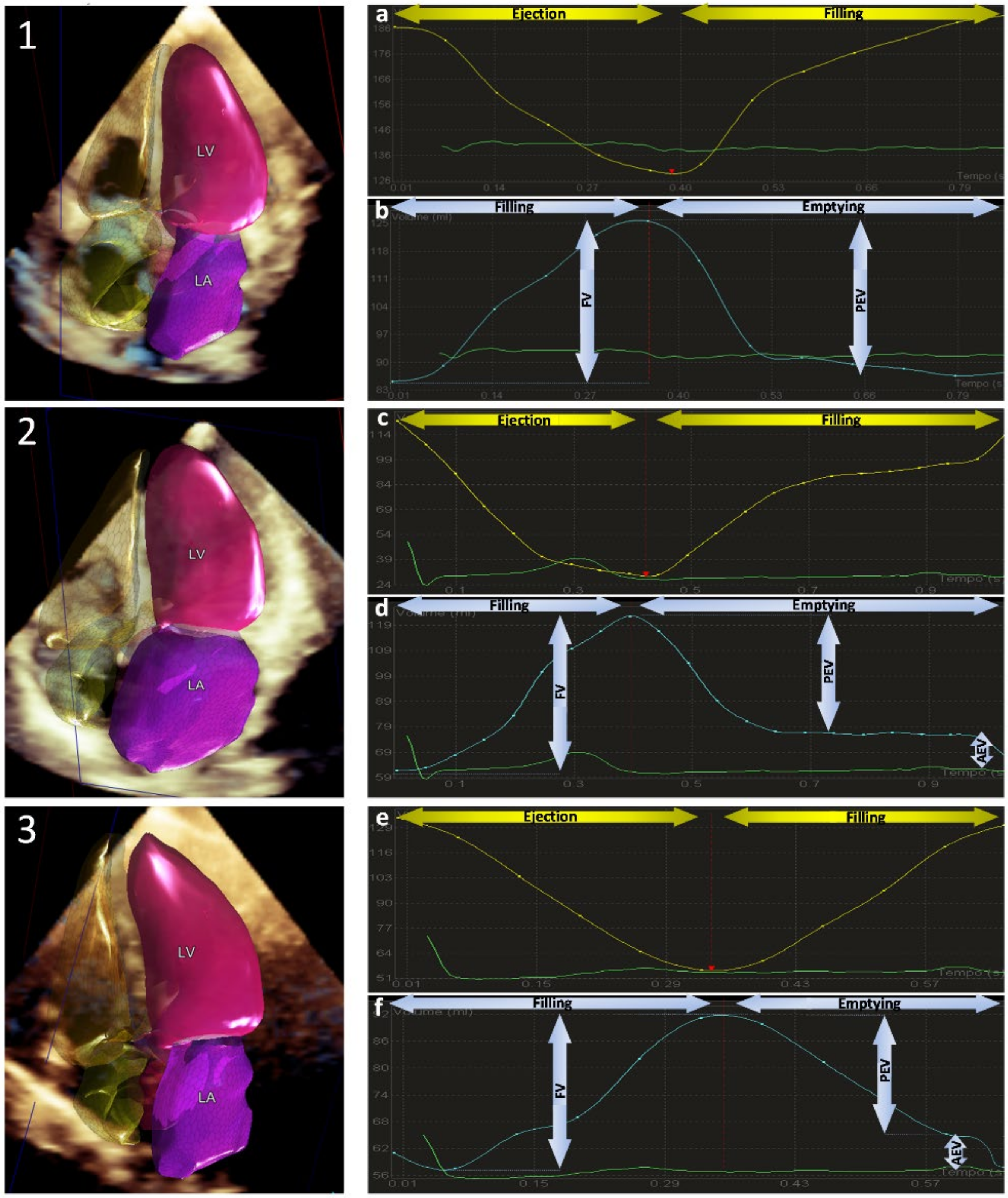
| 2D TTE | CMR | |
|---|---|---|
| Number of patients (n) | 600 | 140 |
| Age (y) | 63 ± 14 | 59 ± 14 |
| Female, n (%) | 169 (28%) | 34 (24%) |
| BSA (n) | 1.87 ± 0.2 | 1.88 ± 0.20 |
| Normal subjects, n (%) | 40/600 (7%) | 3/140 (2%) |
| Atrial fibrillation, n (%) | 73/600 (12%) | 6/140 (4%) |
| Valvular disease, n (%) | 312/600 (52%) | 80/140 (57%) |
| Coronary artery disease, n (%) | 58/600 (10%) | 6/140 (4%) |
| Dilated cardiomyopathy, n (%) | 113/600 (19%) | 35/140 (25%) |
| Miscellaneous, n (%) | 25/600 (4%) | 10/140 (7%) |
| DHM Quality | Adjustment | Time Required (sec) | ||||
|---|---|---|---|---|---|---|
| Feasibility | Good | Suboptimal | Minor | Major | ||
| Total of patients (n, %) | 522/600 (87%) | 327/522 (62%) | 195/522 (28%) | 149/522 (29%) | 38/522 (6%) | 41.3 ± 12.5 |
| Normal subjects (n, %) | 39/40 (97%) | 23/39 (57%) | 16/39 (40%) | 9/39 (21%) | 1/39 (3%) | 31.2 ± 7.9 |
| Atrial fibrillation (n, %) | 59/73 (81%) * | 28/59 (47%) | 31/59 (53%) | 15/59 (25%) | 6/59 (10%) | 45.7 ± 14.1 |
| Valvular disease (n, %) | 271/312 (87%) | 120/271 (%) | 151/271 (%) | 65/271 (24%) | 16/271 (6%) | 44.5 ± 8.2 |
| Coronary artery disease (n, %) | 47/58 (81%) * | 26/47 (46%) | 21/47 (37%) | 16/47 (34%) | 5/47 (11%) | 35.2 ± 10.2 |
| Dilated cardiomyopathy (n, %) | 98/113 (87%) | 56/98 (57%) | 42/98 (43%) | 51/98 (52%) | 12/98 (12%) | 47.4 ± 16.3 |
| Miscellaneous (n, %) | 24/25 (96%) | 18/24 (75%) | 6/24 (25%) | 5/24 (21%) | 3/24 (12%) | 38.5 ± 11.2 |
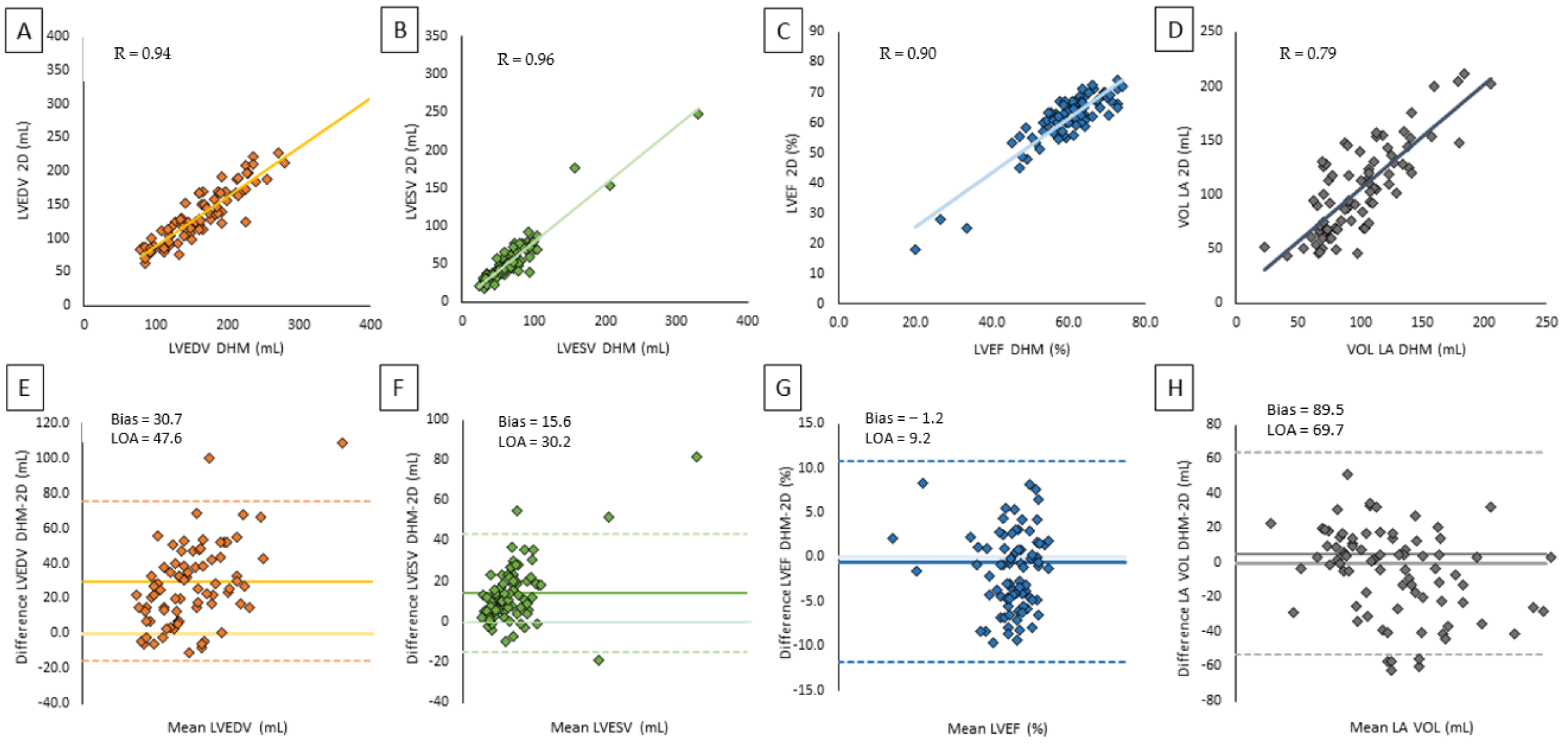
| 2D TTE | DHM | Correlation | Bias | LOA |
| LVEDV (mL) | 141.9 ± 58.4 | 172.6 ± 63.8 | 0.94 | 30.7 | 47.6 |
| LVESV (mL) | 67.2 ± 49.8 | 82.8 ± 53.9 | 0.96 | 15.6 | 30.2 |
| LVEF (%) | 55.8 ± 13.7 | 54.6 ± 12.4 | 0.90 | −1.2 | 9.2 |
| LA volume (mL) | 89.0 ± 38.4 | 90.7 ± 37.4 | 0.79 | 5.5 | 69.7 |
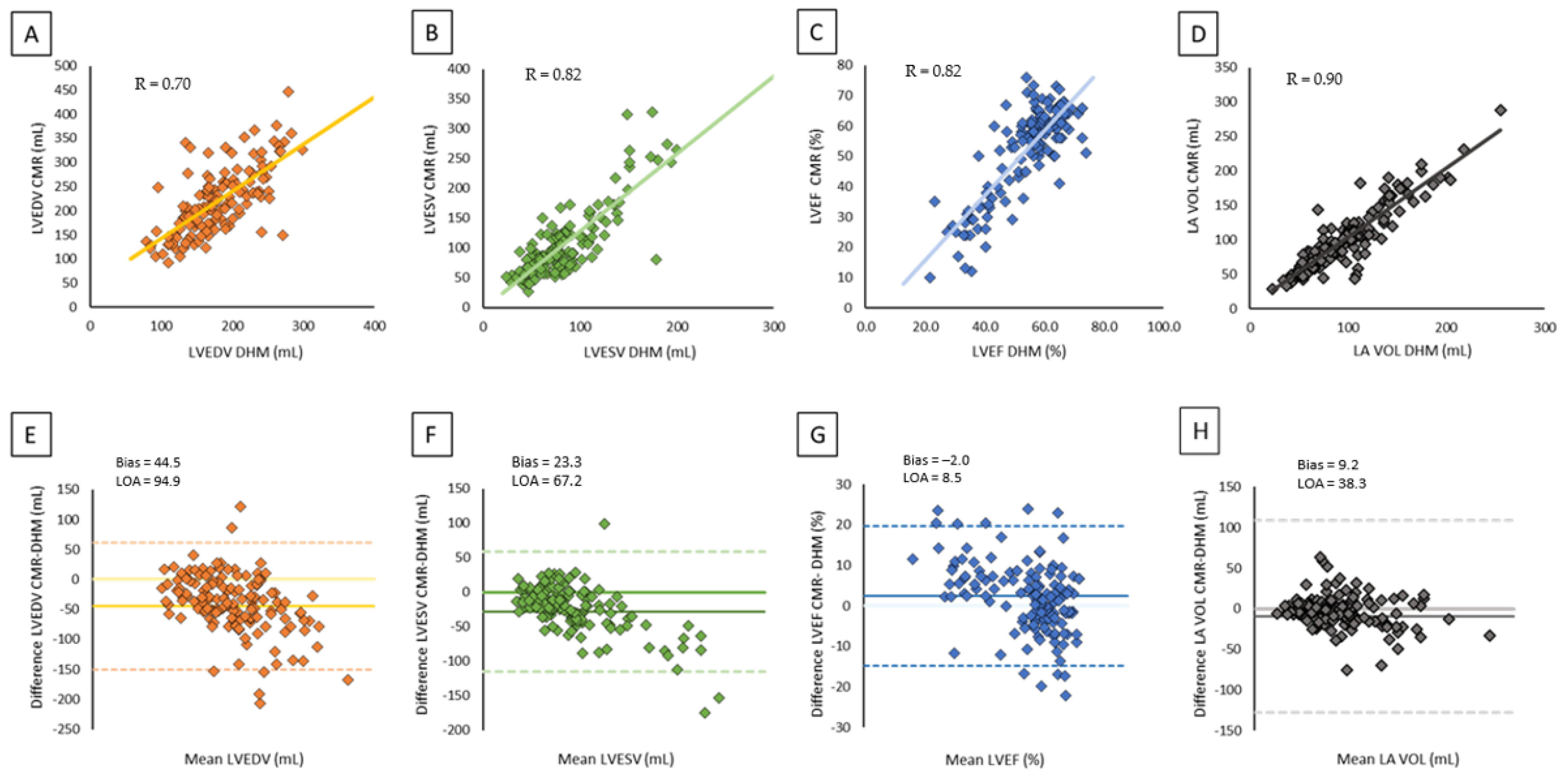
| CMR | DHM | Correlation | Bias | LOA |
| LVEDV (mL) | 226.1 ± 81.0 | 184.7 ± 55.8 | 0.70 | −40.3 | 94.9 |
| LVESV (mL) | 115.2 ± 74.8 | 88.4 ± 43.3 | 0.82 | −23.3 | 67.2 |
| LVEF (%) | 50.9 ± 15.4 | 53.3 ± 11.7 | 0.82 | 2.0 | 8.5 |
| LA volume (mL) | 101.3 ± 45.4 | 90.7 ± 37.4 | 0.90 | −9.2 | 38.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Italiano, G.; Tamborini, G.; Fusini, L.; Mantegazza, V.; Doldi, M.; Celeste, F.; Gripari, P.; Muratori, M.; Lang, R.M.; Pepi, M. Feasibility and Accuracy of the Automated Software for Dynamic Quantification of Left Ventricular and Atrial Volumes and Function in a Large Unselected Population. J. Clin. Med. 2021, 10, 5030. https://doi.org/10.3390/jcm10215030
Italiano G, Tamborini G, Fusini L, Mantegazza V, Doldi M, Celeste F, Gripari P, Muratori M, Lang RM, Pepi M. Feasibility and Accuracy of the Automated Software for Dynamic Quantification of Left Ventricular and Atrial Volumes and Function in a Large Unselected Population. Journal of Clinical Medicine. 2021; 10(21):5030. https://doi.org/10.3390/jcm10215030
Chicago/Turabian StyleItaliano, Gianpiero, Gloria Tamborini, Laura Fusini, Valentina Mantegazza, Marco Doldi, Fabrizio Celeste, Paola Gripari, Manuela Muratori, Roberto M. Lang, and Mauro Pepi. 2021. "Feasibility and Accuracy of the Automated Software for Dynamic Quantification of Left Ventricular and Atrial Volumes and Function in a Large Unselected Population" Journal of Clinical Medicine 10, no. 21: 5030. https://doi.org/10.3390/jcm10215030
APA StyleItaliano, G., Tamborini, G., Fusini, L., Mantegazza, V., Doldi, M., Celeste, F., Gripari, P., Muratori, M., Lang, R. M., & Pepi, M. (2021). Feasibility and Accuracy of the Automated Software for Dynamic Quantification of Left Ventricular and Atrial Volumes and Function in a Large Unselected Population. Journal of Clinical Medicine, 10(21), 5030. https://doi.org/10.3390/jcm10215030








