Decrease in CD14++CD16+ Monocytes in Low-Immunological-Risk Kidney Transplant Patients with Subclinical Borderline Inflammation
Definition
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Collection
2.3. Fine-Needle-Aspiration Biopsy
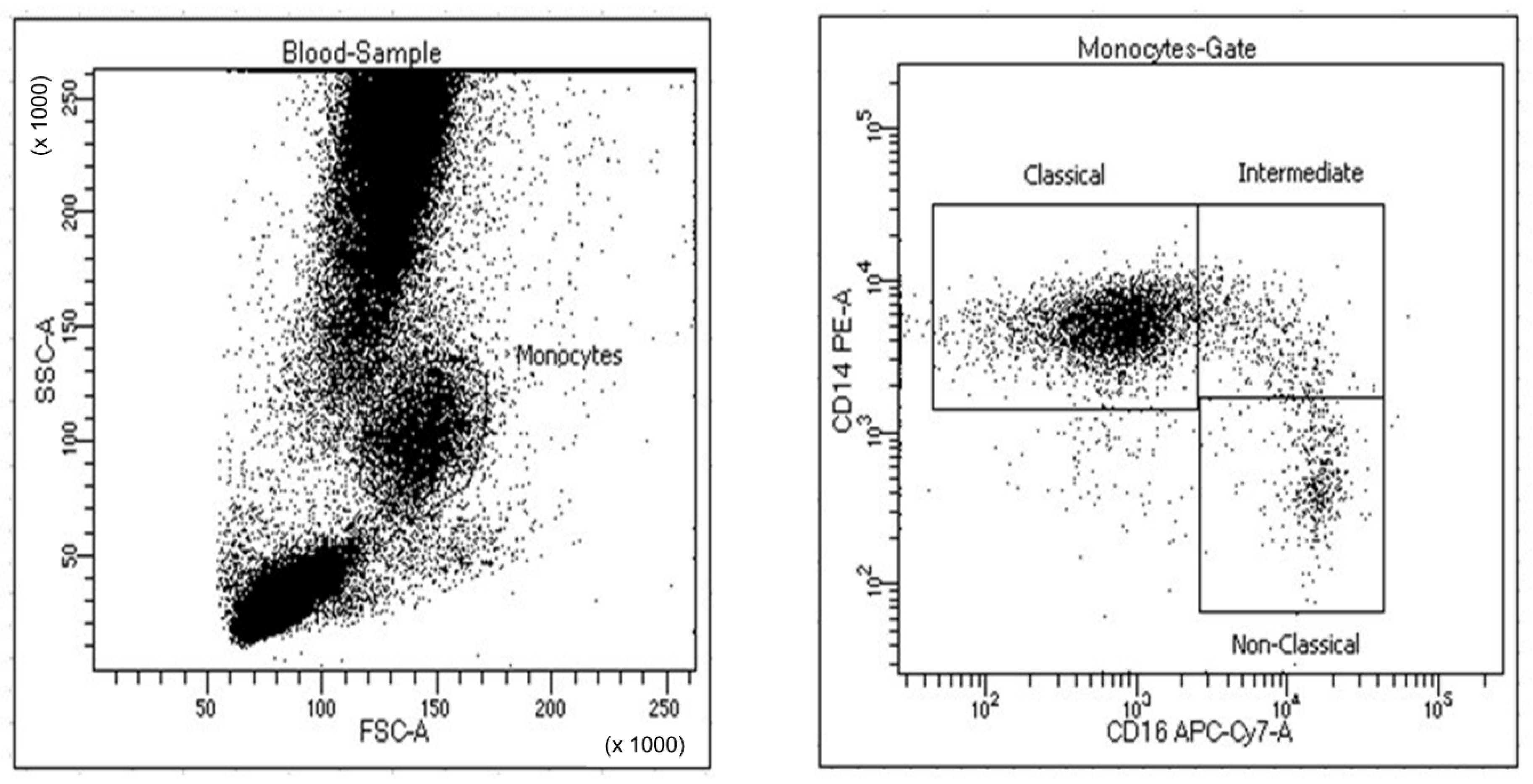
2.4. Flow Cytometry Analysis of the CD14++CD16+ Monocyte Subpopulation
2.5. Outcome
2.6. Statistical Analysis
3. Results
3.1. Donor and Recipient Clinical and Demographic Characteristics
3.2. Histological Score of the Protocol Biopsy
3.3. CD14++CD16+ Monocytes
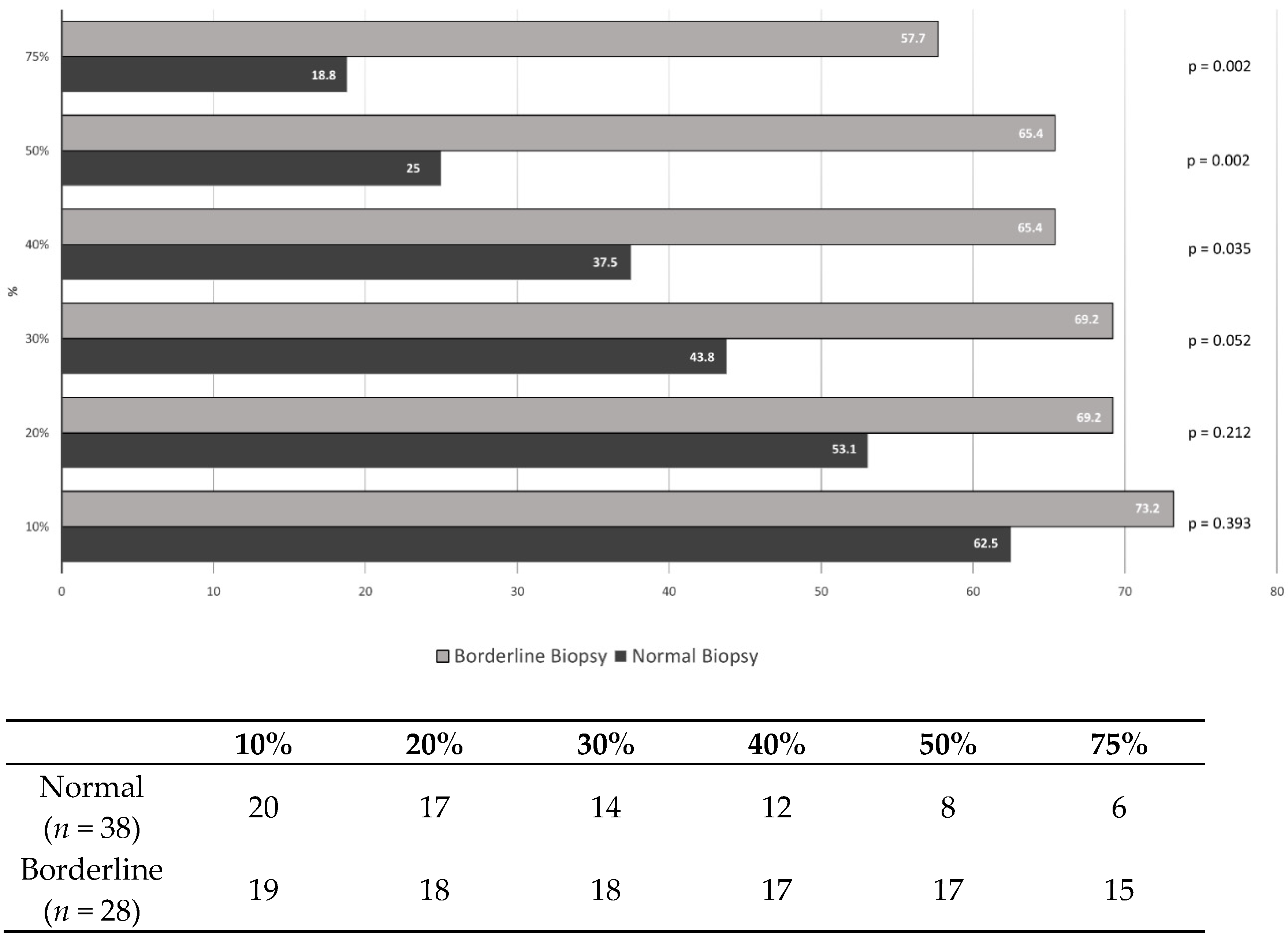
3.4. Percentage Change of the CD14++CD16+ Monocytes
3.5. Risk Factors for Borderline Lesions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brent, L.; Brown, J.; Medawar, P.B. Skin transplantation immunity in relation to hypersensitivity. Lancet 1958, 2, 561–564. [Google Scholar] [CrossRef]
- Rowshani, A.T.; Vereyken, E.J. The role of macrophage lineage cells in kidney graft rejection and survival. Transplantation 2012, 94, 309–318. [Google Scholar] [CrossRef]
- Ziegler-Heitbrock, L.; Ancuta, P.; Crowe, S.; Dalod, M.; Grau, V.; Hart, D.N.; Leenen, P.J.; Liu, Y.J.; MacPherson, G.; Randolph, G.J.; et al. Nomenclature of monocytes and dendritic cells in blood. Blood 2010, 116, e74–e80. [Google Scholar] [CrossRef]
- Wong, K.L.; Yeap, W.H.; Tai, J.J.; Ong, S.M.; Dang, T.M.; Wong, S.C. The three human monocyte subsets: Implications for health and disease. Immunol. Res. 2012, 53, 41–57. [Google Scholar] [CrossRef]
- Seifert, M.E.; Yanik, M.V.; Feig, D.I.; Hauptfeld-Dolejsek, V.; Mroczek-Musulman, E.C.; Kelly, D.R.; Rosenblum, F.; Mannon, R.B. Subclinical inflammation phenotypes and long-term outcomes after pediatric kidney transplantation. Am. J. Transpl. 2018, 18, 2189–2199. [Google Scholar] [CrossRef]
- Mehta, R.; Cherikh, W.; Sood, P.; Hariharan, S. Kidney allograft surveillance biopsy practices across US transplant centers: A UNOS survey. Clin. Transpl. 2017, 31, e12945. [Google Scholar] [CrossRef] [PubMed]
- Cosio, F.G.; Grande, J.P.; Wadei, H.; Larson, T.S.; Griffin, M.D.; Stegall, M.D. Predicting subsequent decline in kidney allograft function from early surveillance biopsies. Am. J. Transpl. 2005, 5, 2464–2472. [Google Scholar] [CrossRef]
- Orandi, B.J.; Chow, E.H.; Hsu, A.; Gupta, N.; Van Arendonk, K.J.; Garonzik-Wang, J.M.; Montgomery, J.R.; Wickliffe, C.; Lonze, B.E.; Bagnasco, S.M.; et al. Quantifying renal allograft loss following early antibody-mediated rejection. Am. J. Transpl. 2015, 15, 489–498. [Google Scholar] [CrossRef]
- Kee, T.Y.; Chapman, J.R.; O’Connell, P.J.; Fung, C.L.; Allen, R.D.; Kable, K.; Vitalone, M.J.; Nankivell, B.J. Treatment of subclinical rejection diagnosed by protocol biopsy of kidney transplants. Transplantation 2006, 82, 36–42. [Google Scholar] [CrossRef]
- Caballero, A.; Palma, E.; Ruiz-Esteban, P.; Sola, E.; López, V.; Fuentes, L.; Rudas, E.; Perea, L.; Hernández, D. Increase in CD8+CD158a+ T Cells in Kidney Graft Blood is Associated with Better Renal Function. Ann. Transpl. 2017, 22, 35–41. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hernández, D.; Alonso-Titos, J.; Vázquez, T.; León, M.; Caballero, A.; Cobo, M.; Sola, E.; López, V.; Ruiz-Esteban, P.; Cruzado, J.; et al. Clinical Relevance of Corticosteroid Withdrawal on Graft Histological Lesions in Low-Immunological-Risk Kidney Transplant Patients. J. Clin. Med. 2021, 10, 2005. [Google Scholar] [CrossRef]
- Hernández, D.; Vázquez, T.; Alonso-Titos, J.; León, M.; Caballero, A.; Cobo, M.; Sola, E.; López, V.; Ruiz-Esteban, P.; Cruzado, J.; et al. Impact of HLA Mismatching on Early Subclinical Inflammation in Low-Immunological-Risk Kidney Transplant Recipients. J. Clin. Med. 2021, 10, 1934. [Google Scholar] [CrossRef]
- Haas, M.; Loupy, A.; Lefaucheur, C.; Roufosse, C.; Glotz, D.; Seron, D.; Nankivell, B.J.; Halloran, P.F.; Colvin, R.B.; Akalin, E.; et al. The Banff 2017 Kidney Meeting Report: Revised diagnostic criteria for chronic active T cell-mediated rejection, antibody-mediated rejection, and prospects for integrative endpoints for next-generation clinical trials. Am. J. Transpl. 2018, 18, 293–307. [Google Scholar] [CrossRef] [PubMed]
- van den Bosch, T.P.P.; Hilbrands, L.B.; Kraaijeveld, R.; Litjens, N.H.R.; Rezaee, F.; Nieboer, D.; Steyerberg, E.W.; van Gestel, J.A.; Roelen, D.L.; Clahsen-van Groningen, M.C.; et al. Pretransplant Numbers of CD16(+) Monocytes as a Novel Biomarker to Predict Acute Rejection After Kidney Transplantation: A Pilot Study. Am. J. Transpl. 2017, 17, 2659–2667. [Google Scholar] [CrossRef]
- Sitoe, N.; Luecke, E.; Tembe, N.; Matavele, R.; Cumbane, V.; Macassa, E.; Vaz, P.; Sheppard, H.; Jani, I.V. Absolute and percent CD4+ T-cell enumeration by flow cytometry using capillary blood. J. Immunol. Methods 2011, 372, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Srisala, S.; Pongsakul, N.; Sahakijpicharn, T.; Hongeng, S.; Chutipongtanate, S.; Apiwattanakul, N. Capillary blood as an alternative specimen for enumeration of percentages of lymphocyte subsets. BMC Res. Notes 2019, 12, 633. [Google Scholar] [CrossRef] [PubMed]
- Dahan, K.; Audard, V.; Roudot-Thoraval, F.; Desvaux, D.; Abtahi, M.; Mansour, H.; Kumal, M.; Lang, P.; Grimbert, P. Renal allograft biopsies with borderline changes: Predictive factors of clinical outcome. Am. J. Transpl. 2006, 6, 1725–1730. [Google Scholar] [CrossRef]
- de Freitas, D.G.; Sellarés, J.; Mengel, M.; Chang, J.; Hidalgo, L.G.; Famulski, K.S.; Sis, B.; Einecke, G.; Halloran, P.F. The nature of biopsies with „borderline rejection” and prospects for eliminating this category. Am. J. Transpl. 2012, 12, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Kraaij, M.D.; Vereyken, E.J.; Leenen, P.J.; van den Bosch, T.P.; Rezaee, F.; Betjes, M.G.; Baan, C.C.; Rowshani, A.T. Human monocytes produce interferon-gamma upon stimulation with LPS. Cytokine 2014, 67, 7–12. [Google Scholar] [CrossRef]
- Oberbarnscheidt, M.H.; Zeng, Q.; Li, Q.; Dai, H.; Williams, A.L.; Shlomchik, W.D.; Rothstein, D.M.; Lakkis, F.G. Non-self recognition by monocytes initiates allograft rejection. J. Clin. Investig. 2014, 124, 3579–3589. [Google Scholar] [CrossRef]
- Van den Bosch, T.P.; Caliskan, K.; Kraaij, M.D.; Constantinescu, A.A.; Manintveld, O.C.; Leenen, P.J.; von der Thüsen, J.H.; Clahsen-van Groningen, M.C.; Baan, C.C.; Rowshani, A.T. CD16+ Monocytes and Skewed Macrophage Polarization toward M2 Type Hallmark Heart Transplant Acute Cellular Rejection. Front. Immunol. 2017, 8, 346. [Google Scholar] [CrossRef] [PubMed]
| Total n = 66 | Borderline (n = 28) | Normal (n = 38) | p Value | |
|---|---|---|---|---|
| Donor age (years) | 53.7 ± 11.3 | 55.3 ± 11.0 | 52.6 ± 11.6 | 0.357 |
| Recipient age (years) | 53.9 ± 11.9 | 57.8 ± 9.5 | 51.1 ± 12.8 | 0.045 |
| Recipient weight (Kg) | 76.9 ± 14.6 | 76.9 ± 14.6 | 76.9 ± 14.7 | 0.989 |
| Donor sex (M) % | 65.2 | 75.0 | 57.9 | 0.149 |
| Recipient sex (M) % | 68.2 | 67.9 | 68.4 | 0.961 |
| Donor type (Deceased) % | 81.8 | 89.3 | 76.3 | 0.177 |
| Replacement therapy (HD) % | 58.5 | 64.3 | 54.1 | 0.702 |
| Cause of kidney disease (%) | ||||
| Glomerular | 22.7 | 21.4 | 23.7 | 0.676 |
| Diabetes | 12.1 | 14.3 | 10.5 | |
| Polycystosis | 25.8 | 35.7 | 18.4 | |
| Interstitial nephropathy | 7.6 | 7.1 | 7.9 | |
| Autoimmune | 1.5 | 0.0 | 2.6 | |
| Nephroangiosclerosis | 7.6 | 3.6 | 10.5 | |
| No determined | 10.3 | 10.7 | 10.5 | |
| Other | 12.1 | 7.1 | 15.8 | |
| Induction (%) | 87.9 | 84.6 | 90.6 | 0.485 |
| Cold ischemia time (hours) | 10.7 ± 5.0 | 10.9 ± 3.7 | 10.6 ± 5.8 | 0.787 |
| Transfusions (Yes) % | 9.7 | 7.7 | 11.1 | 0.653 |
| PRA pre-transplant (%) | 0.2 ± 1.7 | 0.5 ± 2.6 | 0.0 | 0.326 |
| Total HLA mismatches (n) | 6.3 ± 2.5 | 6.6 ± 2.2 | 6.1 ± 2.7 | 0.425 |
| HLA-DR mismatches (n) | 1.2 ± 0.7 | 1.2 ± 0.6 | 1.2 ± 0.7 | 0.982 |
| Delayed graft function (%) | 22.7 | 25.0 | 21.1 | 0.705 |
| Creatinine (mg/d) | 1.6 ± 0.5 | 1.7 ± 0.5 | 1.6 ± 0.6 | 0.536 |
| Proteinuria (mg/24 h) | 268.7 ± 214.7 | 269.3 ± 239.8 | 268.2 ± 197.9 | 0.986 |
| Diastolic blood pressure (mmHg) | 71.6 ± 6.9 | 73.1 ± 6.0 | 70.8 ± 7.3 | 0.280 |
| Systolic blood pressure (mmHg) | 125.9 ± 11.5 | 124.8 ± 9.3 | 126.4 ± 12.6 | 0.639 |
| Tacrolimus levels (ng/mL) | 9.7 ± 2.4 | 9.3 ± 2.6 | 9.9 ± 2.2 | 0.290 |
| Total (n = 66) | Borderline (n = 28) | Normal (n = 38) | p Value | |
|---|---|---|---|---|
| g (0–3) | 0.11 ± 0.31 | 0.14 ± 0.36 | 0.08 ± 0.27 | 0.412 |
| ptc (0–3) | 1.25 ± 0.49 | 0.27 ± 0.72 | 0.03 ± 0.16 | 0.104 |
| t (0–3) | 0.46 ± 0.50 | 1.0 ± 0.0 | 0.05 ± 0.23 | 0.000 |
| i (0–3) | 0.68 ± 0.47 | 1.0 ± 0.0 | 0.45 ± 0.50 | 0.000 |
| v (0–3) | 0.0 | 0.0 | 0.0 | |
| ci (0–3) | 0.49 ± 0.50 | 0.57 ± 0.50 | 0.42 ± 0.50 | 0.233 |
| ct (0–3) | 0.39 ± 0.49 | 0.50 ± 0.51 | 0.31 ± 0.47 | 0.139 |
| cg (0–3) | 0.0 | 0.0 | 0.0 | |
| cv (0–3) | 0.47 ± 0.61 | 0.54 ± 0.69 | 0.42 ± 0.55 | 0.457 |
| ah (0–3) | 0.36 ± 0.62 | 0.50 ± 0.75 | 0.26 ± 0.50 | 0.153 |
| ct + ci | 0.88 ± 0.95 | 1.07 ± 0.98 | 0.74 ± 0.92 | 0.160 |
| ct + ci + cg + cv | 1.35 ± 1.23 | 1.61 ± 1.40 | 1.16 ± 1.08 | 0.162 |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| Difference of 10% | 1.629 (0.529–5.011) | 0.395 | ||||
| Difference of 20% | 1.985 (0.671–5.871) | 0.215 | ||||
| Difference of 30% | 2.893 (0.976–8.578) | 0.055 | ||||
| Difference of 40% | 3.148 (1.070–9.264) | 0.037 | ||||
| Difference of 50% | 5.667 (1.818–17.667) | 0.003 | 7.325 (2.079–25.807) | 0.002 | ||
| Difference of 75% | 5.909 (1.815–19.238) | 0.003 | 7.708 (2.096–28.351) | 0.002 | ||
| Sex (M) | 0.974 (0.342–2.777) | 0.961 | ||||
| Recipient age (years) | 1.054 (1.006–1.105) | 0.028 | 1.071 (1.010–1.135) | 0.022 | 1.069 (1.011–1.131) | 0.020 |
| Delayed graft function (Yes/No) | 1.250 (0.393–3.978) | 0.706 | ||||
| Cold ischemia time (hours) | 1.014 (0.913–1.125) | 0.796 | ||||
| Transfusions (Yes/No) | 0.667 (0.113–3.945) | 0.655 | ||||
| Donor age (years) | 1.021 (0.977–1.068) | 0.352 | ||||
| Induction therapy (Yes/No) | 0.569 (0.115–2.807) | 0.489 | ||||
| Total HLA mismatches (n) | 1.088 (0.886–1.337) | 0.420 | ||||
| HLA–DR mismatches (n) | 1.009 (0.483–2.106) | 0.982 | ||||
| Creatinine | 1.352 (0.528–3.464) | 0.530 | ||||
| Proteinuria | 1.000 (0.997–1.003) | 0.986 | ||||
| Tacrolimus levels | 0.887 (0.712–1.106) | 0.287 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caballero, A.; Vazquez-Sanchez, T.; Ruiz-Esteban, P.; Leon, M.; Alonso-Titos, J.; Lopez, V.; Sola, E.; Gutierrez, E.; Cabello, M.; Casas-Gonzalez, C.; et al. Decrease in CD14++CD16+ Monocytes in Low-Immunological-Risk Kidney Transplant Patients with Subclinical Borderline Inflammation. J. Clin. Med. 2021, 10, 5051. https://doi.org/10.3390/jcm10215051
Caballero A, Vazquez-Sanchez T, Ruiz-Esteban P, Leon M, Alonso-Titos J, Lopez V, Sola E, Gutierrez E, Cabello M, Casas-Gonzalez C, et al. Decrease in CD14++CD16+ Monocytes in Low-Immunological-Risk Kidney Transplant Patients with Subclinical Borderline Inflammation. Journal of Clinical Medicine. 2021; 10(21):5051. https://doi.org/10.3390/jcm10215051
Chicago/Turabian StyleCaballero, Abelardo, Teresa Vazquez-Sanchez, Pedro Ruiz-Esteban, Myriam Leon, Juana Alonso-Titos, Veronica Lopez, Eugenia Sola, Elena Gutierrez, Mercedes Cabello, Cristina Casas-Gonzalez, and et al. 2021. "Decrease in CD14++CD16+ Monocytes in Low-Immunological-Risk Kidney Transplant Patients with Subclinical Borderline Inflammation" Journal of Clinical Medicine 10, no. 21: 5051. https://doi.org/10.3390/jcm10215051
APA StyleCaballero, A., Vazquez-Sanchez, T., Ruiz-Esteban, P., Leon, M., Alonso-Titos, J., Lopez, V., Sola, E., Gutierrez, E., Cabello, M., Casas-Gonzalez, C., Pozo-Alvarez, R., Delgado-Burgos, J., & Hernandez, D. (2021). Decrease in CD14++CD16+ Monocytes in Low-Immunological-Risk Kidney Transplant Patients with Subclinical Borderline Inflammation. Journal of Clinical Medicine, 10(21), 5051. https://doi.org/10.3390/jcm10215051






