Immunologic Dysregulation and Hypercoagulability as a Pathophysiologic Background in COVID-19 Infection and the Immunomodulating Role of Colchicine
Abstract
:1. Introduction: Coronavirus Disease 19 (COVID-19) Pandemic
2. COVID-19 Clinical and Pathophysiological Aspects
2.1. COVID-19 Infection: Clinical Course
2.2. COVID-19 Pathophysiology: Immunologic Dysregulation and Hypercoagulability
3. Colchicine and COVID-19 Infection
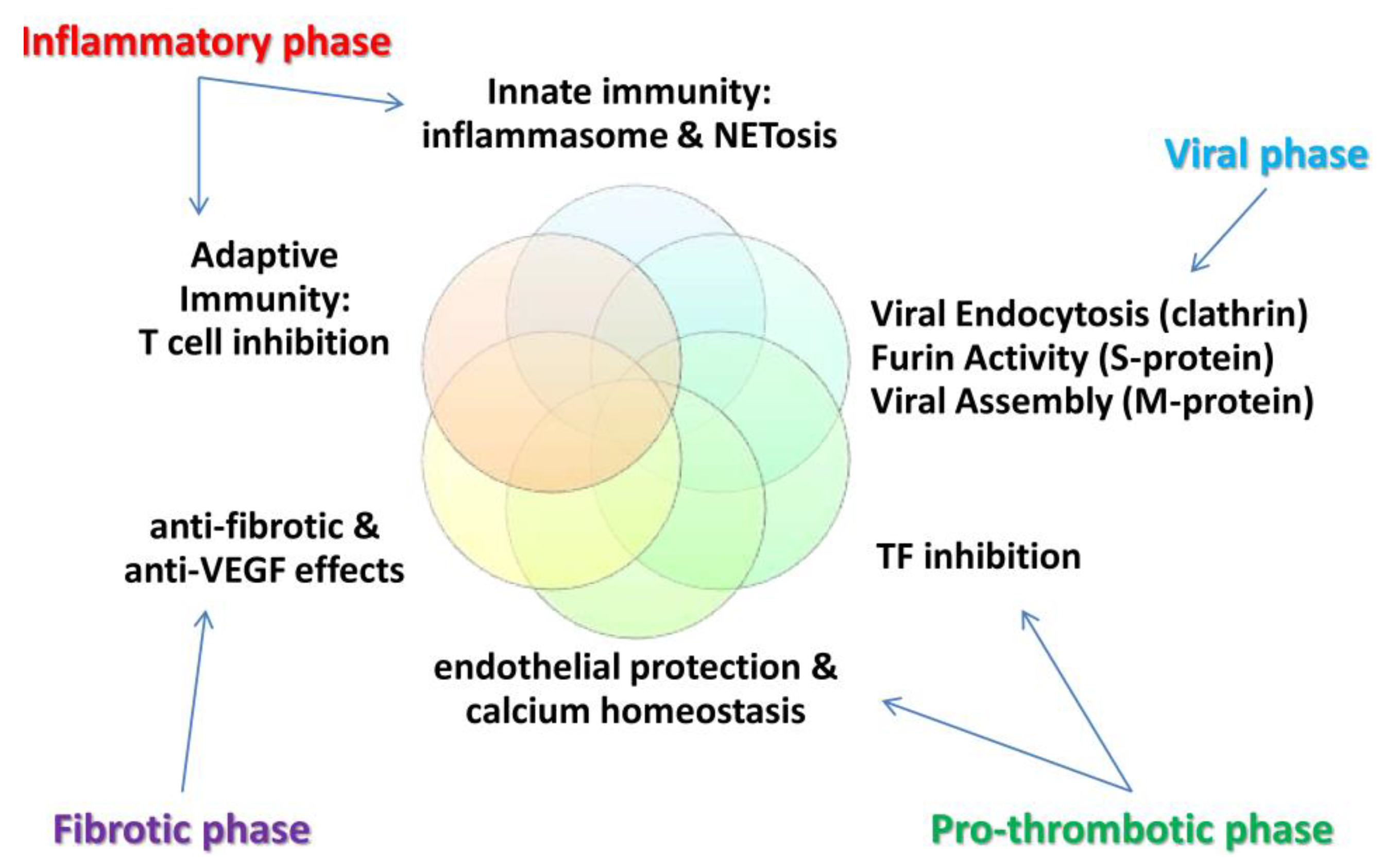
3.1. Brief History, Pharmacology and COVID-19 Hypothesis
3.2. Clinical Results with Colchicine in COVID-19 Patients
3.2.1. Studies Evaluating Colchicine as Prevention against COVID-19
3.2.2. Colchicine for the Treatment or Prevention of COVID-19 Complications
3.2.3. Meta-Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harapan, H.; Itoh, N.; Yufika, A.; Winardi, W.; Keam, S.; Te, H.; Megawati, D.; Hayati, Z.; Wagner, A.L.; Mudatsir, M. Coronavirus disease 2019 (COVID-19): A literature review. J. Infect. Public Health 2020, 13, 667–673. [Google Scholar] [CrossRef]
- COVID-19 Map—Johns Hopkins Coronavirus Resource Center n.d. Available online: https://coronavirus.jhu.edu/map.html (accessed on 2 September 2021).
- Peacock, T.P.; Penrice-Randal, R.; Hiscox, J.A.; Barclay, W.S. SARS-CoV-2 one year on: Evidence for ongoing viral adaptation. J. Gen. Virol. 2021, 102, 001584. [Google Scholar] [CrossRef]
- Fricke-Galindo, I.; Falfán-Valencia, R. Genetics Insight for COVID-19 Susceptibility and Severity: A Review. Front. Immunol. 2021, 12, 622176. [Google Scholar] [CrossRef] [PubMed]
- Beniac, D.R.; Andonov, A.; Grudeski, E.; Booth, T.F. Architecture of the SARS coronavirus prefusion spike. Nat. Struct. Mol. Biol. 2006, 13, 751–752. [Google Scholar] [CrossRef] [Green Version]
- Gilzad-Kohan, H.; Jamali, F. Anti-Inflammatory Properties of Drugs Used to Control COVID-19 and their Effects on the Renin-Angiotensin System and Angiotensin-Converting Enzyme-2. J. Pharm. Pharm. Sci. 2020, 23, 259–277. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Chen, K.; Zou, J.; Han, P.; Hao, J.; Han, Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front. Med. 2020, 14, 185–192. [Google Scholar] [CrossRef] [Green Version]
- Zou, L.; Ruan, F.; Huang, M.; Liang, L.; Huang, H.; Hong, Z.; Yu, J.; Kang, M.; Song, Y.; Xia, J.; et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N. Engl. J. Med. 2020, 382, 1177–1179. [Google Scholar] [CrossRef] [PubMed]
- Korakas, E.; Ikonomidis, I.; Kousathana, F.; Balampanis, K.; Kountouri, A.; Raptis, A.; Palaiodimou, L.; Kokkinos, A.; Lambadiari, V. Obesity and COVID-19: Immune and metabolic derangement as a possible link to adverse clinical outcomes. Am. J. Physiol. Metab. 2020, 319, E105–E109. [Google Scholar] [CrossRef]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases from the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Berlin, D.A.; Gulick, R.M.; Martinez, F.J. Severe Covid-19. N. Engl. J. Med. 2020, 383, 2451–2460. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Cantini, F.; Goletti, D.; Petrone, L.; Najafi Fard, S.; Niccoli, L.; Foti, R. Immune Therapy, or Antiviral Therapy, or Both for COVID-19: A Systematic Review. Drugs 2020, 80, 1929–1946. [Google Scholar] [CrossRef] [PubMed]
- Kabir, M.A.; Ahmed, R.; Chowdhury, R.; Asher Iqbal, S.M.; Paulmurugan, R.; Demirci, U.; Asghar, W. Management of COVID-19: Current Status and Future Prospects. Microbes Infect. 2021, 23, 104832. [Google Scholar] [CrossRef] [PubMed]
- Elhusseiny, K.M.; Abd-Elhay, F.A.-E.; Kamel, M.G. Possible therapeutic agents for COVID-19: A comprehensive review. Expert Rev. Anti. Infect. Ther. 2020, 18, 1005–1020. [Google Scholar] [CrossRef]
- Fragkou, P.C.; Belhadi, D.; Peiffer-Smadja, N.; Moschopoulos, C.D.; Lescure, F.-X.; Janocha, H.; Karofylakis, E.; Yazdanpanah, Y.; Mentré, F.; Skevaki, C.; et al. Review of trials currently testing treatment and prevention of COVID-19. Clin. Microbiol. Infect. 2020, 26, 988–998. [Google Scholar] [CrossRef] [PubMed]
- Magro, G. COVID-19: Review on latest available drugs and therapies against SARS-CoV-2. Coagulation and inflammation cross-talking. Virus Res. 2020, 286, 198070. [Google Scholar] [CrossRef] [PubMed]
- Andreou, A.; Trantza, S.; Filippou, D.; Filippou, D.; Sipsas, N.; Tsiodras, S. COVID-19: The potential role of copper and N-acetylcysteine (NAC) in a combination of candidate antiviral treatments against SARS-CoV-2. In Vivo 2020, 34, 1567–1588. [Google Scholar] [CrossRef] [PubMed]
- Rizk, J.G.; Kalantar-Zadeh, K.; Mehra, M.R.; Lavie, C.J.; Rizk, Y.; Forthal, D.N. Pharmaco-Immunomodulatory Therapy in COVID-19. Drugs 2020, 80, 1267–1292. [Google Scholar] [CrossRef] [PubMed]
- Soy, M.; Keser, G.; Atagündüz, P.; Tabak, F.; Atagündüz, I.; Kayhan, S. Cytokine storm in COVID-19: Pathogenesis and overview of anti-inflammatory agents used in treatment. Clin. Rheumatol. 2020, 39, 2085–2094. [Google Scholar] [CrossRef] [PubMed]
- Schnappauf, O.; Chae, J.J.; Kastner, D.L.; Aksentijevich, I. The Pyrin Inflammasome in Health and Disease. Front. Immunol. 2019, 10, 1745. [Google Scholar] [CrossRef]
- Ribeiro, S.A.; Lopes, C.; Amaral, R.; Amaral, A. The therapeutic potential of colchicine in the complications of COVID19. Could the immunometabolic properties of an old and cheap drug help? Metab. Open 2020, 7, 100045. [Google Scholar] [CrossRef] [PubMed]
- Yuki, K.; Fujiogi, M.; Koutsogiannaki, S. COVID-19 pathophysiology: A review. Clin. Immunol. 2020, 215, 108427. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Rana, S. The role of human C5a as a non-genomic target in corticosteroid therapy for management of severe COVID19. Comput. Biol. Chem. 2021, 92, 107482. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento-Monroy, J.C.; Parra-Medina, R.; Garavito, E.; Rojas-Villarraga, A. T Helper 17 Response to Severe Acute Respiratory Syndrome Coronavirus 2: A Type of Immune Response with Possible Therapeutic Implications. Viral Immunol. 2021, 34, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-F.; Hsieh, S.-M.; Pan, S.-C.; Huang, Y.-S.; Chang, S.-C. Dose-Related Aberrant Inhibition of Intracellular Perforin Expression by S1 Subunit of Spike Glycoprotein That Contains Receptor-Binding Domain from SARS-CoV-2. Microorganisms 2021, 9, 1303. [Google Scholar] [CrossRef]
- Jenner, A.L.; Aogo, R.A.; Alfonso, S.; Crowe, V.; Deng, X.; Smith, A.P.; Morel, P.A.; Davis, C.L.; Smith, A.M.; Craig, M. COVID-19 virtual patient cohort suggests immune mechanisms driving disease outcomes. PLoS Pathog. 2021, 17, e1009753. [Google Scholar] [CrossRef] [PubMed]
- Severa, M.; Diotti, R.A.; Etna, M.P.; Rizzo, F.; Fiore, S.; Ricci, D.; Iannetta, M.; Sinigaglia, A.; Lodi, A.; Mancini, N.; et al. Differential plasmacytoid dendritic cell phenotype and type I Interferon response in asymptomatic and severe COVID-19 infection. PLoS Pathog. 2021, 17, e1009878. [Google Scholar] [CrossRef] [PubMed]
- Barnes, B.J.; Adrover, J.M.; Baxter-Stoltzfus, A.; Borczuk, A.; Cools-Lartigue, J.; Crawford, J.M.; Daßler-Plenker, J.; Guerci, P.; Huynh, C.; Knight, J.S.; et al. Targeting potential drivers of COVID-19: Neutrophil extracellular traps. J. Exp. Med. 2020, 217, e20200652. [Google Scholar] [CrossRef]
- Obermayer, A.; Jakob, L.-M.; Haslbauer, J.D.; Matter, M.S.; Tzankov, A.; Stoiber, W. Neutrophil Extracellular Traps in Fatal COVID-19-Associated Lung Injury. Dis. Markers 2021, 2021, 1–10. [Google Scholar] [CrossRef]
- Ferreira, A.C.; Soares, V.C.; de Azevedo-Quintanilha, I.G.; Dias, S.d.S.G.; Fintelman-Rodrigues, N.; Sacramento, C.Q.; Mattos, M.; de Freitas, C.S.; Temerozo, J.R.; Teixeira, L.; et al. SARS-CoV-2 engages inflammasome and pyroptosis in human primary monocytes. Cell Death Discov. 2021, 7, 43. [Google Scholar] [CrossRef]
- Tang, N.; Li, D.; Wang, X.; Sun, Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020, 18, 844–847. [Google Scholar] [CrossRef] [Green Version]
- Foret, T.; Dufrost, V.; Salomon Du Mont, L.; Costa, P.; Lefevre, B.; Lacolley, P.; Regnault, V.; Zuily, S.; Wahl, D. Systematic Review of Antiphospholipid Antibodies in COVID-19 Patients: Culprits or Bystanders? Curr. Rheumatol. Rep. 2021, 23, 65. [Google Scholar] [CrossRef] [PubMed]
- Hollerbach, A.; Müller-Calleja, N.; Pedrosa, D.; Canisius, A.; Sprinzl, M.F.; Falter, T.; Rossmann, H.; Bodenstein, M.; Werner, C.; Sagoschen, I.; et al. Pathogenic lipid-binding antiphospholipid antibodies are associated with severity of COVID-19. J. Thromb. Haemost. 2021, 19, 2335–2347. [Google Scholar] [CrossRef] [PubMed]
- Spiezia, L.; Boscolo, A.; Poletto, F.; Cerruti, L.; Tiberio, I.; Campello, E.; Navalesi, P.; Simioni, P. COVID-19-Related Severe Hypercoagulability in Patients Admitted to Intensive Care Unit for Acute Respiratory Failure. Thromb. Haemost. 2020, 120, 998–1000. [Google Scholar] [CrossRef]
- Ozolina, A.; Sarkele, M.; Sabelnikovs, O.; Skesters, A.; Jaunalksne, I.; Serova, J.; Ievins, T.; Bjertnaes, L.J.; Vanags, I. Activation of Coagulation and Fibrinolysis in Acute Respiratory Distress Syndrome: A Prospective Pilot Study. Front. Med. 2016, 3, 64. [Google Scholar] [CrossRef]
- Fogarty, H.; Townsend, L.; Ni Cheallaigh, C.; Bergin, C.; Martin-Loeches, I.; Browne, P.; Bacon, C.L.; Gaule, R.; Gillett, A.; Byrne, M.; et al. COVID19 coagulopathy in Caucasian patients. Br. J. Haematol. 2020, 189, 1044–1049. [Google Scholar] [CrossRef]
- Risitano, A.M.; Mastellos, D.C.; Huber-Lang, M.; Yancopoulou, D.; Garlanda, C.; Ciceri, F.; Lambris, J.D. Complement as a target in COVID-19? Nat. Rev. Immunol. 2020, 20, 343–344. [Google Scholar] [CrossRef] [Green Version]
- Bye, A.P.; Hoepel, W.; Mitchell, J.L.; Jégouic, S.; Loureiro, S.; Sage, T.; de Taeye, S.; van Gils, M.; Kriek, N.; Cooper, N.; et al. Aberrant glycosylation of anti-SARS-CoV-2 IgG is a pro-thrombotic stimulus for platelets. bioRxiv 2021. [Google Scholar] [CrossRef]
- Nazy, I.; Jevtic, S.D.; Moore, J.C.; Huynh, A.; Smith, J.W.; Kelton, J.G.; Arnold, D.M. Platelet-activating immune complexes identified in critically ill COVID-19 patients suspected of heparin-induced thrombocytopenia. J. Thromb. Haemost. 2021, 19, 1342–1347. [Google Scholar] [CrossRef] [PubMed]
- González-Gay, M.A.; Mayo, J.; Castañeda, S.; Cifrián, J.M.; Hernández-Rodríguez, J. Tocilizumab: From the rheumatology practice to the fight against COVID-19, a virus infection with multiple faces. Expert Opin. Biol. Ther. 2020, 20, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Slobodnick, A.; Shah, B.; Krasnokutsky, S.; Pillinger, M.H. Update on colchicine, 2017. Rheumatology 2018, 57, i4–i11. [Google Scholar] [CrossRef] [Green Version]
- Bayes-Genis, A.; Adler, Y.; De Luna, A.B.; Imazio, M. Colchicine in Pericarditis. Eur. Heart J. 2017, 38, 1706–1709. [Google Scholar] [CrossRef] [Green Version]
- Katsilabros, L. La colchicine et ses dérivés contre les viroses. Arch. Inst. Pasteur Hell. 1958, 4, 139–145. [Google Scholar]
- McEwan, T.; Robinson, P.C. A systematic review of the infectious complications of colchicine and the use of colchicine to treat infections. Semin. Arthritis Rheum. 2021, 51, 101–112. [Google Scholar] [CrossRef]
- Deftereos, S.G.; Siasos, G.; Giannopoulos, G.; Vrachatis, D.A.; Angelidis, C.; Giotaki, S.G.; Gargalianos, P.; Giamarellou, H.; Gogos, C.; Daikos, G.; et al. The Greek study in the effects of colchicine in COvid-19 complications prevention (GRECCO-19 study): Rationale and study design. Hell. J. Cardiol. 2020, 61, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Deftereos, S.G.; Vrachatis, D.A.; Angelidis, C.; Vrettou, A.-R.; Sarri, E.K.; Giotaki, S.G.; Varytimiadi, E.; Kossyvakis, C.; Kotsia, E.; Deftereos, G.S.; et al. The Role of Colchicine in Treating Postoperative and Post-catheter Ablation Atrial Fibrillation. Clin. Ther. 2019, 41, 21–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schenone, A.L.; Menon, V. Colchicine in Pericardial Disease: From the Underlying Biology and Clinical Benefits to the Drug-Drug Interactions in Cardiovascular Medicine. Curr. Cardiol. Rep. 2018, 20, 62. [Google Scholar] [CrossRef] [PubMed]
- Vrachatis, D.A.; Giannopoulos, G.; Deftereos, S.G. Colchicine: Conventional and Contemporary Indications. Curr. Pharm. Des. 2018, 24, 647. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, C.; Patoulias, D.; Teperikidis, E.; Mouselimis, D.; Tsarouchas, A.; Toumpourleka, M.; Boulmpou, A.; Bakogiannis, C.; Doumas, M.; Vassilikos, V.P. Colchicine as a Potential Therapeutic Agent Against Cardiovascular Complications of COVID-19: An Exploratory Review. SN Compr. Clin. Med. 2020, 2, 1419–1429. [Google Scholar] [CrossRef] [PubMed]
- Angelidis, C.; Kotsialou, Z.; Kossyvakis, C.; Vrettou, A.-R.; Zacharoulis, A.; Kolokathis, F.; Kekeris, V.; Giannopoulos, G. Colchicine Pharmacokinetics and Mechanism of Action. Curr. Pharm. Des. 2018, 24, 659–663. [Google Scholar] [CrossRef] [PubMed]
- Leung, Y.Y.; Yao Hui, L.L.; Kraus, V.B. Colchicine-Update on mechanisms of action and therapeutic uses. Semin. Arthritis Rheum. 2015, 45, 341–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, S.; Yang, K.C.K.; Atkins, K.; Dalbeth, N.; Robinson, P.C. Adverse events during oral colchicine use: A systematic review and meta-analysis of randomised controlled trials. Arthritis Res. Ther. 2020, 22, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreis, A.; Imazio, M.; Avondo, S.; Casula, M.; Paneva, E.; Piroli, F.; De Ferrari, G.M. Adverse events of colchicine for cardiovascular diseases. J. Cardiovasc. Med. 2021, 22, 637–644. [Google Scholar] [CrossRef]
- Tardif, J.C.; Kouz, S.; Waters, D.D.; Bertrand, O.F.; Diaz, R.; Maggioni, A.P.; Pinto, F.J.; Ibrahim, R.; Gamra, H.; Kiwan, G.S.; et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N. Engl. J. Med. 2019, 381, 2497–2505. [Google Scholar] [CrossRef] [PubMed]
- Nidorf, S.M.; Fiolet, A.T.L.; Mosterd, A.; Eikelboom, J.W.; Schut, A.; Opstal, T.S.J.; The, S.H.K.; Xu, X.-F.; Ireland, M.A.; Lenderink, T.; et al. Colchicine in Patients with Chronic Coronary Disease. N. Engl. J. Med. 2020, 383, 1838–1847. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.; Allen, N.; Harchandani, B.; Pillinger, M.; Katz, S.; Sedlis, S.P.; Echagarruga, C.; Samuels, S.K.; Morina, P.; Singh, P.; et al. Effect of Colchicine on Platelet-Platelet and Platelet-Leukocyte Interactions: A Pilot Study in Healthy Subjects. Inflammation 2016, 39, 182–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deftereos, S.; Giannopoulos, G.; Papoutsidakis, N.; Panagopoulou, V.; Kossyvakis, C.; Raisakis, K.; Cleman, M.W.; Stefanadis, C. Colchicine and the Heart. J. Am. Coll. Cardiol. 2013, 62, 1817–1825. [Google Scholar] [CrossRef] [Green Version]
- Sandbo, N.; Ngam, C.; Torr, E.; Kregel, S.; Kach, J.; Dulin, N. Control of Myofibroblast Differentiation by Microtubule Dynamics through a Regulated Localization of mDia2. J. Biol. Chem. 2013, 288, 15466–15473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, U.N. Bioactive Lipids in COVID-19-Further Evidence. Arch. Med. Res. 2021, 52, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Perico, N.; Ostermann, D.; Bontempeill, M.; Morigi, M.; Amuchastegui, C.S.; Zoja, C.; Akalin, E.; Sayegh, M.H.; Remuzzi, G. Colchicine interferes with L-selectin and leukocyte function-associated antigen-1 expression on human T lymphocytes and inhibits T cell activation. J. Am. Soc. Nephrol. 1996, 7, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Atta, H.M.; El-Rehany, M.A.; Abdel Raheim, S.R.; Fouad, R.; Galal, A.M.F. Colchicine inhibits intimal hyperplasia and leukocyte VEGF expression in dogs. J. Surg. Res. 2008, 146, 184–189. [Google Scholar] [CrossRef]
- Marzo-Mas, A.; Falomir, E.; Murga, J.; Carda, M.; Marco, J.A. Effects on tubulin polymerization and down-regulation of c-Myc, hTERT and VEGF genes by colchicine haloacetyl and haloaroyl derivatives. Eur. J. Med. Chem. 2018, 150, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.Y.; Chen, Y.C.; Kao, Y.H.; Lin, Y.K.; Yeh, Y.H.; Chen, S.A.; Chen, Y.J. Colchicine modulates calcium homeostasis and electrical property of HL-1 cells. J. Cell. Mol. Med. 2016, 20, 1182–1190. [Google Scholar] [CrossRef] [PubMed]
- Kow, C.S.; Hasan, S.S. Colchicine as an adjunct to heparin for prophylaxis of venous thromboembolism in patients with COVID-19. Rheumatol. Int. 2021, 41, 677–678. [Google Scholar] [CrossRef]
- Apostolidou, E.; Skendros, P.; Kambas, K.; Mitroulis, I.; Konstantinidis, T.; Chrysanthopoulou, A.; Nakos, K.; Tsironidou, V.; Koffa, M.; Boumpas, D.T.; et al. Neutrophil extracellular traps regulate IL-1β-mediated inflammation in familial Mediterranean fever. Ann. Rheum. Dis. 2016, 75, 269–277. [Google Scholar] [CrossRef]
- Kaksonen, M.; Roux, A. Mechanisms of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 2018, 19, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Milewska, A.; Nowak, P.; Owczarek, K.; Szczepanski, A.; Zarebski, M.; Hoang, A.; Berniak, K.; Wojarski, J.; Zeglen, S.; Baster, Z.; et al. Entry of Human Coronavirus NL63 into the Cell. J. Virol. 2017, 92, e01933-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AbdelMassih, A.F.; Ye, J.; Kamel, A.; Mishriky, F.; Ismail, H.-A.; Ragab, H.A.; El Qadi, L.; Malak, L.; Abdu, M.; El-Husseiny, M.; et al. A multicenter consensus: A role of furin in the endothelial tropism in obese patients with COVID-19 infection. Obes. Med. 2020, 19, 100281. [Google Scholar] [CrossRef] [PubMed]
- Peele, K.A.; Kumar, V.; Parate, S.; Srirama, K.; Lee, K.W.; Venkateswarulu, T.C. Insilico drug repurposing using FDA approved drugs against Membrane protein of SARS-CoV-2. J. Pharm. Sci. 2021, 110, 2346–2354. [Google Scholar] [CrossRef]
- Cañas, C.A.; Cañas, F.; Bautista-Vargas, M.; Bonilla-Abadía, F. Role of Tissue Factor in the Pathogenesis of COVID-19 and the Possible Ways to Inhibit It. Clin. Appl. Thromb. 2021, 27, 107602962110039. [Google Scholar] [CrossRef]
- Dupuis, J.; Sirois, M.G.; Rhéaume, E.; Nguyen, Q.T.; Clavet-Lanthier, M.-É.; Brand, G.; Mihalache-Avram, T.; Théberge-Julien, G.; Charpentier, D.; Rhainds, D.; et al. Colchicine reduces lung injury in experimental acute respiratory distress syndrome. PLoS ONE 2020, 15, e0242318. [Google Scholar] [CrossRef] [PubMed]
- Bourguiba, R.; Delplanque, M.; Vinit, C.; Ackermann, F.; Savey, L.; Grateau, G.; Hentgen, V.; Georgin-lavialle, S. Clinical course of COVID-19 in a cohort of 342 familial Mediterranean fever patients with a long-term treatment by colchicine in a French endemic area. Ann. Rheum. Dis. 2021, 80, 539–540. [Google Scholar] [CrossRef] [PubMed]
- Güven, S.C.; Erden, A.; Karakaş, Ö.; Armağan, B.; Usul, E.; Omma, A.; Küçükşahin, O. COVID-19 outcomes in patients with familial Mediterranean fever: A retrospective cohort study. Rheumatol. Int. 2021, 41, 715–719. [Google Scholar] [CrossRef] [PubMed]
- Gendelman, O.; Amital, H.; Bragazzi, N.L.; Watad, A.; Chodick, G. Continuous hydroxychloroquine or colchicine therapy does not prevent infection with SARS-CoV-2: Insights from a large healthcare database analysis. Autoimmun. Rev. 2020, 19, 102566. [Google Scholar] [CrossRef]
- Madrid-García, A.; Pérez, I.; Colomer, J.I.; León-Mateos, L.; Jover, J.A.; Fernández-Gutiérrez, B.; Abásolo-Alcazar, L.; Rodríguez-Rodríguez, L. Influence of colchicine prescription in COVID-19-related hospital admissions: A survival analysis. Ther. Adv. Musculoskelet. Dis. 2021, 13, 1759720X2110026. [Google Scholar] [CrossRef]
- Haslak, F.; Yildiz, M.; Adrovic, A.; Sahin, S.; Koker, O.; Aliyeva, A.; Barut, K.; Kasapcopur, O. Management of childhood-onset autoinflammatory diseases during the COVID-19 pandemic. Rheumatol. Int. 2020, 40, 1423–1431. [Google Scholar] [CrossRef]
- Günendi, Z.; Yurdakul, F.G.; Bodur, H.; Cengiz, A.K.; Uçar, Ü.; Çay, H.F.; Şen, N.; Keskin, Y.; Gürer, G.; Melikoğlu, M.A.; et al. The impact of COVID-19 on familial Mediterranean fever: A nationwide study. Rheumatol. Int. 2021, 41, 1447–1455. [Google Scholar] [CrossRef] [PubMed]
- Kharouf, F.; Ishay, Y.; Kenig, A.; Bitan, M.; Ben-Chetrit, E. Incidence and course of COVID-19 hospitalizations among patients with familial Mediterranean fever. Rheumatology 2021, 69, SI85–SI89. [Google Scholar] [CrossRef]
- Deftereos, S.; Giannopoulos, G.; Vrachatis, D.A.; Siasos, G.; Giotaki, S.G.; Cleman, M.; Dangas, G.; Stefanadis, C. Colchicine as a potent anti-inflammatory treatment in COVID-19: Can we teach an old dog new tricks? Eur. Hear. J. Cardiovasc. Pharmacother. 2020, 6, 255. [Google Scholar] [CrossRef]
- Giannopoulos, G.; Vrachatis, D.A.; Deftereos, S.G. Myocardial Injury in COVID-19—Can We Successfully Target Inflammation? JAMA Cardiol. 2020, 5, 1069. [Google Scholar] [CrossRef]
- Dabbagh, M.F.; Aurora, L.; D’Souza, P.; Weinmann, A.J.; Bhargava, P.; Basir, M.B. Cardiac Tamponade Secondary to COVID-19. JACC Case Rep. 2020, 2, 1326–1330. [Google Scholar] [CrossRef] [PubMed]
- Recalcati, S.; Piconi, S.; Franzetti, M.; Barbagallo, T.; Prestinari, F.; Fantini, F. Colchicin treatment of COVID-19 presenting with cutaneous rash and myopericarditis. Dermatol. Ther. 2020, 33, e13891. [Google Scholar] [CrossRef]
- Montealegre-Gómez, G.; Garavito, E.; Gómez-López, A.; Rojas-Villarraga, A.; Parra-Medina, R. Colchicine: A potential therapeutic tool against COVID-19. Experience of 5 patients. Reumatol. Clínica 2020, 17, 371–375. [Google Scholar] [CrossRef]
- Gandolfini, I.; Delsante, M.; Fiaccadori, E.; Zaza, G.; Manenti, L.; Degli Antoni, A.; Peruzzi, L.; Riella, L.V.; Cravedi, P.; Maggiore, U. COVID-19 in kidney transplant recipients. Am. J. Transplant. 2020, 20, 1941–1943. [Google Scholar] [CrossRef]
- Della-Torre, E.; Della-Torre, F.; Kusanovic, M.; Scotti, R.; Ramirez, G.A.; Dagna, L.; Tresoldi, M. Treating COVID-19 with colchicine in community healthcare setting. Clin. Immunol. 2020, 217, 108490. [Google Scholar] [CrossRef]
- Deftereos, S.G.; Giannopoulos, G.; Vrachatis, D.A.; Siasos, G.D.; Giotaki, S.G.; Gargalianos, P.; Metallidis, S.; Sianos, G.; Baltagiannis, S.; Panagopoulos, P.; et al. Effect of Colchicine vs. Standard Care on Cardiac and Inflammatory Biomarkers and Clinical Outcomes in Patients Hospitalized With Coronavirus Disease 2019. JAMA Netw. Open 2020, 3, e2013136. [Google Scholar] [CrossRef] [PubMed]
- Bikdeli, B.; Madhavan, M.V.; Jimenez, D.; Chuich, T.; Dreyfus, I.; Driggin, E.; Nigoghossian, C.D.; Ageno, W.; Madjid, M.; Guo, Y.; et al. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-Up: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 2950–2973. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Tardif, J.-C.; Bouabdallaoui, N.; L’Allier, P.L.; Gaudet, D.; Shah, B.; Pillinger, M.H.; Lopez-Sendon, J.; da Luz, P.; Verret, L.; Audet, S.; et al. Colchicine for community-treated patients with COVID-19 (COLCORONA): A phase 3, randomised, double-blinded, adaptive, placebo-controlled, multicentre trial. Lancet Respir. Med. 2021, 9, 924–932. [Google Scholar] [CrossRef]
- Horby, P.W.; Campbell, M.; Spata, E.; Emberson, J.R.; Staplin, N.; Pessoa-Amorim, G.; Peto, L.; Wiselka, M.; Wiffen, L.; Tiberi, S.; et al. Colchicine in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. medRxiv 2021. [Google Scholar] [CrossRef]
- Colchicine in Patients Hospitalized with COVID-19—ECLA PHRI COLCOVID n.d. Available online: https://www.acc.org/Latest-in-Cardiology/Clinical-Trials/2021/08/26/01/09/ECLA-PHRI-COLCOVID (accessed on 29 August 2021).
- Lopes, M.I.; Bonjorno, L.P.; Giannini, M.C.; Amaral, N.B.; Menezes, P.I.; Dib, S.M.; Gigante, S.L.; Benatti, M.N.; Rezek, U.C.; Emrich-Filho, L.L.; et al. Beneficial effects of colchicine for moderate to severe COVID-19: A randomised, double-blinded, placebo-controlled clinical trial. RMD Open 2021, 7, e001455. [Google Scholar] [CrossRef]
- Mareev, V.Y.; Orlova, Y.A.; Plisyk, A.G.; Pavlikova, E.P.; Akopyan, Z.A.; Matskeplishvili, S.T.; Malakhov, P.S.; Krasnova, T.N.; Seredenina, E.M.; Potapenko, A.V.; et al. Proactive anti-inflammatory therapy with colchicine in the treatment of advanced stages of new coronavirus infection. The first results of the COLORIT study. Kardiologiia 2021, 61, 15–27. [Google Scholar] [CrossRef]
- Brunetti, L.; Diawara, O.; Tsai, A.; Firestein, B.L.; Nahass, R.G.; Poiani, G.; Schlesinger, N. Colchicine to Weather the Cytokine Storm in Hospitalized Patients with COVID-19. J. Clin. Med. 2020, 9, 2961. [Google Scholar] [CrossRef]
- Scarsi, M.; Piantoni, S.; Colombo, E.; Airó, P.; Richini, D.; Miclini, M.; Bertasi, V.; Bianchi, M.; Bottone, D.; Civelli, P.; et al. Association between treatment with colchicine and improved survival in a single-centre cohort of adult hospitalised patients with COVID-19 pneumonia and acute respiratory distress syndrome. Ann. Rheum. Dis. 2020, 79, 1286–1289. [Google Scholar] [CrossRef]
- Sandhu, T.; Tieng, A.; Chilimuri, S.; Franchin, G. A Case Control Study to Evaluate the Impact of Colchicine on Patients Admitted to the Hospital with Moderate to Severe COVID-19 Infection. Can. J. Infect. Dis. Med. Microbiol. 2020, 2020, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kevorkian, J.-P.; Lopes, A.; Sène, D.; Riveline, J.-P.; Vandiedonck, C.; Féron, F.; Nassarmadji, K.; Mouly, S.; Mauvais-Jarvis, F.; Gautier, J.-F.; et al. Oral corticoid, aspirin, anticoagulant, colchicine, and furosemide to improve the outcome of hospitalized COVID-19 patients—The COCAA-COLA cohort study. J. Infect. 2021, 82, 276–316. [Google Scholar] [CrossRef]
- Manenti, L.; Maggiore, U.; Fiaccadori, E.; Meschi, T.; Antoni, A.D.; Nouvenne, A.; Ticinesi, A.; Cerundolo, N.; Prati, B.; Delsante, M.; et al. Reduced mortality in COVID-19 patients treated with colchicine: Results from a retrospective, observational study. PLoS ONE 2021, 16, e0248276. [Google Scholar] [CrossRef] [PubMed]
- García-Posada, M.; Aruachan-Vesga, S.; Mestra, D.; Humánez, K.; Serrano-Coll, H.; Cabrales, H.; Faccini, Á.; Mattar, S. Clinical outcomes of patients hospitalized for COVID-19 and evidence-based on the pharmacological management reduce mortality in a region of the Colombian Caribbean. J. Infect. Public Health 2021, 14, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Karatza, E.; Ismailos, G.; Karalis, V. Colchicine for the treatment of COVID-19 patients: Efficacy, safety, and model informed dosage regimens. Xenobiotica 2021, 51, 643–656. [Google Scholar] [CrossRef]
- Vrachatis, D.A.; Giannopoulos, G.V.; Giotaki, S.G.; Raisakis, K.; Kossyvakis, C.; Iliodromitis, K.E.; Reimers, B.; Tousoulis, D.; Cleman, M.; Stefanadis, C.; et al. Impact of colchicine on mortality in patients with COVID-19: A meta-analysis. Hell. J. Cardiol. 2021. [Epub ahead of print]. [Google Scholar] [CrossRef] [PubMed]
- Salah, H.M.; Mehta, J.L. Meta-analysis of the Effect of Colchicine on Mortality and Mechanical Ventilation in COVID-19. Am. J. Cardiol. 2021, 145, 170–172. [Google Scholar] [CrossRef]
- Hariyanto, T.I.; Halim, D.A.; Jodhinata, C.; Yanto, T.A.; Kurniawan, A. Colchicine treatment can improve outcomes of coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. Clin. Exp. Pharmacol. Physiol. 2021, 48, 823–830. [Google Scholar] [CrossRef]
- Chiu, L.; Chow, R.; Chiu, N.; Lo, C.-H.; Aggarwal, R.; Lee, J.; Choi, Y.-G.; Lam, H.; Prsic, E.H.; Shin, H.J. Colchicine use in patients with COVID-19: A systematic review and meta-analysis. medRxiv 2021. [Google Scholar] [CrossRef]
- Golpour, M.; Mousavi, T.; Alimohammadi, M.; Mosayebian, A.; Shiran, M.; Alizadeh Navaei, R.; Rafiei, A. The effectiveness of Colchicine as an anti-inflammatory drug in the treatment of coronavirus disease 2019: Meta-analysis. Int. J. Immunopathol. Pharmacol. 2021, 35, 205873842110317. [Google Scholar] [CrossRef] [PubMed]
- Elshafei, M.N.; El-Bardissy, A.; Khalil, A.; Danjuma, M.; Mubasher, M.; Abubeker, I.Y.; Mohamed, M.F.H. Colchicine use might be associated with lower mortality in COVID-19 patients: A meta-analysis. Eur. J. Clin. Invest. 2021, 51, e13645. [Google Scholar] [CrossRef] [PubMed]
- Nawangsih, E.N.; Kusmala, Y.Y.; Rakhmat, I.I.; Handayani, D.R.; Juliastuti, H.; Wibowo, A.; Lim, M.A.; Pranata, R. Colchicine and mortality in patients with coronavirus disease 2019 (COVID-19) pneumonia: A systematic review, meta-analysis, and meta-regression. Int. Immunopharmacol. 2021, 96, 107723. [Google Scholar] [CrossRef] [PubMed]
- Lien, C.-H.; Lee, M.-D.; Weng, S.-L.; Lin, C.-H.; Liu, L.Y.-M.; Tai, Y.-L.; Lei, W.-T.; Liu, J.-M.; Huang, Y.-N.; Chi, H.; et al. Repurposing Colchicine in Treating Patients with COVID-19: A Systematic Review and Meta-Analysis. Life 2021, 11, 864. [Google Scholar] [CrossRef]
- Bassetti, M.; Giacobbe, D.R.; Bruzzi, P.; Barisione, E.; Centanni, S.; Castaldo, N.; Corcione, S.; De Rosa, F.G.; Di Marco, F.; Gori, A.; et al. Clinical Management of Adult Patients with COVID-19 Outside Intensive Care Units: Guidelines from the Italian Society of Anti-Infective Therapy (SITA) and the Italian Society of Pulmonology (SIP). Infect. Dis. Ther. 2021, 30, 1–49. [Google Scholar] [CrossRef]


| Therapeutic Strategy | Agent/Approach Tested |
|---|---|
| Immunization | active immunity: vaccines passive immunity: plasma, immunoglobulins |
| Antiviral Agents | fusion inhibitors RNA polymerase inhibitors protease inhibitors endosome acidification inhibitors |
| Supportive Care | ECMO artificial liver system (ALS) cytokine filters thromboprophylaxis |
| Immunomodulation | Interferons Steroids Colchicine Macrolides JAK inhibitors BTK inhibitors IL-6 inhibitors IL-1 inhibitors Anti-GM-CSF Abs Thalidomide Cell therapies |
| Author Country Year | Design | n | Age | ♀ (%) | BMI (kg/m2) | DM (%) | Colchicine Dosage | SoC | Findings | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Randomized Controlled Trials | ||||||||||
| Deftereos SG [87] Greece April 2020 | prospective, open-label, RCT | 105 | 64 | 41.9 | 27.5 | 10.5 | L:2 mg M: 0.5 mg BD up to 21 days | HCQ ± AZM ± LPV/r ± tocilizumab | WHO-OSCD: 1.8% vs. 14% OR 0.11; 95% CI: 0.01–0.96; p: 0.02 | Positive |
| Tardif JC et al. [90] Multinational March 2020–January 2021 | double-blinded RCT non-hospitalized patients | 4488 | 54 | 53.9 | 30 | 19.9 | 0.5 mg BD 3d 0.5 mg OD 27d | HCQ OACs Anti-platelets | Composite of death or hospitalization 4.7% vs. 5.8% OR:0.79; 95% CI: 0.61–1.03; p: 0.081 Secondary endpoint, Mechanical ventilation OR: 0.53; 95% CI: 0.25–1.09 secondary analysis PCR-confirmed COVID-19 Composite of death or hospitalization 4.6% vs. 6% OR: 0.75; 95% CI: 0.57–0.99; p: 0.042 Any hospital admission (not pre-specified) OR: 0.76; 95% CI: 0.58–0.99; p: 0.04 | Neutral |
| Lopes M. et al. [93] Brazil April–August 2020 | double blinded RCT | 72 | 55 | 54 | 31.6 | 39 | 0.5 mg q 8 h 5 days 0.5 mg BD 5 days | AZM 500mg OD up to 7 days HCQ 400 mg BD daily for 2 days, then 400 mg OD up to 8 days | Need for supplemental oxygen 4.0 days vs. 6.5 days; p < 0.001 Time of hospitalization 7.0 days vs. 9.0 days; p: 0.003 | Positive |
| Mareev VY et al. [94] (COLORIT study) Russia | prospective comparative RCT | 43 | 61 | 30 | 30.4 | 11.6 | 1 mg/day for 3 days 0.5 mg/day for 14 days | NA | Primary endpoint SHOCS-COVID score change day 12 8 to 2 vs. 7 to 7 (p: 0.002) Secondary outcomes Hospital stay: 13 vs. 17.5; p: 0.079 Any oxygen support: decreased from 50% to 9.5%; p: 0.011 Deaths: 0 vs. 2; p: 0.467 | Positive |
| Horby PW et al. [91] Pre-print RECOVERY NCT04381936 Multinational November 2020–March 2021 | open-label, RCT | 11340 | 63.4 | 30.5 | NA | 25.5 | L: 1 mg + 0.5 mg 12 h later M: 0.5 mg BD up to 10 days | corticosteroids remdesivir tocilizumab convalescent plasma baricitinib aspirin | Primary outcome 28-day all-cause mortality RR: 1.01; 95% CI: 0.93–1.0; p: 0.77 Secondary outcomes time to discharge 10 vs. 10 d invasive mechanical ventilation 11 vs. 11% | Neutral |
| Observational Studies | ||||||||||
| Brunetti L et al. [95] USA March–May 2020 | propensity matched retrospective observational cohort | 66 | 62.9 | 34.8 | 30.7 | 21.2 | L: 1.2 mg M: 0.6 mg BD | AZM, HCQ ± remdesivir or tocilizumab | 28-days mortality OR:0.20; 95% Cl:0.05–0.80; p: 0.023 WHO-OSCI days 14 and 28 57.6% vs. 51.5%; p: 0.621 Not requiring supplemental oxygen on days 14 and 28 54.5% vs. 54.5%, p: 1.0 Hospital discharge by day 28 OR: 5.0; 95% CI: 1.25–20.1; p: 0.023 | Positive |
| Scarsi M et al. [96] Italy March–April 2020 | retrospective, case-control observational study | 262 | 70 | 36.5 | NA | NA | M: 1 mg/day | HCQ ± dexamethasone ± LPV/r | 21 days survival rate: 84.2% vs. 63.6%, p: 0.001 adj HR: 0.151; 95% CI: 0.062–0.368; p < 0.0001 | Positive |
| Sandhu T et al. [97] USA March–May 2020 | prospective comparative cohort study | 112 | 67 | 43 | 27.5 | 42 | 0.6 mg BD 3 days 0.6 mg OD for 9 days | HCQ, steroids, oseltmamivir Excluded if on: lamivudine dolutegravir, tocilizumab convalescent plasma | Mortality: 49.1% vs. 72.9%; p: 0.002 Intubations: 52.8% vs. 73.6%; p: 0.006 Discharge rate: 50.9% vs. 27.1%; p: 0.002 | Positive |
| Kevorkian JP et al. [98] (COCAA-COLA study) France January–November 2020 | observational cohort study | 68 | 66 | 22 | 27 | 44 | Prednisone 1 mg/kg/day Furosemide 80 mg/day Salicylate 75 mg/day Colchicine L: 1.5 mg M: 0.5 mg q 8 h Rivaroxaban or Apixaban | dexamethasone (6 mg OD for up to 10 days) LMWH | Primary composite endpoint OR: 0.097; 95% CI: 0.001–0.48; p: 0.0009
| Positive |
| Manenti L et al. [99] Italy February–April 2020 | retrospective cohort study age & sex matched | 141 | 61.5 | 29 | 27.5 | 17 | M: 1 mg/day up to 21 days | ABX, antivirals, HCQ, i.v steroids, tocilizumab | 21 days mortality adj HR: 0.24; 95% CI: 0.09–0.67; p: 0.006 WHO-OSCI: adj relative improvement rate 1.80 95% CI: 1.00–3.22; p: 0.048 | Positive |
| García-Posada M et al. [100] Colombia May–August 2020 | descriptive observational study | 209 | 60 | 39 | NA | 25.3 | L:2 mg M: 0.5 mg BD up to 20 days | varying combinations of ABX corticosteroids LMWH or tocilizumab | All-cause mortality: (combination of ABX, LMWH, colchicine, corticosteroids) OR: 0.26; 95% CI: 0.08−0.71; p < 0.05 | positive |
| Meta-Analysis | Journal | n | Studies Included | Mortality Effect |
|---|---|---|---|---|
| Vrachatis et al., 2021 [102] | Hell J Cardiol | 881 | 6 studies 3 cohorts 2 RCTs 1 case-control | OR 0.35 (95% CI: 0.24–0.52; p < 0.05) |
| Salah et al., 2021 [103] | Am J Cardiol | 5259 | 8 studies 4 cohorts 3 RCTs 1 case-control | RR 0.62 (95% CI: 0.48–0.81; p = 0.0005) |
| Hariyanto et al., 2021 [104] | Clin Exp Pharmacol Physiol | 5778 | 8 studies 4 cohorts 3 RCTs 1 case-control | OR 0.43 (95% CI: 0.32–0.58; p = n/R) |
| Chiu et al., 2021 [105] | medRxiv (pre-print) | 5033 | 6 studies 3 cohorts 3 RCTs | OR 0.36 (95% CI: 0.17–0.76; p = n/R) |
| Golpour et al., 2021 [106] | Int J Immunopathol Pharmacol | 5678 | 10 studies 5 cohorts 4 RCTs 1 case-control | RR 0.365 (95% CI: 0.555–0.748; p < 0.05) |
| Elshafei et al., 2021 [107] | Eur J Clin Invest | 5522 | 9 studies 4 cohorts 4 RCTs 1 case-control | OR 0.35 (95% CI: 0.25–0.48; p = n/R) |
| Nawangsih et al., 2021 [108] | Int Immunopharmacol | 5530 | 8 studies 5 cohorts 3 RCTs | OR 0.47 (95% CI: 0.31–0.72; p = 0.001) |
| Lien et al., 2021 [109] | Life (Basel) | 17,205 | 11 studies 7 cohorts 4 RCTs | OR 0.57 (95% CI: 0.38–0.87; p < 0.01) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vrachatis, D.A.; Papathanasiou, K.A.; Giotaki, S.G.; Raisakis, K.; Kossyvakis, C.; Kaoukis, A.; Kolokathis, F.; Deftereos, G.; Iliodromitis, K.E.; Avramides, D.; et al. Immunologic Dysregulation and Hypercoagulability as a Pathophysiologic Background in COVID-19 Infection and the Immunomodulating Role of Colchicine. J. Clin. Med. 2021, 10, 5128. https://doi.org/10.3390/jcm10215128
Vrachatis DA, Papathanasiou KA, Giotaki SG, Raisakis K, Kossyvakis C, Kaoukis A, Kolokathis F, Deftereos G, Iliodromitis KE, Avramides D, et al. Immunologic Dysregulation and Hypercoagulability as a Pathophysiologic Background in COVID-19 Infection and the Immunomodulating Role of Colchicine. Journal of Clinical Medicine. 2021; 10(21):5128. https://doi.org/10.3390/jcm10215128
Chicago/Turabian StyleVrachatis, Dimitrios A., Konstantinos A. Papathanasiou, Sotiria G. Giotaki, Konstantinos Raisakis, Charalampos Kossyvakis, Andreas Kaoukis, Fotis Kolokathis, Gerasimos Deftereos, Konstantinos E. Iliodromitis, Dimitrios Avramides, and et al. 2021. "Immunologic Dysregulation and Hypercoagulability as a Pathophysiologic Background in COVID-19 Infection and the Immunomodulating Role of Colchicine" Journal of Clinical Medicine 10, no. 21: 5128. https://doi.org/10.3390/jcm10215128
APA StyleVrachatis, D. A., Papathanasiou, K. A., Giotaki, S. G., Raisakis, K., Kossyvakis, C., Kaoukis, A., Kolokathis, F., Deftereos, G., Iliodromitis, K. E., Avramides, D., Bogossian, H., Siasos, G., Giannopoulos, G., Reimers, B., Lansky, A., Tardif, J.-C., & Deftereos, S. (2021). Immunologic Dysregulation and Hypercoagulability as a Pathophysiologic Background in COVID-19 Infection and the Immunomodulating Role of Colchicine. Journal of Clinical Medicine, 10(21), 5128. https://doi.org/10.3390/jcm10215128








