The Impact of Superficial Vessel Density on Glaucoma Progression according to the Stage of Glaucoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Optical Coherence Tomography Angiography
2.3. Assessment of Visual Field Progression
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tham, Y.-C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.-Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Hood, D.C.; Slobodnick, A.; Raza, A.S.; de Moraes, C.G.; Teng, C.C.; Ritch, R. Early glaucoma involves both deep local, and shallow widespread, retinal nerve fiber damage of the macular region. Investig. Ophthalmol. Vis. Sci. 2014, 55, 632–649. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.S.; Cho, J.; de Moraes, C.G.; Wang, M.; Zhang, X.; Kardon, R.H.; Liebmann, J.M.; Ritch, R.; Hood, D.C. Retinal ganglion cell layer thickness and local visual field sensitivity in glaucoma. Arch. Ophthalmol. 2011, 129, 1529–1536. [Google Scholar] [CrossRef] [PubMed]
- Susanna, R.; Nicolela, M.T.; Soriano, D.S.; Carvalho, C. Automated perimetry: A study of the glaucoma hemifield test for the detection of early glaucomatous visual field loss. J. Glaucoma 1994, 3, 12–16. [Google Scholar] [CrossRef]
- Tatemichi, M.; Nakano, T.; Tanaka, K.; Hayashi, T.; Nawa, T.; Miyamoto, T.; Hiro, H.; Iwasaki, A.; Sugita, M. Performance of glaucoma mass screening with only a visual field test using frequency-doubling technology perimetry. Am. J. Ophthalmol. 2002, 134, 529–537. [Google Scholar] [CrossRef]
- Asano, S.; Murata, H.; Matsuura, M.; Fujino, Y.; Asaoka, R. Early detection of glaucomatous visual field progression using pointwise linear regression with binomial test in the central 10 degrees. Am. J. Ophthalmol. 2019, 199, 140–149. [Google Scholar] [CrossRef]
- Mwanza, J.-C.; Budenz, D.L.; Warren, J.L.; Webel, A.D.; Reynolds, C.E.; Barbosa, D.T.; Lin, S. Retinal nerve fibre layer thickness floor and corresponding functional loss in glaucoma. Br. J. Ophthalmol. 2015, 99, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Banister, K.R.; Boachie, C.; Bourne, R.; Cook, J.; Burr, J.M.; Ramsay, C.; Garway-Heath, D.; Gray, J.; McMeekin, P.; Hernández, R.; et al. Can automated imaging for optic disc and retinal nerve fiber layer analysis aid glaucoma detection? Ophthalmology 2016, 123, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Yu, M.; Leung, C. Impact of segmentation errors and retinal blood vessels on retinal nerve fibre layer measurements using spectral-domain optical coherence tomography. Acta Ophthalmol. 2016, 94, e211–e219. [Google Scholar] [CrossRef] [PubMed]
- Hood, D.C.; Kardon, R. A framework for comparing structural and functional measures of glaucomatous damage. Prog. Retin. Eye Res. 2007, 26, 688–710. [Google Scholar] [CrossRef] [PubMed]
- Heijl, A.; Lindgren, A.; Lindgren, G. Test-retest variability in glaucomatous visual fields. Am. J. Ophthalmol. 1989, 108, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Wall, M.; Woodward, K.R.; Doyle, C.K.; Artes, P. Repeatability of automated perimetry: A comparison between standard automated perimetry with stimulus size III and V, matrix, and motion perimetry. Investig. Ophthalmol. Vis. Sci. 2009, 50, 974–979. [Google Scholar] [CrossRef]
- Yarmohammadi, A.; Zangwill, L.M.; Diniz-Filho, A.; Suh, M.H.; Manalastas, P.I.; Fatehee, N.; Yousefi, S.; Belghith, A.; Saunders, L.J.; Medeiros, F.A.; et al. Optical coherence tomography angiography vessel density in healthy, glaucoma suspect, and glaucoma eyes. Investig. Ophthalmol. Vis. Sci. 2016, 57, OCT451–OCT459. [Google Scholar] [CrossRef] [PubMed]
- Rao, H.L.; Pradhan, Z.S.; Weinreb, R.N.; Riyazuddin, M.; Dasari, S.; Venugopal, J.P.; Puttaiah, N.K.; Rao, D.A.S.; Devi, S.; Mansouri, K.; et al. A comparison of the diagnostic ability of vessel density and structural measurements of optical coherence tomography in primary open angle glaucoma. PLoS ONE 2017, 12, e0173930. [Google Scholar] [CrossRef]
- Ghahari, E.; Bowd, C.; Zangwill, L.M.; Proudfoot, J.; Hasenstab, K.A.; Hou, H.; Penteado, R.C.; Manalastas, P.I.C.; Moghimi, S.; Shoji, T.; et al. Association of macular and circumpapillary microvasculature with visual field sensitivity in advanced glaucoma. Am. J. Ophthalmol. 2019, 204, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.W.; Lee, J.; Kwon, J.; Jo, Y.; Jeong, D.; Shon, G.; Kook, M.S. Relationship between macular vessel density and central visual field sensitivity at different glaucoma stages. Br. J. Ophthalmol. 2019, 103, 1827–1833. [Google Scholar] [CrossRef]
- Martucci, A.; Giannini, C.; Di Marino, M.; Sorge, R.P.; Aiello, F.; Scuteri, D.; Mancino, R.; Nucci, C.; Cesareo, M. Evaluation of putative differences in vessel density and flow area in normal tension and high-pressure glaucoma using OCT-angiography. Prog. Brain Res. 2020, 257, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Rao, H.L.; Pradhan, Z.S.; Suh, M.H.; Moghimi, S.; Mansouri, K.; Weinreb, R.N. Optical coherence tomography angiography in glaucoma. J. Glaucoma 2020, 29, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Hodapp, E.; Parish, K.R.; Douglas, R.A. Clinical Decisions in Glaucoma; Mosby: St. Louis, MO, USA, 1993; pp. 52–61. [Google Scholar]
- Jeon, S.J.; Shin, D.-Y.; Park, H.-Y.L.; Park, C.K. Association of retinal blood flow with progression of visual field in glaucoma. Sci. Rep. 2019, 9, 16813. [Google Scholar] [CrossRef]
- Jeon, S.J.; Park, H.-Y.L.; Park, C.K. Effect of macular vascular density on central visual function and macular structure in glaucoma patients. Sci. Rep. 2018, 8, 16009. [Google Scholar] [CrossRef]
- Wu, H.; de Boer, J.; Chen, L.; Chen, T.C. Correlation of localized glaucomatous visual field defects and spectral domain optical coherence tomography retinal nerve fiber layer thinning using a modified structure–function map for OCT. Eye 2015, 29, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.M.; Hong, S.; Kim, C.Y.; Seong, G.J. Relationship between peripapillary retinal nerve fiber layer thickness measured by optical coherence tomography and visual field severity indices. Korean J. Ophthalmol. 2015, 29, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Bambo, M.P.; Güerri, N.; Ferrandez, B.; Cameo, B.; Fuertes, I.; Polo, V.; Garcia-Martin, E. Evaluation of the macular ganglion cell-inner plexiform layer and the circumpapillary retinal nerve fiber layer in early to severe stages of glaucoma: Correlation with central visual function and visual field indexes. Ophthalmic Res. 2017, 57, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Moghimi, S.; Bowd, C.; Zangwill, L.M.; Penteado, R.C.; Hasenstab, K.; Hou, H.; Ghahari, E.; Manalastas, P.I.C.; Proudfoot, J.; Weinreb, R.N. Measurement floors and dynamic ranges of OCT and OCT angiography in glaucoma. Ophthalmology 2019, 126, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.W.; Lee, J.; Kwon, J.; Choi, J.; Kook, M.S. Regional vascular density–visual field sensitivity relationship in glaucoma according to disease severity. Br. J. Ophthalmol. 2017, 101, 1666–1672. [Google Scholar] [CrossRef]
- Yarmohammadi, A.; Zangwill, L.M.; Diniz-Filho, A.; Suh, M.H.; Yousefi, S.; Saunders, L.J.; Belghith, A.; Manalastas, P.I.C.; Medeiros, F.A.; Weinreb, R.N. Relationship between optical coherence tomography angiography vessel density and severity of visual field loss in glaucoma. Ophthalmology 2016, 123, 2498–2508. [Google Scholar] [CrossRef]
- Munguba, G.C.; Galeb, S.; Liu, Y.; Landy, D.; Lam, D.; Camp, A.; Samad, S.; Tapia, M.L.; Lee, R.K. Nerve fiber layer thinning lags retinal ganglion cell density following crush axonopathy. Investig. Ophthalmol. Vis. Sci. 2014, 55, 6505–6513. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, B.C.; Stevens, K.T.; Levesque, J.M.; Nuschke, A.C.; Sharpe, G.P.; O’Leary, N.; Archibald, M.L.; Wang, X. Longitudinal in vivo imaging of retinal ganglion cells and retinal thickness changes following optic nerve injury in mice. PLoS ONE 2012, 7, e40352. [Google Scholar] [CrossRef]
- Yan, D.B.; Coloma, F.M.; Metheetrairut, A.; Trope, G.E.; Heathcote, J.G.; Ethier, C.R. Deformation of the lamina cribrosa by elevated intraocular pressure. Br. J. Ophthalmol. 1994, 78, 643–648. [Google Scholar] [CrossRef]
- Sommer, A.; Tielsch, J.M.; Katz, J.; Quigley, H.A.; Gottsch, J.D.; Javitt, J.; Singh, K. Relationship Between intraocular pressure and primary open angle glaucoma among white and black Americans. The Baltimore Eye Survey. Arch. Ophthalmol. 1991, 109, 1090–1095. [Google Scholar] [CrossRef] [PubMed]
- Leskea, M.C.; Heijl, A.; Hyman, L.; Bengtsson, B.; Komaroff, E. Factors for progression and glaucoma treatment: The early manifest glaucoma trial. Curr. Opin. Ophthalmol. 2004, 15, 102–106. [Google Scholar] [CrossRef]
- Leske, M.C.; Heijl, A.; Hussein, M.; Bengtsson, B.; Hyman, L.; Komaroff, E. Factors for glaucoma progression and the effect of treatment: The early manifest glaucoma trial. Arch. Ophthalmol. 2003, 121, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Moghimi, S.; Zangwill, L.M.; Penteado, R.C.; Hasenstab, K.; Ghahari, E.; Hou, H.; Christopher, M.; Yarmohammadi, A.; Manalastas, P.I.C.; Shoji, T.; et al. Macular and optic nerve head vessel density and progressive retinal nerve fiber layer loss in glaucoma. Ophthalmology 2018, 125, 1720–1728. [Google Scholar] [CrossRef]
- Ding, X.; Lu, L.; Yang, J.; Chen, Y.; Ma, J. The peripapillary retinal capillary density is highly correlated with its nerve fibre layer in normal population. Clin. Hemorheol. Microcirc. 2020, 74, 231–239. [Google Scholar] [CrossRef]
- Mansoori, T.; Sivaswamy, J.; Gamalapati, J.S.; Balakrishna, N. Topography and correlation of radial peripapillary capillary density network with retinal nerve fibre layer thickness. Int. Ophthalmol. 2018, 38, 967–974. [Google Scholar] [CrossRef] [PubMed]
- She, X.; Guo, J.; Liu, X.; Zhu, H.; Li, T.; Zhou, M.; Wang, F.; Sun, X. Reliability of vessel density measurements in the peripapillary retina and correlation with retinal nerve fiber layer thickness in healthy subjects using optical coherence tomography angiography. Ophthalmologica 2018, 240, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Takusagawa, H.L.; Liu, L.; Ma, K.N.; Jia, Y.; Gao, S.; Zhang, M.; Edmunds, B.; Parikh, M.; Tehrani, S.; Morrison, J.C.; et al. Projection-resolved optical coherence tomography angiography of macular retinal circulation in glaucoma. Ophthalmology 2017, 124, 1589–1599. [Google Scholar] [CrossRef]
- Liu, L.; Edmunds, B.; Takusagawa, H.L.; Tehrani, S.; Lombardi, L.H.; Morrison, J.C.; Jia, Y.; Huang, D. Projection-resolved optical coherence tomography angiography of the peripapillary retina in glaucoma. Am. J. Ophthalmol. 2019, 207, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Al-Sheikh, M.; Tepelus, T.C.; Nazikyan, T.; Sadda, S.R. Repeatability of automated vessel density measurements using optical coherence tomography angiography. Br. J. Ophthalmol. 2017, 101, 449–452. [Google Scholar] [CrossRef]
- Fernández-Vigo, J.I.; Kudsieh, B.; Macarro-Merino, A.; Arriola-Villalobos, P.; Martínez-De-La-Casa, J.M.; García-Feijóo, J.; Fernández-Vigo, J. Reproducibility of macular and optic nerve head vessel density measurements by swept-source optical coherence tomography angiography. Eur. J. Ophthalmol. 2020, 30, 756–763. [Google Scholar] [CrossRef] [PubMed]
| Early (n, 50) | Moderate to Advanced (n, 52) | p Value | |
|---|---|---|---|
| Age (years) | 54.90 ± 13.93 | 59.15 ± 10.88 | 0.090 a |
| Male (n, %) | 18 (36.0%) | 27 (51.9%) | 0.105 b |
| DM (Yes, %) | 4 (8.0%) | 3 (5.8%) | 0.656 b |
| HTN (Yes, %) | 10 (20.0%) | 8 (19.2%) | 0.922 b |
| Total Follow-up Time (months) | 99.64 ± 38.30 | 91.35 ± 32.92 | 0.243 a |
| Baseline IOP (mmHg) | 15.72 ± 2.72 | 15.92 ± 3.85 | 0.760 a |
| CCT (μm) | 532.57 ± 28.24 | 528.31 ± 34.85 | 0.515 a |
| Axial Length (mm) | 25.39 ± 1.71 | 24.74 ± 4.26 | 0.369 a |
| Baseline MD (dB) | −2.17 ± 1.99 | −11.84 ± 3.83 | <0.001 a |
| Baseline PSD (dB) | 3.33 ± 2.19 | 10.86 ± 3.43 | <0.001a |
| Final MD (dB) | −3.26 ± 3.21 | −14.30 ± 5.99 | <0.001a |
| Final PSD (dB) | 4.85 ± 3.24 | 12.04 ± 2.91 | <0.001a |
| Rate of MD Progression (dB/year) | −0.16 ± 0.36 | −0.35 ± 0.56 | 0.049 a |
| Baseline Rim Area | 0.85 ± 0.16 | 0.63 ± 0.26 | <0.001a |
| Baseline Disc Area | 1.95 ± 0.42 | 1.94 ± 0.49 | 0.892 a |
| Baseline Average CD Ratio | 0.73 ± 0.08 | 0.79 ± 0.09 | <0.001a |
| Baseline Average RNFLT (μm) | 77.76 ± 9.68 | 65.02 ± 10.61 | <0.001a |
| Baseline Average GCIPLT (μm) | 71.64 ± 7.35 | 63.80 ± 7.86 | <0.001a |
| Final Rim Area | 0.83 ± 0.15 | 0.67 ± 0.22 | <0.001a |
| Final Disc Area | 1.96 ± 0.48 | 1.92 ± 0.49 | 0.676 a |
| Final Average CD ratio | 0.74 ± 0.08 | 0.79 ± 0.10 | 0.005 a |
| Final Average RNFLT (μm) | 73.02 ± 9.90 | 62.88 ± 10.09 | <0.001a |
| Final Average GCIPLT (μm) | 68.68 ± 8.50 | 63.16 ± 7.44 | 0.001a |
| Early (n, 50) | Moderate to Advanced (n, 52) | p Value | |
|---|---|---|---|
| OCTA SSI | 67.31 ± 5.43 | 64.88 ± 7.19 | 0.129 |
| Circumpapillary Superficial VD (%) | 38.86 ± 2.83 | 34.39 ± 5.08 | <0.001 |
| Circumpapillary Deep VD (%) | 52.15 ± 5.85 | 53.79 ± 4.91 | 0.143 |
| Macular Superficial VD (%) | 35.51 ± 2.23 | 33.04 ± 3.02 | <0.001 |
| Macular Deep VD (%) | 39.42 ± 2.30 | 38.97 ± 2.23 | 0.312 |
| Univariate | Multivariate: Model 1 | Multivariate: Model 2 | ||||
|---|---|---|---|---|---|---|
| B | p Value | B | p Value | B | p Value | |
| Age (year) | −0.005 | 0.152 | ||||
| Baseline IOP (mmHg) | −0.028 | 0.050 | −0.027 | 0.104 | −0.017 | 0.247 |
| Baseline MD (dB) | 0.022 | 0.010 | −0.006 | 0.689 | 0.003 | 0.757 |
| OCTA | ||||||
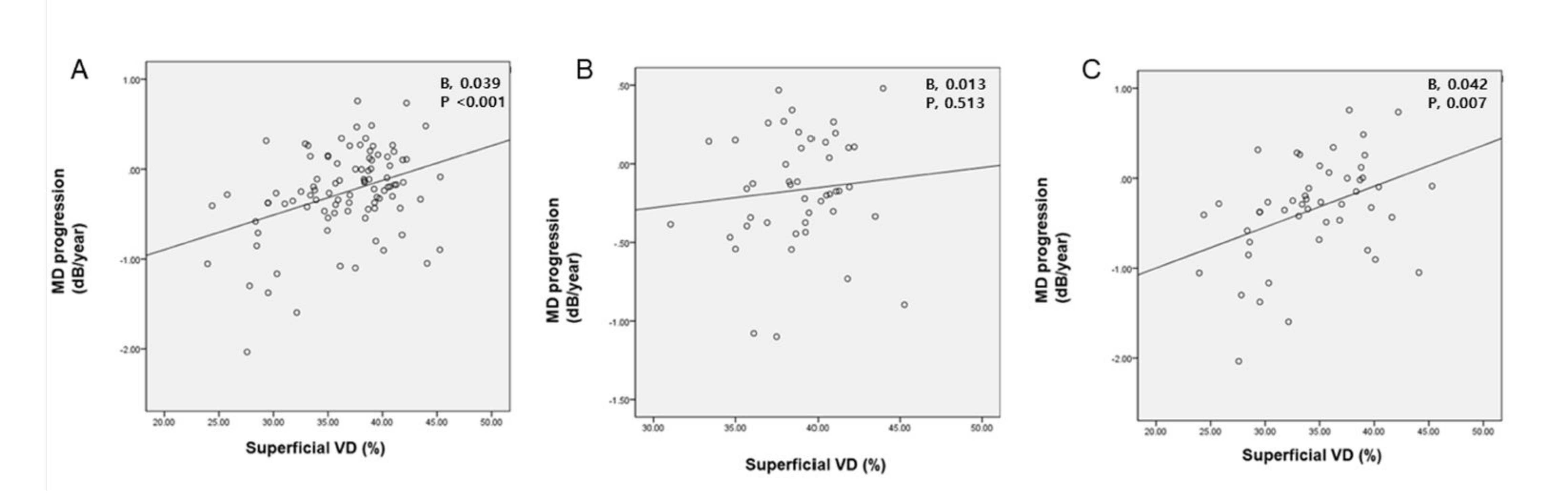
| Circumpapillary Superficial VD (%) | 0.039 | <0.001 | 0.006 | 0.756 | 0.039 | <0.001 |
| Circumpapillary Deep VD (%) | −0.009 | 0.353 | ||||
| Macular Superficial VD (%) | 0.042 | 0.011 | 0.021 | 0.290 | 0.016 | 0.402 |
| Macular Deep VD (%) | 0.023 | 0.284 | ||||
| Baseline OCT | ||||||
| Rim Area | 0.432 | 0.028 | −0.211 | 0.436 | ||
| Average CD Ratio | −0.461 | 0.403 | ||||
| Average RNFLT (μm) | 0.015 | <0.001 | 0.020 | <0.001 | ||
| Average GCIPLT (μm) | 0.017 | 0.021 | −0.003 | 0.744 | ||
| Univariate | Multivariate: Model 1 | Multivariate: Model 2 | ||||
|---|---|---|---|---|---|---|
| B | p Value | B | p Value | B | p Value | |
| Age (year) | 0.002 | 0.628 | ||||
| Baseline IOP (mmHg) | −0.054 | 0.003 | −0.054 | 0.004 | −0.054 | 0.003 |
| Baseline MD (dB) | 0.012 | 0.659 | ||||
| OCTA | ||||||
| Circumpapillary Superficial VD (%) | 0.013 | 0.513 | ||||
| Circumpapillary Deep VD (%) | 0.003 | 0.758 | ||||
| Macular Superficial VD (%) | 0.017 | 0.472 | ||||
| Macular Deep VD (%) | −0.028 | 0.220 | ||||
| Baseline OCT | ||||||
| Rim Area | 0.581 | 0.070 | 0.567 | 0.055 | ||
| Average CD Ratio | −0.027 | 0.968 | ||||
| Average RNFLT (μm) | 0.004 | 0.451 | ||||
| Average GCIPLT (μm) | 0.002 | 0.880 | ||||
| Univariate | Multivariate: Model 1 | Multivariate: Model 2 | ||||
|---|---|---|---|---|---|---|
| B | p Value | B | p Value | B | p Value | |
| Age (year) | −0.014 | 0.052 | −0.004 | 0.592 | −0.009 | 0.198 |
| Baseline IOP (mmHg) | −0.014 | 0.487 | ||||
| Baseline MD (dB) | 0.031 | 0.135 | ||||
| OCTA | ||||||
| Circumpapillary Superficial VD (%) | 0.045 | 0.004 | 0.009 | 0.694 | 0.042 | 0.007 |
| Circumpapillary Deep VD (%) | −0.019 | 0.272 | ||||
| Macular Superficial VD (%) | 0.043 | 0.100 | ||||
| Macular Deep VD (%) | 0.066 | 0.060 | 0.070 | 0.052 | 0.063 | 0.061 |
| Baseline OCT | ||||||
| Rim Area | 0.238 | 0.433 | ||||
| Average CD ratio | −0.169 | 0.855 | ||||
| Average RNFLT (μm) | 0.024 | 0.001 | 0.027 | 0.001 | ||
| Average GCIPLT (μm) | 0.019 | 0.093 | −0.004 | 0.729 | ||
| Eyes with Superficial VD Lower 50% (n, 35) | Eyes with Superficial VD Upper 50% (n, 30) | p Value | |
|---|---|---|---|
| Baseline MD (dB) | −10.78 ± 5.92 | −4.38 ± 4.90 | <0.001 a |
| Baseline PSD (dB) | 8.91 ± 4.47 | 5.58 ± 4.52 | 0.004 a |
| Final MD (dB) | −14.93 ± 7.38 | −6.61 ± 5.18 | <0.001 a |
| Final PSD (dB) | 10.52 ± 3.50 | 8.0 ± 4.95 | 0.024 a |
| Rate of MD Progression (dB/year) | −0.58 ± 0.45 | −0.38 ± 0.30 | 0.041 a |
| Baseline Rim Area | 0.58 ± 0.28 | 0.81 ± 0.18 | <0.001 a |
| Baseline Disc Area | 1.97 ± 0.43 | 1.90 ± 0.36 | 0.479 a |
| Baseline Average CD Ratio | 0.81 ± 0.08 | 0.74 ± 0.07 | <0.001 a |
| Baseline Average RNFLT (μm) | 62.26 ± 9.89 | 76.40 ± 9.53 | <0.001 a |
| Baseline Average GCIPLT (μm) | 61.69 ± 7.64 | 71.35 ± 8.07 | <0.001 a |
| Final Rim Area | 0.65 ± 0.26 | 0.77 ± 0.14 | 0.031 a |
| Final Disc Area | 2.02 ± 0.48 | 1.86 ± 0.40 | 0.173 a |
| Final Average CD Ratio | 0.81 ± 0.09 | 0.76 ± 0.07 | 0.010 a |
| Final Average RNFLT (μm) | 59.60 ± 8.19 | 71.90 ± 9.54 | <0.001 a |
| Final Average GCIPLT (μm) | 60.70 ± 7.72 | 67.93 ± 8.97 | 0.001 a |
| Eyes with Superficial VD Lower 50% (n, 35) | Eyes with Superficial VD Upper 50% (n, 30) | p Value | |
|---|---|---|---|
| OCTA SSI | 61.82 ± 8.05 | 61.0 ± 8.18 | 0.814 |
| Circumpapillary Superficial VD (%) | 32.05 ± 3.64 | 40.30 ± 2.11 | <0.001 |
| Circumpapillary Deep VD (%) | 54.08 ± 5.02 | 52.49 ± 5.20 | 0.218 |
| Macular Superficial VD (%) | 32.35 ± 2.73 | 35.18 ± 2.69 | <0.001 |
| Macular Deep VD (%) | 39.51 ± 2.66 | 39.13 ± 2.32 | 0.546 |
| Univariate | Multivariate: Model 1 | Multivariate: Model 2 | ||||
|---|---|---|---|---|---|---|
| B | p Value | B | p Value | B | p Value | |
| Age (year) | −0.003 | 0.658 | ||||
| Baseline IOP (mmHg) | 0.012 | 0.567 | ||||
| Baseline MD (dB) | 0.018 | 0.166 | ||||
| OCTA | ||||||
| Circumpapillary Superficial VD (%) | 0.049 | 0.021 | 0.047 | 0.016 | 0.049 | 0.021 |
| Circumpapillary Deep VD (%) | −0.003 | 0.832 | ||||
| Macular superficial VD (%) | 0.046 | 0.108 | ||||
| Macular Deep VD (%) | 0.066 | 0.022 | 0.058 | 0.026 | ||
| Baseline OCT | ||||||
| Rim Area | −0.383 | 0.173 | ||||
| Average CD Ratio | 1.685 | 0.068 | 2.451 | 0.003 | ||
| Average RNFLT (μm) | 0.009 | 0.253 | ||||
| Average GCIPLT (μm) | 0.009 | 0.440 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Park, C.K.; Park, H.-Y.L. The Impact of Superficial Vessel Density on Glaucoma Progression according to the Stage of Glaucoma. J. Clin. Med. 2021, 10, 5150. https://doi.org/10.3390/jcm10215150
Lee J, Park CK, Park H-YL. The Impact of Superficial Vessel Density on Glaucoma Progression according to the Stage of Glaucoma. Journal of Clinical Medicine. 2021; 10(21):5150. https://doi.org/10.3390/jcm10215150
Chicago/Turabian StyleLee, Jiyun, Chan Kee Park, and Hae-Young Lopilly Park. 2021. "The Impact of Superficial Vessel Density on Glaucoma Progression according to the Stage of Glaucoma" Journal of Clinical Medicine 10, no. 21: 5150. https://doi.org/10.3390/jcm10215150
APA StyleLee, J., Park, C. K., & Park, H.-Y. L. (2021). The Impact of Superficial Vessel Density on Glaucoma Progression according to the Stage of Glaucoma. Journal of Clinical Medicine, 10(21), 5150. https://doi.org/10.3390/jcm10215150





