Viral Myocarditis—From Pathophysiology to Treatment
Abstract
:1. Introduction
2. General Pathophysiological Aspects
2.1. Phases of Viral-Mediated Myocarditis
2.2. Autoimmunity in Viral Myocarditis
3. Clinical Presentations and Diagnostics
3.1. Endomyocardial Biopsy
3.2. Liquid Biopsy
4. Viruses
4.1. Enteroviruses/Adenoviruses
4.2. Erythroparvovirus
4.3. Herpesviridae
4.4. Hepatitis C Virus and Human Immunodeficiency Virus
4.5. SARS-CoV-2
COVID-19 Vaccine and Myocarditis
4.6. Other Rare Virus Infections
5. Treatment Options
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caforio, A.L.P.; Pankuweit, S.; Arbustini, E.; Basso, C.; Blanes, J.G.; Felix, S.B.; Fu, M.; Heliö, T.; Heymans, S.; Jahns, R.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 2636–2648. [Google Scholar] [CrossRef] [PubMed]
- Bock, C.-T.; Klingel, K.; Kandolf, R. Human Parvovirus B19–Associated Myocarditis. N. Engl. J. Med. 2010, 362, 1248–1249. [Google Scholar] [CrossRef] [PubMed]
- Sagar, S.; Liu, P.P.; Cooper, L.T., Jr. Myocarditis. Lancet 2012, 379, 738–747. [Google Scholar] [CrossRef] [Green Version]
- Schultheiss, H.-P.; Fairweather, D.; Caforio, A.L.P.; Escher, F.; Hershberger, R.; Lipshultz, S.E.; Liu, P.P.; Matsumori, A.; Mazzanti, A.; McMurray, J.; et al. Dilated cardiomyopathy. Nat. Rev. Dis. Prim. 2019, 5, 32. [Google Scholar] [CrossRef]
- Blauwet, L.A.; Cooper, L.T. Myocarditis. Prog. Cardiovasc. Dis. 2010, 52, 274–288. [Google Scholar] [CrossRef] [Green Version]
- Pauschinger, M.; Bowles, N.E.; Fuentes-Garcia, F.J.; Pham, V.; Kühl, U.; Schwimmbeck, P.L.; Schultheiss, H.-P.; Towbin, J.A. Detection of Adenoviral Genome in the Myocardium of Adult Patients with Idiopathic Left Ventricular Dysfunction. Circulation 1999, 99, 1348–1354. [Google Scholar] [CrossRef] [Green Version]
- Schultheiss, H.-P.; Piper, C.; Sowade, O.; Waagstein, F.; Kapp, J.-F.; Wegscheider, K.; Groetzbach, G.; Pauschinger, M.; Escher, F.; Arbustini, E.; et al. Betaferon in chronic viral cardiomyopathy (BICC) trial: Effects of interferon-β treatment in patients with chronic viral cardiomyopathy. Clin. Res. Cardiol. 2016, 105, 763–773. [Google Scholar] [CrossRef]
- Watanabe, M.; Panetta, G.L.; Piccirillo, F.; Spoto, S.; Myers, J.; Serino, F.M.; Costantino, S.; Di Sciascio, G. Acute Epstein-Barr related myocarditis: An unusual but life-threatening disease in an immunocompetent patient. J. Cardiol. Cases 2020, 21, 137–140. [Google Scholar] [CrossRef]
- Ilyas, S.Z.; Tabassum, R.; Hamed, H.; Rehman, S.U.; Qadri, I. Hepatitis C Virus-Associated Extrahepatic Manifestations in Lung and Heart and Antiviral Therapy-Related Cardiopulmonary Toxicity. Viral Immunol. 2017, 30, 633–641. [Google Scholar] [CrossRef]
- Matsumori, A.; Matoba, Y.; Sasayama, S. Dilated Cardiomyopathy Associated with Hepatitis C Virus Infection. Circulation 1995, 92, 2519–2525. [Google Scholar] [CrossRef]
- Matsumori, A.; Shimada, T.; Chapman, N.M.; Tracy, S.M.; Mason, J.W. Myocarditis and Heart Failure Associated with Hepatitis C Virus Infection. J. Card. Fail. 2006, 12, 293–298. [Google Scholar] [CrossRef]
- Escher, F.; Kühl, U.; Gross, U.; Westermann, D.; Poller, W.; Tschöpe, C.; Lassner, D.; Schultheiss, H.-P. Aggravation of left ventricular dysfunction in patients with biopsy-proven cardiac human herpesvirus A and B infection. J. Clin. Virol. 2015, 63, 1–5. [Google Scholar] [CrossRef]
- Kühl, U.; Lassner, D.; Wallaschek, N.; Gross, U.M.; Krueger, G.R.; Seeberg, B.; Kaufer, B.B.; Escher, F.; Poller, W.; Schultheiss, H.-P. Chromosomally integrated human herpesvirus 6 in heart failure: Prevalence and treatment. Eur. J. Heart Fail. 2015, 17, 9–19. [Google Scholar] [CrossRef]
- Bonavita, C.; Cardin, R. Don’t Go Breaking My Heart: MCMV as a Model for HCMV-Associated Cardiovascular Diseases. Pathogens 2021, 10, 619. [Google Scholar] [CrossRef]
- Kho, M.M.L.; Roest, S.; Bovée, D.M.; Metselaar, H.J.; Hoek, R.A.S.; van der Eijk, A.A.; Manintveld, O.C.; Roodnat, J.I.; van Besouw, N.M. Herpes Zoster in Solid Organ Transplantation: Incidence and Risk Factors. Front. Immunol. 2021, 12, 774. [Google Scholar] [CrossRef]
- Palmeira, M.M.; Ribeiro, H.Y.U.; Lira, Y.G.; Neto, F.O.M.J.; Rodrigues, I.A.D.S.; Da Paz, L.N.F.; Pinheiro, M.D.C.N. Heart failure due to cytomegalovirus myocarditis in immunocompetent young adults: A case report. BMC Res. Notes 2016, 9, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Roubille, C.; Brunel, A.-S.; Gahide, G.; Kovacsik, H.V.; Le Quellec, A. Cytomegalovirus (CMV) and Acute Myocarditis in an Immunocompetent Patient. Intern. Med. 2010, 49, 131–133. [Google Scholar] [CrossRef] [Green Version]
- Schmidt-Lucke, C.; Spillmann, F.; Bock, C.-T.; Kühl, U.; Van Linthout, S.; Schultheiss, H.; Tschöpe, C. Interferon Beta Modulates Endothelial Damage in Patients with Cardiac Persistence of Human Parvovirus B19 Infection. J. Infect. Dis. 2010, 201, 936–945. [Google Scholar] [CrossRef]
- Schultheiss, H.-P.; Bock, T.; Pietsch, H.; Aleshcheva, G.; Baumeier, C.; Fruhwald, F.; Escher, F. Nucleoside Analogue Reverse Transcriptase Inhibitors Improve Clinical Outcome in Transcriptional Active Human Parvovirus B19-Positive Patients. J. Clin. Med. 2021, 10, 1928. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Cotella, J.I.; Sauce, A.L.; Saldarriaga, C.I.; Perez, G.E.; Farina, J.M.; Wyss, F.; Liprandi, A.S.; Mendoza, I.; Múnera, A.G.; Alexander, B.; et al. Chikungunya and the Heart. Cardiology 2021, 146, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Farias, L.A.B.G.; Beserra, F.L.C.N.; Fernandes, L.; Teixeira, A.A.R.; Ferragut, J.M.; Girão, E.S.; Neto, R.D.J.P. Myocarditis Following Recent Chikungunya and Dengue Virus Coinfection: A Case Report. Arq. Bras. Cardiol. 2019, 113, 783–786. [Google Scholar] [CrossRef] [PubMed]
- Simon, F.; Paule, P.; Oliver, M. Chikungunya Virus–Induced Myopericarditis: Toward an Increase of Dilated Cardiomyopathy in Countries with Epidemics? Am. J. Trop. Med. Hyg. 2008, 78, 212–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tahir, H.; Daruwalla, V.; Hayat, S. Myocarditis Leading to Severe Dilated Cardiomyopathy in a Patient with Dengue Fever. Case Rep. Cardiol. 2015, 2015, 319312. [Google Scholar] [CrossRef]
- Miranda, C.; Borges, M.; Schmidt, A.; Pazin-Filho, A.; Rossi, M.A.; Ramos, S.G.; Fonseca, B. A case presentation of a fatal dengue myocarditis showing evidence for dengue virus-induced lesion. Eur. Heart J. Acute Cardiovasc. Care 2013, 2, 127–130. [Google Scholar] [CrossRef] [Green Version]
- Ntusi, N.; O’Dwyer, E.; Dorrell, L.; Wainwright, E.; Piechnik, S.; Clutton, G.; Hancock, G.; Ferreira, V.; Cox, P.; Badri, M.; et al. HIV-1–Related Cardiovascular Disease Is Associated with Chronic Inflammation, Frequent Pericardial Effusions, and Probable Myocardial Edema. Circ. Cardiovasc. Imaging 2016, 9, e004430. [Google Scholar] [CrossRef] [Green Version]
- Sengupta, P.; Biswas, S.; Roy, T. Hepatitis E-Induced Acute Myocarditis in an Elderly Woman. Case Rep. Gastroenterol. 2019, 13, 342–349. [Google Scholar] [CrossRef]
- Mamas, M.; Fraser, D.; Neyses, L. Cardiovascular manifestations associated with influenza virus infection. Int. J. Cardiol. 2008, 130, 304–309. [Google Scholar] [CrossRef]
- Kodama, M. Influenza Myocarditis. Circ. J. 2010, 74, 2060–2061. [Google Scholar] [CrossRef]
- Frustaci, A.; Abdulla, A.K.; Caldarulo, M.; Buffon, A. Fatal measles myocarditis. Cardiologia 1990, 35, 347–349. [Google Scholar]
- Choi, M.J.; Song, J.Y.; Yang, T.U.; Jeon, J.H.; Noh, J.Y.; Hong, K.-W.; Cheong, H.J.; Kim, W.J. Acute Myopericarditis caused by Human Metapneumovirus. Infect. Chemother. 2016, 48, 36–40. [Google Scholar] [CrossRef] [Green Version]
- Calabrese, F.; Rigo, E.; Milanesi, O.; Boffa, G.M.; Angelini, A.; Valente, M.; Thiene, G. Molecular Diagnosis of Myocarditis and Dilated Cardiomyopathy in Children: Clinicopathologic Features and Prognostic Implications. Diagn. Mol. Pathol. 2002, 11, 212–221. [Google Scholar] [CrossRef]
- Jemail, L.; Miyao, M.; Hamayasu, H.; Minami, H.; Abiru, H.; Baba, S.; Osamura, T.; Tamaki, K.; Kotani, H. Fatal Mumps Myocarditis Associated with Left Ventricular Non-Compaction. Am. J. Case Rep. 2020, 21, e921177-1. [Google Scholar] [CrossRef]
- Park, S.C.; Crane, I.M.; Pal, K.; Cagnina, R.E. Rabies Encephalitis with Myocarditis Mimicking ST-Elevation Myocardial Infarction. Open Forum Infect. Dis. 2019, 6, ofz260. [Google Scholar] [CrossRef] [Green Version]
- Raman, G.V.; Prosser, A.; Spreadbury, P.; Cockcroft, P.; Okubadejo, O. Rabies presenting with myocarditis and encephalitis. J. Infect. 1988, 17, 155–158. [Google Scholar] [CrossRef]
- Kawashima, H.; Inagaki, N.; Nakayama, T.; Morichi, S.; Nishimata, S.; Yamanaka, G.; Kashiwagi, Y. Cardiac Complications Caused by Respiratory Syncytial Virus Infection: Questionnaire Survey and a Literature Review. Glob. Pediatr. Health 2021, 8. [Google Scholar] [CrossRef]
- Miura, H.; Hattori, F.; Uchida, H.; Hata, T.; Kudo, K.; Sato, M.; Yoshikawa, T. Case report of severe myocarditis in an immunocompromised child with Respiratory Syncytial Virus infection. BMC Pediatr. 2018, 18, 51. [Google Scholar] [CrossRef] [Green Version]
- Harada, T.; Ohtaki, E.; Tobaru, T.; Kitahara, K.; Sumiyoshi, T.; Hosoda, S. Rubella-Associated Perimyocarditis: A case report. Angiology 2002, 53, 727–732. [Google Scholar] [CrossRef]
- Ioannou, A.; Tsappa, I.; Metaxa, S.; Missouris, C.G. Ventricular Fibrillation following Varicella Zoster Myocarditis. Case Rep. Cardiol. 2017, 2017, 1017686. [Google Scholar] [CrossRef] [Green Version]
- Aletti, M.; Lecoules, S.; Kanczuga, V.; Soler, C.; Maquart, M.; Simon, F.; Leparc-Goffart, I. Transient myocarditis associated with acute Zika virus infection. Clin. Infect. Dis. 2016, 64, 678–679. [Google Scholar] [CrossRef]
- Krittanawong, C.; Zhang, H.; Sun, T. Cardiovascular complications after Zika virus infection. Int. J. Cardiol. 2016, 221, 859. [Google Scholar] [CrossRef]
- Minhas, A.M.; Nayab, A.; Iyer, S.; Narmeen, M.; Fatima, K.; Khan, M.S.; Constantin, J. Association of Zika Virus with Myocarditis, Heart Failure, and Arrhythmias: A Literature Review. Cureus 2017, 9. [Google Scholar] [CrossRef] [Green Version]
- Kühl, U.; Pauschinger, M.; Noutsias, M.; Seeberg, B.; Bock, T.; Lassner, D.; Poller, W.; Kandolf, R.; Schultheiss, H.-P. High Prevalence of Viral Genomes and Multiple Viral Infections in the Myocardium of Adults with “Idiopathic” Left Ventricular Dysfunction. Circulation 2005, 111, 887–893. [Google Scholar] [CrossRef] [Green Version]
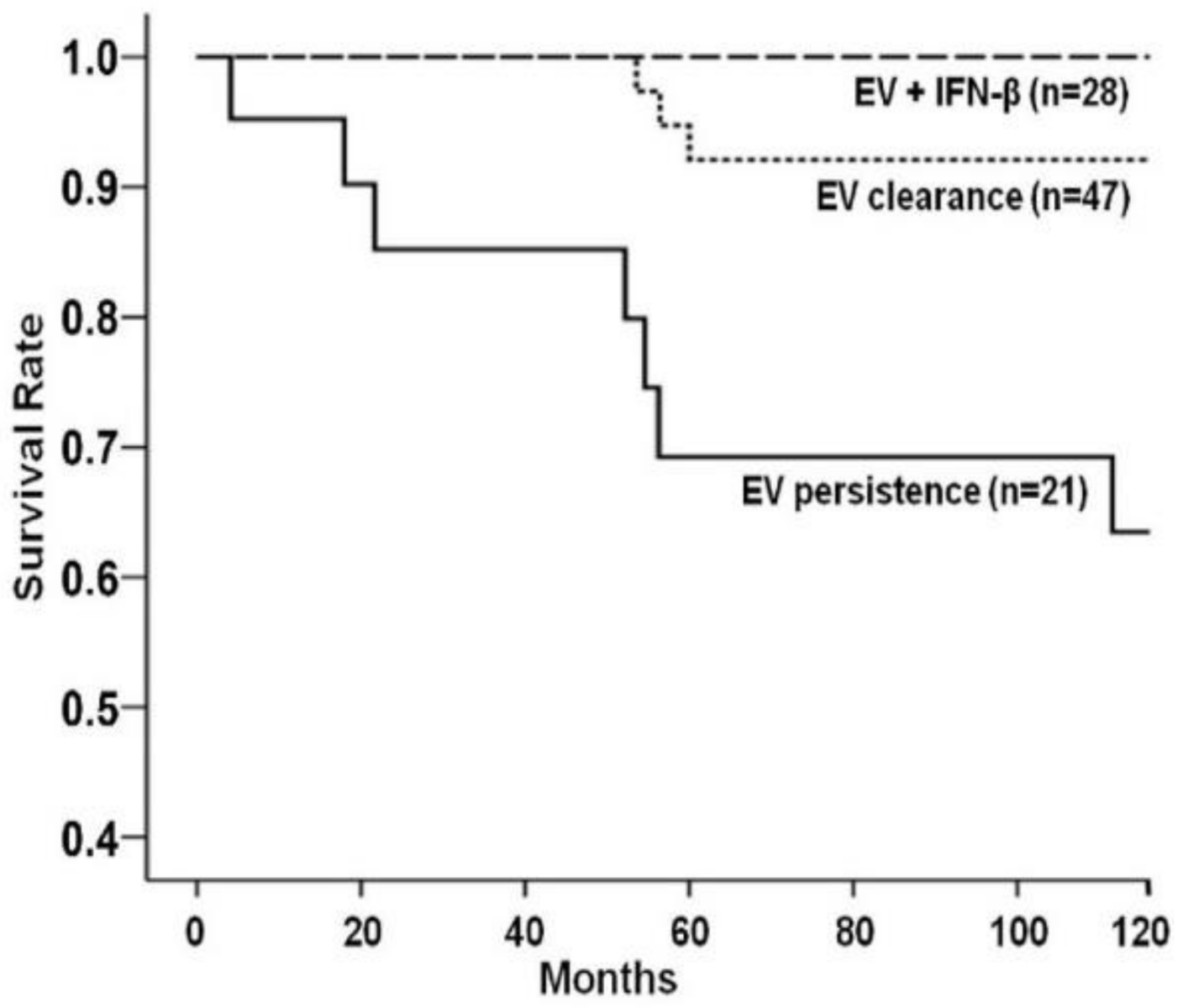
- Kühl, U.; Lassner, D.; von Schlippenbach, J.; Poller, W.; Schultheiss, H.-P. Interferon-Beta Improves Survival in Enterovirus-Associated Cardiomyopathy. J. Am. Coll. Cardiol. 2012, 60, 1295–1296. [Google Scholar] [CrossRef] [Green Version]
- Pauschinger, M.; Doerner, A.; Kuehl, U.; Schwimmbeck, P.L.; Poller, W.; Kandolf, R.; Schultheiss, H.-P. Enteroviral RNA Replication in the Myocardium of Patients with Left Ventricular Dysfunction and Clinically Suspected Myocarditis. Circulation 1999, 99, 889–895. [Google Scholar] [CrossRef] [Green Version]
- Pietsch, H.; Escher, F.; Aleshcheva, G.; Lassner, D.; Bock, C.-T.; Schultheiss, H.-P. Detection of parvovirus mRNAs as markers for viral activity in endomyocardial biopsy-based diagnosis of patients with unexplained heart failure. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Kühl, U.; Lassner, D.; Dorner, A.; Rohde, M.; Escher, F.; Seeberg, B.; Hertel, E.; Tschope, C.; Skurk, C.; Gross, U.M.; et al. A distinct subgroup of cardiomyopathy patients characterized by transcriptionally active cardiotropic erythrovirus and altered cardiac gene expression. Basic Res. Cardiol. 2013, 108, 372. [Google Scholar] [CrossRef]
- Bracamonte-Baran, W.; Čiháková, D. Cardiac Autoimmunity: Myocarditis. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2017; Volume 1003, pp. 187–221. [Google Scholar]
- Rose, N.R. Viral myocarditis. Curr. Opin. Rheumatol. 2016, 28, 383–389. [Google Scholar] [CrossRef] [Green Version]
- Huber, S.A. Viral Myocarditis and Dilated Cardiomyopathy: Etiology and Pathogenesis. Curr. Pharm. Des. 2016, 22, 408–426. [Google Scholar] [CrossRef] [PubMed]
- Esfandiarei, M.; McManus, B.M. Molecular Biology and Pathogenesis of Viral Myocarditis. Annu. Rev. Pathol. Mech. Dis. 2008, 3, 127–155. [Google Scholar] [CrossRef] [PubMed]
- Fairweather, D.; Frisancho-Kiss, S.; Rose, N.R. Viruses as adjuvants for autoimmunity: Evidence from Coxsackievirus-induced myocarditis. Rev. Med. Virol. 2004, 15, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Tschöpe, C.; Ammirati, E.; Bozkurt, B.; Caforio, A.L.P.; Cooper, L.T.; Felix, S.B.; Hare, J.M.; Heidecker, B.; Heymans, S.; Hübner, N.; et al. Myocarditis and inflammatory cardiomyopathy: Current evidence and future directions. Nat. Rev. Cardiol. 2021, 18, 169–193. [Google Scholar] [CrossRef] [PubMed]
- Schultheiss, H.-P.; Kühl, U.; Cooper, L. The management of myocarditis. Eur. Heart J. 2011, 32, 2616–2625. [Google Scholar] [CrossRef] [Green Version]
- Thiene, G.; Bruneval, P.; Veinot, J.; Leone, O. Diagnostic use of the endomyocardial biopsy: A consensus statement. Virchows Arch. 2013, 463, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Leone, O.; Pieroni, M.; Rapezzi, C.; Olivotto, I. The spectrum of myocarditis: From pathology to the clinics. Virchows Arch. 2019, 475, 279–301. [Google Scholar] [CrossRef]
- Hanna, R.; Dalvi, S.; Sălăgean, T.; Pop, I.D.; Bordea, I.R.; Benedicenti, S. Understanding COVID-19 Pandemic: Molecular Mechanisms and Potential Therapeutic Strategies. An Evidence-Based Review. J. Inflamm. Res. 2021, 14, 13–56. [Google Scholar] [CrossRef]
- Rose, N.R.; Neu, N.; Neumann, D.A.; Herskowitz, A. Myocarditis: A Postinfectious Autoimmune Disease. In New Concepts in Viral Heart Disease; Springer: Berlin/Heidelberg, Germany, 1988; pp. 139–147. [Google Scholar]
- Root-Bernstein, R.; Fairweather, D. Unresolved issues in theories of autoimmune disease using myocarditis as a framework. J. Theor. Biol. 2015, 375, 101–123. [Google Scholar] [CrossRef] [Green Version]
- Ammirati, E.; Frigerio, M.; Adler, E.D.; Basso, C.; Birnie, D.H.; Brambatti, M.; Friedrich, M.G.; Klingel, K.; Lehtonen, J.; Moslehi, J.J.; et al. Management of Acute Myocarditis and Chronic Inflammatory Cardiomyopathy. Circ. Heart Fail. 2020, 13, e007405. [Google Scholar] [CrossRef]
- Westermann, D.; Lindner, D.; Kasner, M.; Zietsch, C.; Savvatis, K.; Escher, F.; von Schlippenbach, J.; Skurk, C.; Steendijk, P.; Riad, A.; et al. Cardiac Inflammation Contributes to Changes in the Extracellular Matrix in Patients with Heart Failure and Normal Ejection Fraction. Circ. Heart Fail. 2011, 4, 44–52. [Google Scholar] [CrossRef] [Green Version]
- Kawai, C. From Myocarditis to Cardiomyopathy: Mechanisms of Inflammation and Cell Death. Circulation 1999, 99, 1091–1100. [Google Scholar] [CrossRef] [Green Version]
- Heymans, S.; Eriksson, U.; Lehtonen, J.; Cooper, L.T. The Quest for New Approaches in Myocarditis and Inflammatory Cardiomyopathy. J. Am. Coll. Cardiol. 2016, 68, 2348–2364. [Google Scholar] [CrossRef]
- Monda, E.; Palmiero, G.; Rubino, M.; Verrillo, F.; Amodio, F.; Di Fraia, F.; Pacileo, R.; Fimiani, F.; Esposito, A.; Cirillo, A.; et al. Molecular Basis of Inflammation in the Pathogenesis of Cardiomyopathies. Int. J. Mol. Sci. 2020, 21, 6462. [Google Scholar] [CrossRef]
- ElAmm, C.; Fairweather, D.; Cooper, L.T. Republished: Pathogenesis and diagnosis of myocarditis. Postgrad. Med. J. 2012, 88, 539–544. [Google Scholar] [CrossRef] [Green Version]
- Poller, W.; Haas, J.; Klingel, K.; Kühnisch, J.; Gast, M.; Kaya, Z.; Escher, F.; Kayvanpour, E.; Degener, F.; Opgen-Rhein, B.; et al. Familial Recurrent Myocarditis Triggered by Exercise in Patients with a Truncating Variant of the Desmoplakin Gene. J. Am. Heart Assoc. 2020, 9, e015289. [Google Scholar] [CrossRef]
- Mahfoud, F.; Gärtner, B.; Kindermann, M.; Ukena, C.; Gadomski, K.; Klingel, K.; Kandolf, R.; Böhm, M.; Kindermann, I. Virus serology in patients with suspected myocarditis: Utility or futility? Eur. Heart J. 2011, 32, 897–903. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Lurz, P.; Luecke, C.; Eitel, I.; Föhrenbach, F.; Frank, C.; Grothoff, M.; de Waha, S.; Rommel, K.-P.; Lurz, J.A.; Klingel, K.; et al. Comprehensive Cardiac Magnetic Resonance Imaging in Patients with Suspected Myocarditis. J. Am. Coll. Cardiol. 2016, 67, 1800–1811. [Google Scholar] [CrossRef]
- Kasner, M.; Aleksandrov, A.; Escher, F.; Al-Saadi, N.; Makowski, M.; Spillmann, F.; Genger, M.; Schultheiss, H.-P.; Kühl, U.; Pieske, B.; et al. Multimodality imaging approach in the diagnosis of chronic myocarditis with preserved left ventricular ejection fraction (MCpEF): The role of 2D speckle-tracking echocardiography. Int. J. Cardiol. 2017, 243, 374–378. [Google Scholar] [CrossRef]
- Gutberlet, M.; Spors, B.; Thoma, T.; Bertram, H.; Denecke, T.; Felix, R.; Noutsias, M.; Schultheiss, H.-P.; Kühl, U. Suspected Chronic Myocarditis at Cardiac MR: Diagnostic Accuracy and Association with Immunohistologically Detected Inflammation and Viral Persistence. Radiology 2008, 246, 401–409. [Google Scholar] [CrossRef]
- Aretz, H.T. Myocarditis: The Dallas criteria. Hum. Pathol. 1987, 18, 619–624. [Google Scholar] [CrossRef]
- Escher, F.; Kühl, U.; Lassner, D.; Poller, W.; Westermann, D.; Pieske, B.; Tschöpe, C.; Schultheiss, H.-P. Long-term outcome of patients with virus-negative chronic myocarditis or inflammatory cardiomyopathy after immunosuppressive therapy. Clin. Res. Cardiol. 2016, 105, 1011–1020. [Google Scholar] [CrossRef]
- Baumeier, C.; Escher, F.; Aleshcheva, G.; Pietsch, H.; Schultheiss, H.-P. Plasminogen activator inhibitor-1 reduces cardiac fibrosis and promotes M2 macrophage polarization in inflammatory cardiomyopathy. Basic Res. Cardiol. 2021, 116, 1–9. [Google Scholar] [CrossRef]
- Blanco-Domínguez, R.; Sánchez-Díaz, R.; de la Fuente, H.; Jiménez-Borreguero, L.J.; Matesanz-Marín, A.; Relaño, M.; Jiménez-Alejandre, R.; Linillos-Pradillo, B.; Tsilingiri, K.; Martín-Mariscal, M.L.; et al. A Novel Circulating MicroRNA for the Detection of Acute Myocarditis. N. Engl. J. Med. 2021, 384, 2014–2027. [Google Scholar] [CrossRef]
- Ling, H.; Fabbri, M.; Calin, G.A. MicroRNAs and other non-coding RNAs as targets for anticancer drug development. Nat. Rev. Drug Discov. 2013, 12, 847–865. [Google Scholar] [CrossRef] [Green Version]
- Kuehl, U.; Lassner, D.; Gast, M.; Stroux, A.; Rohde, M.; Siegismund, C.; Wang, X.; Escher, F.; Gross, M.; Skurk, C.; et al. Differential Cardiac MicroRNA Expression Predicts the Clinical Course in Human Enterovirus Cardiomyopathy. Circ. Heart Fail. 2015, 8, 605–618. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Kowdley, K.V. MicroRNAs in Common Human Diseases. Genom. Proteom. Bioinform. 2012, 10, 246–253. [Google Scholar] [CrossRef] [Green Version]
- Kühl, U.; Rohde, M.; Lassner, D.; Gross, U.; Escher, F.; Schultheiss, H.-P. miRNA as activity markers in Parvo B19 associated heart disease. Herz 2012, 37, 637–643. [Google Scholar] [CrossRef]
- Corsten, M.F.; Papageorgiou, A.; Verhesen, W.; Carai, P.; Lindow, M.; Obad, S.; Summer, G.; Coort, S.L.M.; Hazebroek, M.; Van Leeuwen, R.; et al. MicroRNA Profiling Identifies MicroRNA-155 as an Adverse Mediator of Cardiac Injury and Dysfunction During Acute Viral Myocarditis. Circ. Res. 2012, 111, 415–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.-F.; Ding, Y.-J.; Zhang, Z.-X.; Wang, Z.-F.; Luo, C.-L.; Li, B.-X.; Shen, Y.-W.; Tao, L.-Y.; Zhao, Z.-Q. MicroRNA-21 regulation of the progression of viral myocarditis to dilated cardiomyopathy. Mol. Med. Rep. 2014, 10, 161–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aleshcheva, G.; Pietsch, H.; Escher, F.; Schultheiss, H. MicroRNA profiling as a novel diagnostic tool for identification of patients with inflammatory and/or virally induced cardiomyopathies. ESC Heart Fail. 2021, 8, 408–422. [Google Scholar] [CrossRef] [PubMed]
- Bowles, N.E.; Ni, J.; Kearney, D.L.; Pauschinger, M.; Schultheiss, H.-P.; McCarthy, R.; Hare, J.; Bricker, J.; Bowles, K.R.; Towbin, J.A. Detection of viruses in myocardial tissues by polymerase chain reaction: Evidence of adenovirus as a common cause of myocarditis in children and adults. J. Am. Coll. Cardiol. 2003, 42, 466–472. [Google Scholar] [CrossRef] [Green Version]
- Garmaroudi, F.S.; Marchant, D.; Hendry, R.; Luo, H.; Yang, D.; Ye, X.; Shi, J.; McManus, B.M. Coxsackievirus B3 replication and pathogenesis. Future Microbiol. 2015, 10, 629–653. [Google Scholar] [CrossRef]
- Westermann, D.; Savvatis, K.; Lindner, D.; Zietsch, C.; Becher, P.M.; Hammer, E.; Heimesaat, M.M.; Bereswill, S.; Völker, U.; Escher, F.; et al. Reduced Degradation of the Chemokine MCP-3 by Matrix Metalloproteinase-2 Exacerbates Myocardial Inflammation in Experimental Viral Cardiomyopathy. Circulation 2011, 124, 2082–2093. [Google Scholar] [CrossRef]
- McManus, B.M.; Chow, L.H.; Wilson, J.E.; Anderson, D.R.; Gulizia, J.M.; Gauntt, C.J.; Klingel, K.E.; Beisel, K.W.; Kandolf, R. Direct Myocardial Injury by Enterovirus: A Central Role in the Evolution of Murine Myocarditis. Clin. Immunol. Immunopathol. 1993, 68, 159–169. [Google Scholar] [CrossRef]
- Chapman, S.J.; Hill, A.V.S. Human genetic susceptibility to infectious disease. Nat. Rev. Genet. 2012, 13, 175–188. [Google Scholar] [CrossRef]
- Belkaya, S.; Kontorovich, A.; Byun, M.; Mulero-Navarro, S.; Bajolle, F.; Cobat, A.; Josowitz, R.; Itan, Y.; Quint, R.; Lorenzo, L.; et al. Autosomal Recessive Cardiomyopathy Presenting as Acute Myocarditis. J. Am. Coll. Cardiol. 2017, 69, 1653–1665. [Google Scholar] [CrossRef]
- Frustaci, A.; Chimenti, C.; Calabrese, F.; Pieroni, M.; Thiene, G.; Maseri, A. Immunosuppressive therapy for active lymphocytic myocarditis: Virological and immunologic profile of responders versus nonresponders. Circulation 2003, 107, 857–863. [Google Scholar] [CrossRef]
- Grün, S.; Schumm, J.; Greulich, S.; Wagner, A.; Schneider, S.; Bruder, O.; Kispert, E.-M.; Hill, S.; Ong, P.; Klingel, K.; et al. Long-Term Follow-Up of Biopsy-Proven Viral Myocarditis: Predictors of Mortality and Incomplete Recovery. J. Am. Coll. Cardiol. 2012, 59, 1604–1615. [Google Scholar] [CrossRef]
- Schenk, T.; Enders, M.; Pollak, S.; Hahn, R.; Huzly, D. High Prevalence of Human Parvovirus B19 DNA in Myocardial Autopsy Samples from Subjects without Myocarditis or Dilative Cardiomyopathy. J. Clin. Microbiol. 2009, 47, 106–110. [Google Scholar] [CrossRef] [Green Version]
- Stewart, G.C.; Lopez-Molina, J.; Gottumukkala, R.V.; Rosner, G.F.; Anello, M.S.; Hecht, J.L.; Winters, G.L.; Padera, R.F.; Baughman, K.L.; Lipes, M.A. Myocardial parvovirus B19 persistence: Lack of association with clinicopathologic phenotype in adults with heart failure. Circ. Heart Fail. 2011, 4, 71–78. [Google Scholar] [CrossRef] [Green Version]
- Verdonschot, J.; Hazebroek, M.; Merken, J.; Debing, Y.; Dennert, R.; Rocca, H.-P.B.-L.; Heymans, S. Relevance of cardiac parvovirus B19 in myocarditis and dilated cardiomyopathy: Review of the literature. Eur. J. Heart Fail. 2016, 18, 1430–1441. [Google Scholar] [CrossRef]
- Hsu, T.-C.; Wu, W.-J.; Chen, M.-C.; Tsay, G.J. Human parvovirus B19 non-structural protein (NS1) induces apoptosis through mitochondria cell death pathway in COS-7 cells. Scand. J. Infect. Dis. 2004, 36, 570–577. [Google Scholar] [CrossRef]
- Ahmed, M.; Honisch, S.; Pelzl, L.; Fezai, M.; Hosseinzadeh, Z.; Bock, C.-T.; Kandolf, R.; Lang, F. Up-regulation of epithelial Na+ channel ENaC by human parvovirus B19 capsid protein VP1. Biochem. Biophys. Res. Commun. 2015, 468, 179–184. [Google Scholar] [CrossRef]
- Woolf, A.D.; Campion, G.V.; Chishick, A.; Wise, S.; Cohen, B.J.; Klouda, P.T.; Caul, O.; A Dieppe, P. Clinical manifestations of human parvovirus B19 in adults. Arch. Intern. Med. 1989, 149, 1153–1156. [Google Scholar] [CrossRef]
- Brown, K.E.; Anderson, S.M.; Young, N.S. Erythrocyte P antigen: Cellular receptor for B19 parvovirus. Science 1993, 262, 114–117. [Google Scholar] [CrossRef]
- Pozzuto, T.; von Kietzell, K.; Bock, T.; Schmidt-Lucke, C.; Poller, W.; Zobel, T.; Lassner, D.; Zeichhardt, H.; Weger, S.; Fechner, H. Transactivation of human parvovirus B19 gene expression in endothelial cells by adenoviral helper functions. Virology 2011, 411, 50–64. [Google Scholar] [CrossRef] [Green Version]
- Bachelier, K.; Biehl, S.; Schwarz, V.; Kindermann, I.; Kandolf, R.; Sauter, M.; Ukena, C.; Yilmaz, A.; Sliwa, K.; Bock, C.-T.; et al. Parvovirus B19-induced vascular damage in the heart is associated with elevated circulating endothelial microparticles. PLoS ONE 2017, 12, e0176311. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Lucke, C.; Zobel, T.; Escher, F.; Tschöpe, C.; Lassner, D.; Kühl, U.; Gubbe, K.; Volk, H.-D.; Schultheiss, H.-P. Human Parvovirus B19 (B19V) Up-regulates CXCR4 Surface Expression of Circulating Angiogenic Cells: Implications for Cardiac Ischemia in B19V Cardiomyopathy. J. Infect. Dis. 2017, 217, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Lucke, C.; Zobel, T.; Schrepfer, S.; Kühl, U.; Wang, D.; Klingel, K.; Becher, P.M.; Fechner, H.; Pozzuto, T.; Van Linthout, S.; et al. Impaired Endothelial Regeneration Through Human Parvovirus B19–Infected Circulating Angiogenic Cells in Patients with Cardiomyopathy. J. Infect. Dis. 2015, 212, 1070–1081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magro, C.M.; Crowson, A.N.; Dawood, M.; Nuovo, G.J. Parvoviral infection of endothelial cells and its possible role in vasculitis and autoimmune diseases. J. Rheumatol. 2002, 29, 1227–1235. [Google Scholar]
- Kerr, J.R. The role of parvovirus B19 in the pathogenesis of autoimmunity and autoimmune disease. J. Clin. Pathol. 2016, 69, 279–291. [Google Scholar] [CrossRef] [Green Version]
- Bogomolovas, J.; Šimoliūnas, E.; Rinkūnaitė, I.; Smalinskaitė, L.; Podkopajev, A.; Bironaitė, D.; Weis, C.-A.; Marx, A.; Bukelskienė, V.; Gretz, N.; et al. A Novel Murine Model of Parvovirus Associated Dilated Cardiomyopathy Induced by Immunization with VP1-Unique Region of Parvovirus B19. BioMed Res. Int. 2016, 2016, 1627184. [Google Scholar] [CrossRef] [Green Version]
- Thammasri, K.; Rauhamäki, S.; Wang, L.; Filippou, A.; Kivovich, V.; Marjomäki, V.; Naides, S.J.; Gilbert, L. Human Parvovirus B19 Induced Apoptotic Bodies Contain Altered Self-Antigens that are Phagocytosed by Antigen Presenting Cells. PLoS ONE 2013, 8, e67179. [Google Scholar] [CrossRef] [Green Version]
- Kühl, U.; Lassner, D.; Pauschinger, M.; Gross, U.; Seeberg, B.; Noutsias, M.; Poller, W.; Schultheiss, H.-P. Prevalence of erythrovirus genotypes in the myocardium of patients with dilated cardiomyopathy. J. Med. Virol. 2008, 80, 1243–1251. [Google Scholar] [CrossRef]
- Hazebroek, M.R.; Henkens, M.T.; Raafs, A.G.; Verdonschot, J.A.; Merken, J.J.; Dennert, R.M.; Eurlings, C.; Hamid, M.A.A.; Wolffs, P.F.; Winkens, B.; et al. Intravenous immunoglobulin therapy in adult patients with idiopathic chronic cardiomyopathy and cardiac parvovirus B19 persistence: A prospective, double-blind, randomized, placebo-controlled clinical trial. Eur. J. Heart Fail. 2021, 23, 302–309. [Google Scholar] [CrossRef]
- Tschöpe, C.; Elsanhoury, A.; Schlieker, S.; Van Linthout, S.; Kühl, U. Immunosuppression in inflammatory cardiomyopathy and parvovirus B19 persistence. Eur. J. Heart Fail. 2019, 21, 1468–1469. [Google Scholar] [CrossRef] [Green Version]
- Callon, D.; Berri, F.; Lebreil, A.-L.; Fornès, P.; Andreoletti, L. Coinfection of Parvovirus B19 with Influenza A/H1N1 Causes Fulminant Myocarditis and Pneumonia. An Autopsy Case Report. Pathogens 2021, 10, 958. [Google Scholar] [CrossRef]
- Yeleti, R.; Guglin, M.; Saleem, K.; Adigopula, S.V.; Sinha, A.; Upadhyay, S.; E Everett, J.; Ballut, K.; Uppuluri, S.; Rao, R.A. Fulminant myocarditis: COVID or not COVID? Reinfection or co-infection? Future Cardiol. 2021. [Google Scholar] [CrossRef]
- Bock, C.-T. Molecular phenotypes of human parvovirus B19 in patients with myocarditis. World J. Cardiol. 2014, 6, 183–195. [Google Scholar] [CrossRef]
- Janovitz, T.; Wong, S.; Young, N.S.; Oliveira, T.; Falck-Pedersen, E. Parvovirus B19 integration into human CD36+ erythroid progenitor cells. Virology 2017, 511, 40–48. [Google Scholar] [CrossRef]
- Prober, C. Sixth Disease and the Ubiquity of Human Herpesviruses. N. Engl. J. Med. 2005, 352, 753–755. [Google Scholar] [CrossRef]
- Kühl, U.; Schultheiss, H.-P. Viral myocarditis. Swiss Med. Wkly. 2014, 144, w14010. [Google Scholar] [CrossRef]
- Mutlu, H.; Alam, M.; Ozbilgin, O.F. A rare case of Epstein-Barr virus-induced dilated cardiomyopathy. Heart Lung 2011, 40, 81–87. [Google Scholar] [CrossRef]
- Chimenti, C.; Verardo, R.; Grande, C.; Francone, M.; Frustaci, A. Infarct-like myocarditis with coronary vasculitis and aneurysm formation caused by Epstein–Barr virus infection. ESC Heart Fail. 2020, 7, 938–941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kytö, V.; Vuorinen, T.; Saukko, P.; Lautenschlager, I.; Lignitz, E.; Saraste, A.; Voipio-Pulkki, L.-M. Cytomegalovirus Infection of the Heart Is Common in Patients with Fatal Myocarditis. Clin. Infect. Dis. 2005, 40, 683–688. [Google Scholar] [CrossRef] [PubMed]
- De, A.; Myridakis, D.; Kerrigan, M.; Kiblawi, F. Varicella Myopericarditis Mimicking Myocardial Infarction in a 17-Year-Old Boy. Tex. Heart Inst. J. 2011, 38, 288–290. [Google Scholar] [PubMed]
- O’Grady, M.J.; Moylett, E. Cardiac-Related Varicella Mortality in Childhood: A Literature Review with Clinical Experience. Pediatr. Cardiol. 2011, 32, 1241–1243. [Google Scholar] [CrossRef]
- Rezk, S.A.; Zhao, X.; Weiss, L.M. Epstein-Barr virus (EBV)–associated lymphoid proliferations, a 2018 update. Hum. Pathol. 2018, 79, 18–41. [Google Scholar] [CrossRef]
- Ace, O.; Domb, S. Myocarditis as the initial presentation of Epstein-Barr virus infection in a 17-year-old male patient. Can. Fam. Physician 2019, 65, 897–899. [Google Scholar]
- Padala, S.K.; Kumar, A.; Padala, S. Fulminant Cytomegalovirus Myocarditis in an Immunocompetent Host: Resolution with Oral Valganciclovir. Tex. Heart Inst. J. 2014, 41, 523–529. [Google Scholar] [CrossRef] [Green Version]
- Scherger, S.; Mathur, S.; Bajrovic, V.; Johnson, S.C.; Benamu, E.; Ramanan, P.; Wolfel, E.; Levi, M.E.; Abidi, M.Z. Cytomegalovirus myocarditis in solid organ transplant recipients: A case series and review of literature. Transpl. Infect. Dis. 2020, 22, e13282. [Google Scholar] [CrossRef]
- Saraca, L.M.; Lazzari, L.; Di Giuli, C.; Lavagna, A.; Mezzetti, P.; Bovelli, D.; Boschetti, E.; Francisci, D. Cytomegalovirus myocarditis in a patient with systemic lupus erythematosus (SLE) successfully treated with ganciclovir. IDCases 2018, 12, 4–6. [Google Scholar] [CrossRef]
- Wang, L.; Verschuuren, E.A.; Paap, D.; Rondaan, C.; Raveling-Eelsing, E.; Westra, J.; Bos, N.A. Prophylactic vaccination with a live-attenuated herpes zoster vaccine in lung transplant candidates. J. Heart Lung Transplant. 2020, 39, 1445–1454. [Google Scholar] [CrossRef]
- Galossi, A.; Guarisco, R.; Bellis, L.; Puoti, C. Extrahepatic manifestations of chronic HCV infection. J. Gastrointest. Liver Dis. 2007, 16, 65–73. [Google Scholar]
- Saleh, A.; Matsumori, A.; Abdelrazek, S.; Eltaweel, S.; Salous, A.; Neumann, F.-J.; Antz, M. Myocardial involvement in coronavirus disease. Herz 2020, 45, 719–725. [Google Scholar] [CrossRef]
- Matsumori, A. Hepatitis C Virus Infection and Cardiomyopathies. Circ. Res. 2005, 96, 144–147. [Google Scholar] [CrossRef] [Green Version]
- Poller, W.; Kaya, Z.; Muche, M.; Kasner, M.; Skurk, C.; Kappert, K.; Tauber, R.; Escher, F.; Schultheiss, H.-P.; Epple, H.-J.; et al. High incidence of cardiac dysfunction and response to antiviral treatment in patients with chronic hepatitis C virus infection. Clin. Res. Cardiol. 2017, 106, 551–556. [Google Scholar] [CrossRef]
- Haykal, M.; Matsumori, A.; Saleh, A.; Fayez, M.; Negm, H.; Shalaby, M.; Bassuony, S. Diagnosis and treatment of HCV heart diseases. Expert Rev. Cardiovasc. Ther. 2021, 19, 493–499. [Google Scholar] [CrossRef]
- Sanchez, M.J.; Bergasa, N.V. Hepatitis C associated cardiomyopathy: Potential pathogenic mechanisms and clinical implications. Med. Sci. Monit. 2008, 14, RA55–RA63. [Google Scholar]
- Belkin, M.N.; Uriel, N. Heart health in the age of highly active antiretroviral therapy: A review of HIV cardiomyopathy. Curr. Opin. Cardiol. 2018, 33, 317–324. [Google Scholar] [CrossRef]
- Yu, C.-M.; Wong, R.S.-M.; Wu, E.B.; Kong, S.-L.; Wong, J.; Yip, G.W.-K.; Soo, Y.O.Y.; Chiu, M.L.S.; Chan, Y.-S.; Hui, D.; et al. Cardiovascular complications of severe acute respiratory syndrome. Postgrad. Med. J. 2006, 82, 140–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alhogbani, T. Acute myocarditis associated with novel Middle East respiratory syndrome coronavirus. Ann. Saudi Med. 2016, 36, 78–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Tay, M.Z.; Poh, C.M.; Rénia, L.; Macary, P.A.; Ng, L.F.P. The trinity of COVID-19: Immunity, inflammation and intervention. Nat. Rev. Immunol. 2020, 20, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Bikdeli, B.; Madhavan, M.V.; Jimenez, D.; Chuich, T.; Dreyfus, I.; Driggin, E.; Nigoghossian, C.D.; Ageno, W.; Madjid, M.; Guo, Y.; et al. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-Up. J. Am. Coll. Cardiol. 2020, 75, 2950–2973. [Google Scholar] [CrossRef] [PubMed]
- Connors, J.M.; Levy, J.H. Thromboinflammation and the hypercoagulability of COVID-19. J. Thromb. Haemost. 2020, 18, 1559–1561. [Google Scholar] [CrossRef]
- Ruan, Q.; Yang, K.; Wang, W.; Jiang, L.; Song, J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensiv. Care Med. 2020, 46, 846–848. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72,314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Bae, S.; Kim, S.R.; Kim, M.-N.; Shim, W.J.; Park, S.-M. Impact of cardiovascular disease and risk factors on fatal outcomes in patients with COVID-19 according to age: A systematic review and meta-analysis. Heart 2021, 107, 373–380. [Google Scholar] [CrossRef]
- Pal, A.; Ahirwar, A.K.; Sakarde, A.; Asia, P.; Gopal, N.; Alam, S.; Kaim, K.; Ahirwar, P.; Sorte, S.R. COVID-19 and cardiovascular disease: A review of current knowledge. Horm. Mol. Biol. Clin. Investig. 2021, 42, 99–104. [Google Scholar] [CrossRef]
- Zhong, Z.; Li, H.; Zhu, J.; Ji, P.; Li, B.; Pang, J.; Zhang, J.; Liang, X. Clinical characteristics of 2,459 severe or critically ill COVID-19 patients. Medicine 2021, 100, e23781. [Google Scholar] [CrossRef]
- Fu, L.; Liu, X.; Su, Y.; Ma, J.; Hong, K. Prevalence and impact of cardiac injury on COVID -19: A systematic review and meta-analysis. Clin. Cardiol. 2021, 44, 276–283. [Google Scholar] [CrossRef]
- Bansal, A.; Kumar, A.; Patel, D.; Puri, R.; Kalra, A.; Kapadia, S.R.; Reed, G.W. Meta-analysis Comparing Outcomes in Patients with and without Cardiac Injury and Coronavirus Disease 2019 (COVID 19). Am. J. Cardiol. 2021, 141, 140–146. [Google Scholar] [CrossRef]
- Puntmann, V.O.; Carerj, M.L.; Wieters, I.; Fahim, M.; Arendt, C.; Hoffmann, J.; Shchendrygina, A.; Escher, F.; Vasa-Nicotera, M.; Zeiher, A.M.; et al. Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered from Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 1265. [Google Scholar] [CrossRef]
- Deng, Q.; Hu, B.; Zhang, Y.; Wang, H.; Zhou, X.; Hu, W.; Cheng, Y.; Yan, J.; Ping, H.; Zhou, Q. Suspected myocardial injury in patients with COVID-19: Evidence from front-line clinical observation in Wuhan, China. Int. J. Cardiol. 2020, 311, 116–121. [Google Scholar] [CrossRef]
- Tavazzi, G.; Pellegrini, C.; Maurelli, M.; Belliato, M.; Sciutti, F.; Bottazzi, A.; Sepe, P.A.; Resasco, T.; Camporotondo, R.; Bruno, R.; et al. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur. J. Heart Fail. 2020, 22, 911–915. [Google Scholar] [CrossRef] [Green Version]
- Escher, F.; Pietsch, H.; Aleshcheva, G.; Bock, C.-T.; Baumeier, C.; Elsaesser, A.; Wenzel, P.; Hamm, C.; Westenfeld, R.; Schultheiss, M.; et al. Detection of viral SARS-CoV-2 genomes and histopathological changes in endomyocardial biopsies. ESC Heart Fail. 2020, 7, 2440–2447. [Google Scholar] [CrossRef]
- Bradley, B.T.; Maioli, H.; Johnston, R.; Chaudhry, I.; Fink, S.L.; Xu, H.; Najafian, B.; Deutsch, G.; Lacy, J.M.; Williams, T.; et al. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: A case series. Lancet 2020, 396, 320–332. [Google Scholar] [CrossRef]
- Lindner, D.; Fitzek, A.; Bräuninger, H.; Aleshcheva, G.; Edler, C.; Meissner, K.; Scherschel, K.; Kirchhof, P.; Escher, F.; Schultheiss, H.-P.; et al. Association of Cardiac Infection with SARS-CoV-2 in Confirmed COVID-19 Autopsy Cases. JAMA Cardiol. 2020, 5, 1281–1285. [Google Scholar] [CrossRef]
- Wenzel, P.; Kopp, S.; Göbel, S.; Jansen, T.; Geyer, M.; Hahn, F.; Kreitner, K.-F.; Escher, F.; Schultheiss, H.-P.; Münzel, T. Evidence of SARS-CoV-2 mRNA in endomyocardial biopsies of patients with clinically suspected myocarditis tested negative for COVID-19 in nasopharyngeal swab. Cardiovasc. Res. 2020, 116, 1661–1663. [Google Scholar] [CrossRef]
- Yang, L.; Nilsson-Payant, B.E.; Han, Y.; Jaffré, F.; Zhu, J.; Wang, P.; Zhang, T.; Redmond, D.; Houghton, S.; Møller, R.; et al. Cardiomyocytes Recruit Monocytes upon SARS-CoV-2 Infection by Secreting CCL2. Stem Cell Rep. 2021, 16, 2274–2288. [Google Scholar] [CrossRef]
- Salah, H.M.; Mehta, J.L. COVID-19 Vaccine and Myocarditis. Am. J. Cardiol. 2021, 157, 146–148. [Google Scholar] [CrossRef]
- Viskin, D.; Topilsky, Y.; Aviram, G.; Mann, T.; Sadon, S.; Hadad, Y.; Flint, N.; Shmilovich, H.; Banai, S.; Havakuk, O. Myocarditis Associated with COVID-19 Vaccination: Echocardiography, Cardiac Tomography, and Magnetic Resonance Imaging Findings. Circ. Cardiovasc. Imaging 2021, 14, e013236. [Google Scholar] [CrossRef]
- Verma, A.K.; Lavine, K.J.; Lin, C.-Y. Myocarditis after Covid-19 mRNA Vaccination. N. Engl. J. Med. 2021, 385, 1332–1334. [Google Scholar] [CrossRef]
- Joob, B.; Wiwanitkit, V. Cardiac problem because of Zika virus infection: A possibility. Anatol. J. Cardiol. 2016, 16, 455–456. [Google Scholar] [CrossRef]
- Traverse, E.; Hopkins, H.; Vaidhyanathan, V.; Barr, K. Cardiomyopathy and Death Following Chikungunya Infection: An Increasingly Common Outcome. Trop. Med. Infect. Dis. 2021, 6, 108. [Google Scholar] [CrossRef]
- Cooper, L.T. Ventricular Arrhythmias and Sudden Cardiac Death in Lymphocytic Myocarditis. J. Am. Coll. Cardiol. 2020, 75, 1058–1060. [Google Scholar] [CrossRef] [PubMed]
- Eckart, R.E.; Scoville, S.L.; Campbell, C.L.; Shry, E.A.; Stajduhar, K.C.; Potter, R.N.; Pearse, L.A.; Virmani, R. Sudden Death in Young Adults: A 25-Year Review of Autopsies in Military Recruits. Ann. Intern. Med. 2004, 141, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Duncker, D.; König, T.; Hohmann, S.; Bauersachs, J.; Veltmann, C. Ventricular arrhythmias in patients with newly diagnosed nonischemic cardiomyopathy: Insights from the PROLONG study. Clin. Cardiol. 2017, 40, 586–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bozkurt, B.; Colvin, M.; Cook, J.; Cooper, L.; Deswal, A.; Fonarow, G.; Francis, G.S.; Lenihan, D.; Lewis, E.F.; McNamara, D.M.; et al. Current Diagnostic and Treatment Strategies for Specific Dilated Cardiomyopathies: A Scientific Statement from the American Heart Association. Circulation 2016, 134, e579–e646. [Google Scholar] [CrossRef]
- ESC Scientific Document Group; Priori, S.G.; Blomström-Lundqvist, C.; Mazzanti, A.; Blom, N.; Borggrefe, M.; Camm, J.; Elliott, P.; Fitzsimons, D.; Hatala, R.; et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC) Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). EP Eur. 2015, 17, 1601–1687. [Google Scholar] [CrossRef]
- Rafailidis, P.I.; Mavros, M.N.; Kapaskelis, A.; Falagas, M.E. Antiviral treatment for severe EBV infections in apparently immunocompetent patients. J. Clin. Virol. 2010, 49, 151–157. [Google Scholar] [CrossRef]
- Remick, J.; Georgiopoulou, V.; Marti, C.; Ofotokun, I.; Kalogeropoulos, A.; Lewis, W.; Butler, J. Heart failure in patients with human immunodeficiency virus infection: Epidemiology, pathophysiology, treatment, and future research. Circulation 2014, 129, 1781–1789. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Gao, B.; Xiong, S. Involvement of NLRP3 inflammasome in CVB3-induced viral myocarditis. Am. J. Physiol. Circ. Physiol. 2014, 307, H1438–H1447. [Google Scholar] [CrossRef] [Green Version]
- Frustaci, A.; Russo, M.A.; Chimenti, C. Randomized study on the efficacy of immunosuppressive therapy in patients with virus-negative inflammatory cardiomyopathy: The TIMIC study. Eur. Heart J. 2009, 30, 1995–2002. [Google Scholar] [CrossRef]
- Chimenti, C.; Verardo, R.; Scopelliti, F.; Grande, C.; Petrosillo, N.; Piselli, P.; De Paulis, R.; Frustaci, A. Myocardial expression of Toll-like receptor 4 predicts the response to immunosuppressive therapy in patients with virus-negative chronic inflammatory cardiomyopathy. Eur. J. Heart Fail. 2017, 19, 915–925. [Google Scholar] [CrossRef]
- Klein, A.L.; Imazio, M.; Brucato, A.; Cremer, P.; LeWinter, M.; Abbate, A.; Lin, D.; Martini, A.; Beutler, A.; Chang, S.; et al. RHAPSODY: Rationale for and design of a pivotal Phase 3 trial to assess efficacy and safety of rilonacept, an interleukin-1α and interleukin-1β trap, in patients with recurrent pericarditis. Am. Heart J. 2020, 228, 81–90. [Google Scholar] [CrossRef]
- Poller, W.; Tank, J.; Skurk, C.; Gast, M. Cardiovascular RNA Interference Therapy. Circ. Res. 2013, 113, 588–602. [Google Scholar] [CrossRef] [Green Version]
- Stein, E.A.; Pinkert, S.; Becher, P.M.; Geisler, A.; Zeichhardt, H.; Klopfleisch, R.; Poller, W.; Tschöpe, C.; Lassner, D.; Fechner, H.; et al. Combination of RNA Interference and Virus Receptor Trap Exerts Additive Antiviral Activity in Coxsackievirus B3-induced Myocarditis in Mice. J. Infect. Dis. 2014, 211, 613–622. [Google Scholar] [CrossRef]
- Tank, J.; Lindner, D.; Wang, X.; Stroux, A.; Gilke, L.; Gast, M.; Zietsch, C.; Skurk, C.; Scheibenbogen, C.; Klingel, K.; et al. Single-target RNA interference for the blockade of multiple interacting proinflammatory and profibrotic pathways in cardiac fibroblasts. J. Mol. Cell. Cardiol. 2014, 66, 141–156. [Google Scholar] [CrossRef] [PubMed]
- Uil, C.A.D.; Akin, S.; Jewbali, L.S.; Miranda, D.D.R.; Brugts, J.J.; Constantinescu, A.A.; Kappetein, A.P.; Caliskan, K. Short-term mechanical circulatory support as a bridge to durable left ventricular assist device implantation in refractory cardiogenic shock: A systematic review and meta-analysis. Eur. J. Cardio-Thorac. Surg. 2017, 52, 14–25. [Google Scholar] [CrossRef] [Green Version]
- Maron, B.J.; Udelson, J.E.; Bonow, R.O.; Nishimura, R.A.; Ackerman, M.J.; Estes, N.M.; Cooper, L.; Link, M.S.; Maron, M.S. Eligibility and Disqualification Recommendations for Competitive Athletes with Cardiovascular Abnormalities: Task Force 3: Hypertrophic Cardiomyopathy, Arrhythmogenic Right Ventricular Cardiomyopathy and Other Cardiomyopathies, and Myocarditis: A Scientific Statement From the American Heart Association and American College of Cardiology. Circulation 2015, 132, e273–e280. [Google Scholar] [CrossRef] [PubMed]
- Eichhorn, C.; Bière, L.; Schnell, F.; Schmied, C.; Wilhelm, M.; Kwong, R.Y.; Gräni, C. Myocarditis in Athletes Is a Challenge: Diagnosis, Risk Stratification, and Uncertainties. JACC Cardiovasc. Imaging 2020, 13, 494–507. [Google Scholar] [CrossRef]


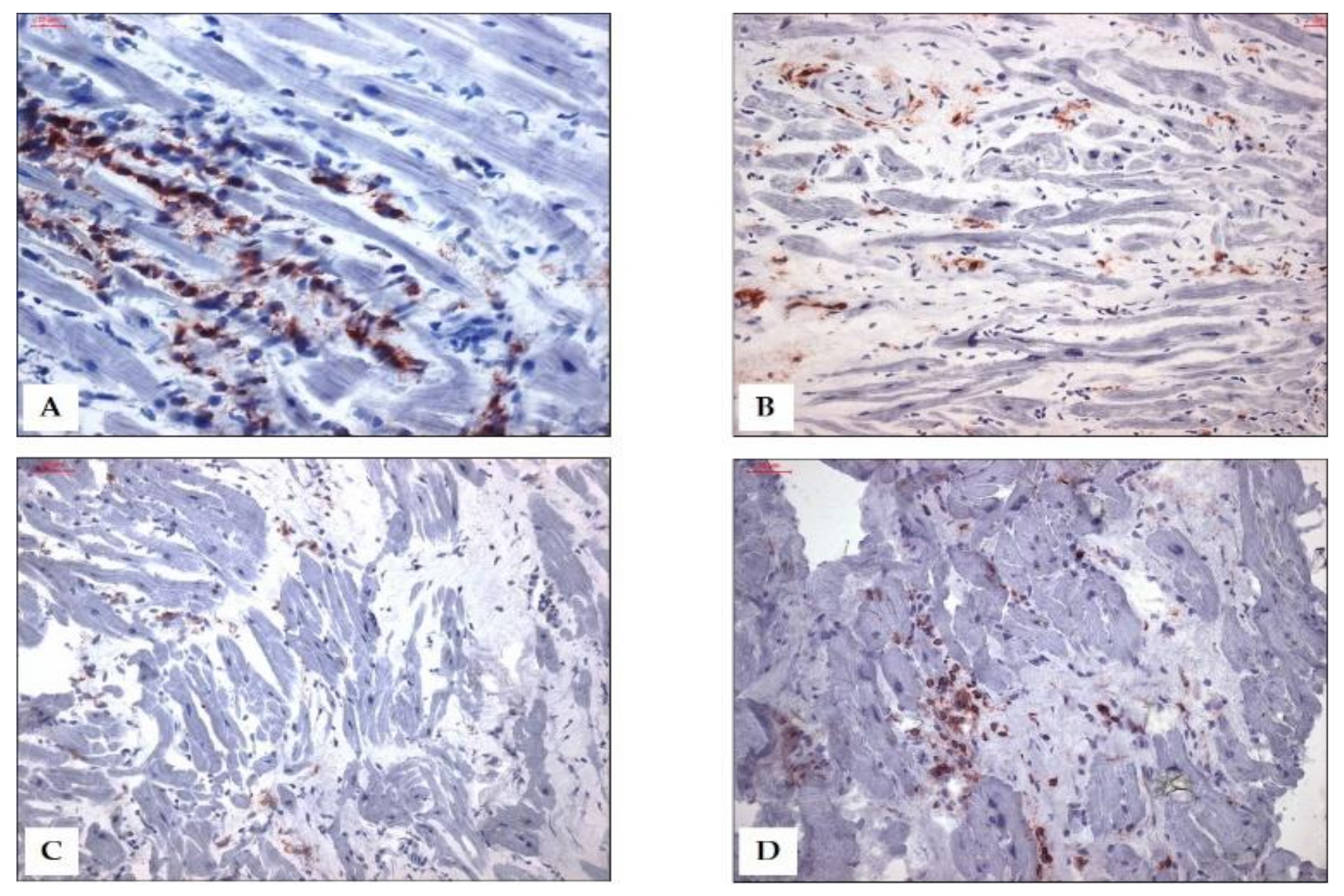





| Virus | Virus Family a | Genome Organization | Target Cells in the Heart Muscle | Detection Frequency | Antiviral Therapy Option b/Prevention | References |
|---|---|---|---|---|---|---|
| Classical cardiotropic viruses | ||||||
| Adenovirus (ADV) | Adenoviridae/human adenovirus | linear dsDNA | cardiomyocytes | rare | β-interferon/n.v.a. | [6,7] |
| Enteroviruses (EV)/particularly Coxsackie viruses B (CVB), EV71 and echoviruses | Picornaviridae | linear (+) ssRNA | cardiomyocytes | moderate | β-interferon/n.v.a. | [7] |
| Epstein–Barr Virus (EBV) | Herpesviridae/human γ-herpesvirus 4 (HHV-4) | linear dsDNA | b lymphocytes, cardiomyocytes (?) | moderate | ganciclovir/n.v.a. | [8] |
| Hepatitis C virus (HCV) | Flaviviridae | linear (+) ssRNA | mononuclear cells/monocytes/CD68+-macrophages | infrequent | polymerase, proteinase, NS5A inhibitors, (e.g., sofosbuvir, simeprevir, daclastavir) etc./n.v.a. | [9,10,11] |
| Human herpesvirus-6 (HHV6) | Herpesviridae/human β-herpesvirus | linear dsDNA | CD4+ T lymphocytes, endothelial cells, T-cell tropism | frequent | ganciclovir, foscarnet, cidofovir/n.v.a. | [12,13] |
| Human cytomegalovirus (HCMV) | Herpesviridae/human β-herpesvirus 5 (HHV-5) | linear dsDNA | epithelial cells, endothelial cells, fibroblasts, smooth muscle cells | moderate | ganciclovir, valganciclovir/n.v.a. | [14,15,16,17] |
| Parvovirus B19 (B19V) | Parvoviridae/erythroparvovirus | linear (−) ssDNA | endothelial cells | frequent | [telbivudine; β-interferon] /n.v.a. | [18,19] |
| Viruses with suspected association to myocarditis and DCM | ||||||
| Coronaviruses/particularly SARS-CoV-2 | Coronaviridae/severe acute respiratory syndrome coronavirus 2 | linear (+) ssRNA | cardiomyocytes, endothelial cells, macrophages | infrequent | n.d.a./licensed vaccines | [20] |
| Chikungunya virus (CHIKV) | Togaviridae | linear (+) ssRNA | epithelial cells, endothelial cells, primary fibroblasts, macrophages | infrequent | n.d.a./n.v.a. | [21,22,23] |
| Dengue virus (DENV) | Flaviviridae | linear (+) ssRNA | CD14+ monocytes | infrequent | n.d.a., symptomatic treatment/licensed vaccines | [24] |
| Herpes simplex virus 1 (HSV1) | Herpesviridae/human α-herpesvirus 1 | linear dsDNA | epithelial cells, sensory neurons, | infrequent | acyclovir, valacyclovir, famciclovir/n.v.a. | [25] |
| Human immunodeficiency virus | Retroviridae | linear (+) ssRNA | dendritic cells, macrophages, osteoclasts | infrequent | antiretroviral therapy (ART)/n.v.a. | [26] |
| Hepatitis E virus (HEV) | Hepeviridae/Orthohepevirus A | linear (+) ssRNA | endometrial stromal cells, hepatocytes | infrequent | ribavirin, PEG-IFN-α/(vaccine licensed in China (HEV 239) | [27] |
| Influenza A and B viruses | Orthomyxoviridae | linear (+/−) ssRNA, segmented | human airway epithelial cells | infrequent | oseltamivir (Tamiflu®), zanamivir (Relenza®), peramivir (Rapivab®); baloxavir (Xofluza®)/licensed vaccine | [28,29] |
| Measles virus | Paramyxoviridae | linear (−) ssRNA | human airway epithelial cells | infrequent | n.d.a./licensed vaccine | [30] |
| Metapneumovirus | Pneumoviridae | linear (−) ssRNA | infrequent | n.d.a./n.v.a | [31] | |
| Mumps virus | Paramyxoviridae | linear (−) ssRNA | neurotropic, CNS | infrequent | n.d.a./licensed vaccine | [32,33] |
| Rabies virus | Rhabdoviridae | linear (−) ssRNA | neurotropic, CNS | infrequent | post-exposure prophylaxis (PEP)/licensed vaccine | [34,35] |
| Respiratory syncytial virus (RSV) | Pneumoviridae | linear (−) ssRNA | human airway epithelial cells | infrequent | ribavirin/n.v.a. | [36,37] |
| Rubella virus | Matonaviridae | linear (+) ssRNA | human airway epithelial cells (?) | infrequent | n.d.a./licensed vaccine | [38] |
| Varicella-zoster virus (VZV) | human α-herpesvirus 3 (HHV-3) | linear dsDNA | sensory neurons | infrequent | acyclovir, famciclovir, valaciclovir/licensed vaccines | [39] |
| Zika virus (ZIKV) | Flaviviridae | linear (+) ssRNA | endometrial stromal cells | infrequent | n.d.a./n.v.a. | [40,41,42] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schultheiss, H.-P.; Baumeier, C.; Aleshcheva, G.; Bock, C.-T.; Escher, F. Viral Myocarditis—From Pathophysiology to Treatment. J. Clin. Med. 2021, 10, 5240. https://doi.org/10.3390/jcm10225240
Schultheiss H-P, Baumeier C, Aleshcheva G, Bock C-T, Escher F. Viral Myocarditis—From Pathophysiology to Treatment. Journal of Clinical Medicine. 2021; 10(22):5240. https://doi.org/10.3390/jcm10225240
Chicago/Turabian StyleSchultheiss, Heinz-Peter, Christian Baumeier, Ganna Aleshcheva, C.-Thomas Bock, and Felicitas Escher. 2021. "Viral Myocarditis—From Pathophysiology to Treatment" Journal of Clinical Medicine 10, no. 22: 5240. https://doi.org/10.3390/jcm10225240
APA StyleSchultheiss, H.-P., Baumeier, C., Aleshcheva, G., Bock, C.-T., & Escher, F. (2021). Viral Myocarditis—From Pathophysiology to Treatment. Journal of Clinical Medicine, 10(22), 5240. https://doi.org/10.3390/jcm10225240







