Effect of DA-9701 on the Gastrointestinal Motility in the Streptozotocin-Induced Diabetic Mice
Abstract
:1. Introduction
2. Materials and Methods
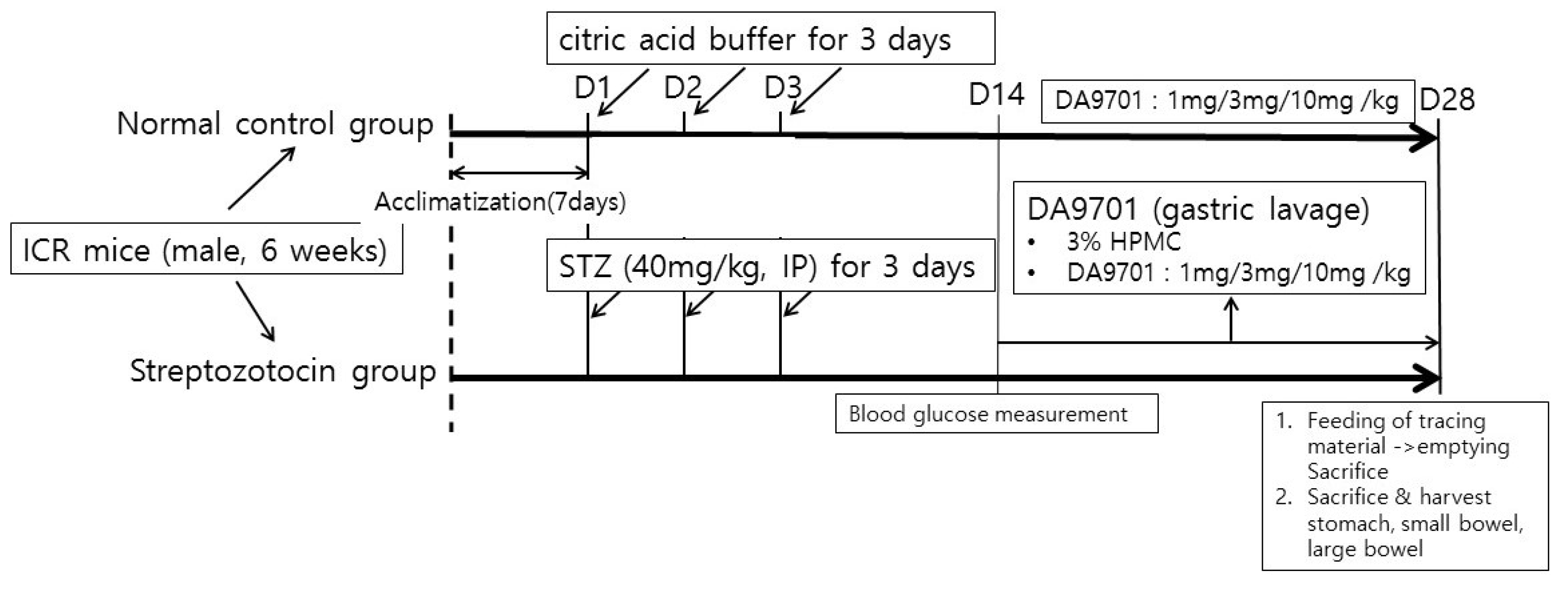
2.1. Streptozotocin (STZ)-Induced Diabetic Mice Model
2.2. DA-9701 Treatment in Experimental Mice
2.3. Assessment of GI Motility Using Isometric Contraction Measurement
2.4. Measurement of Intestinal Transit
2.5. Measurement of GE Rate with Phenol Red Marker
2.6. Statistical Analysis
3. Results
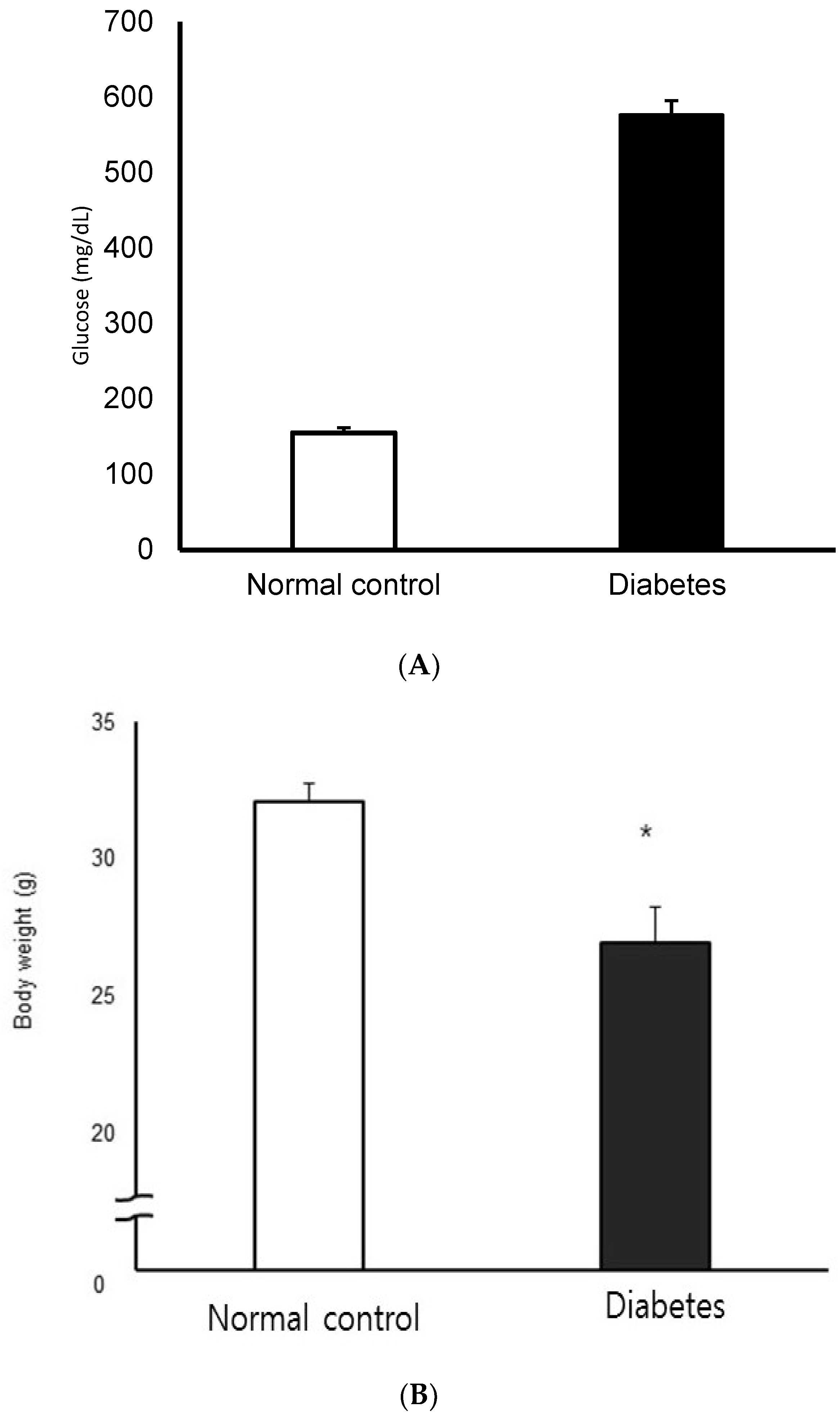
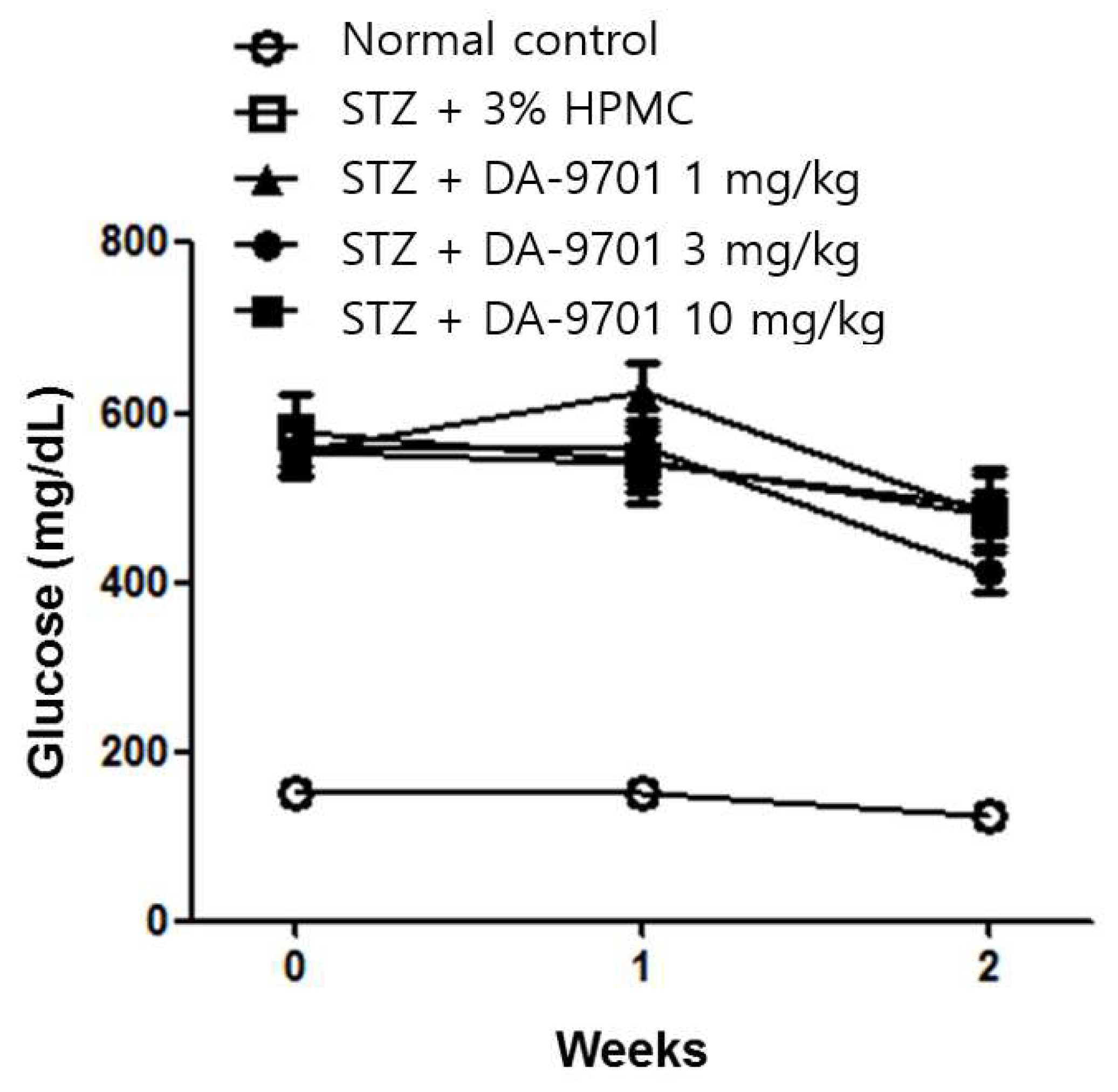
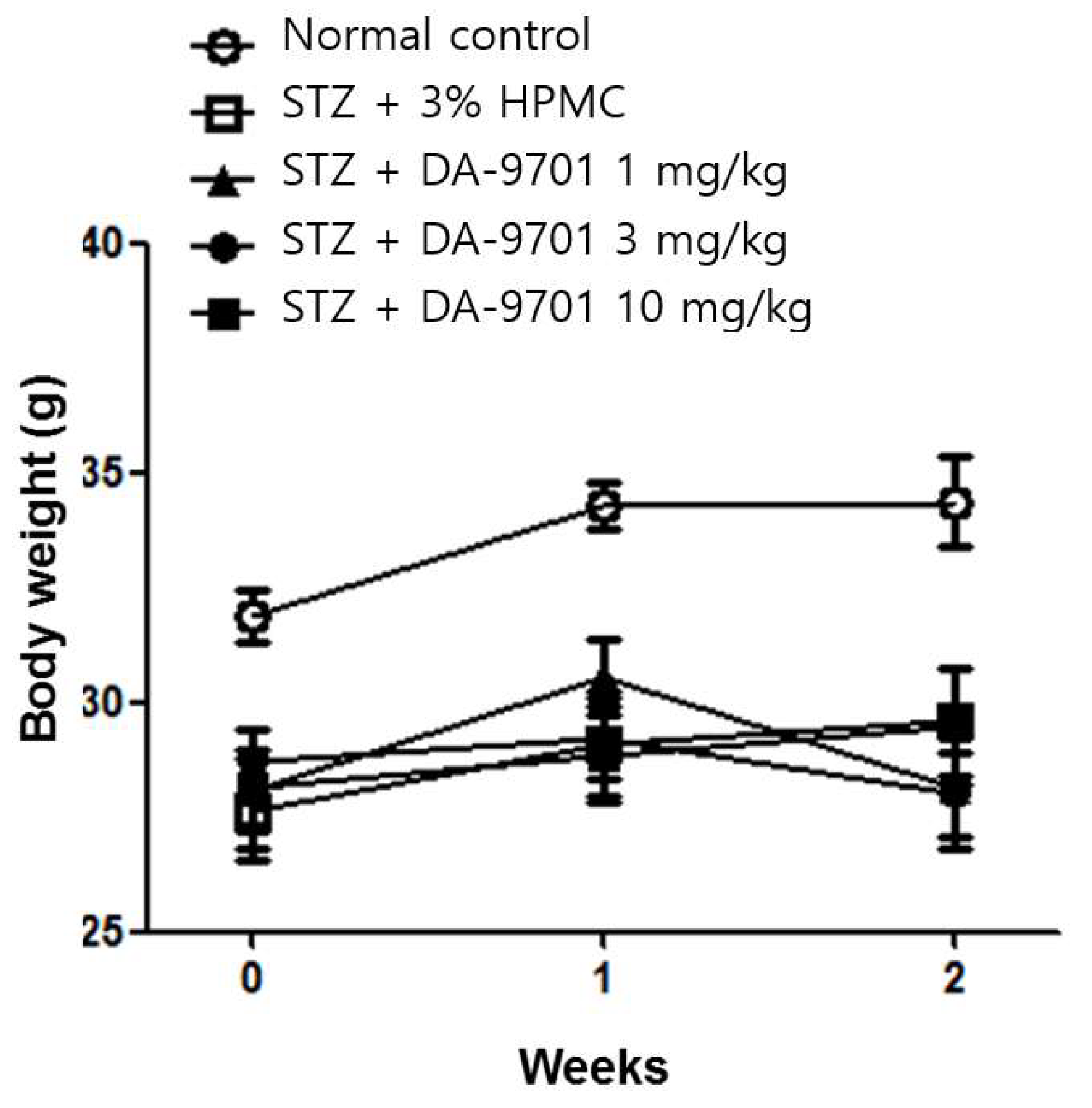
3.1. Blood Glucose and Body Weight in the STZ-Induced Diabetic Mice Model
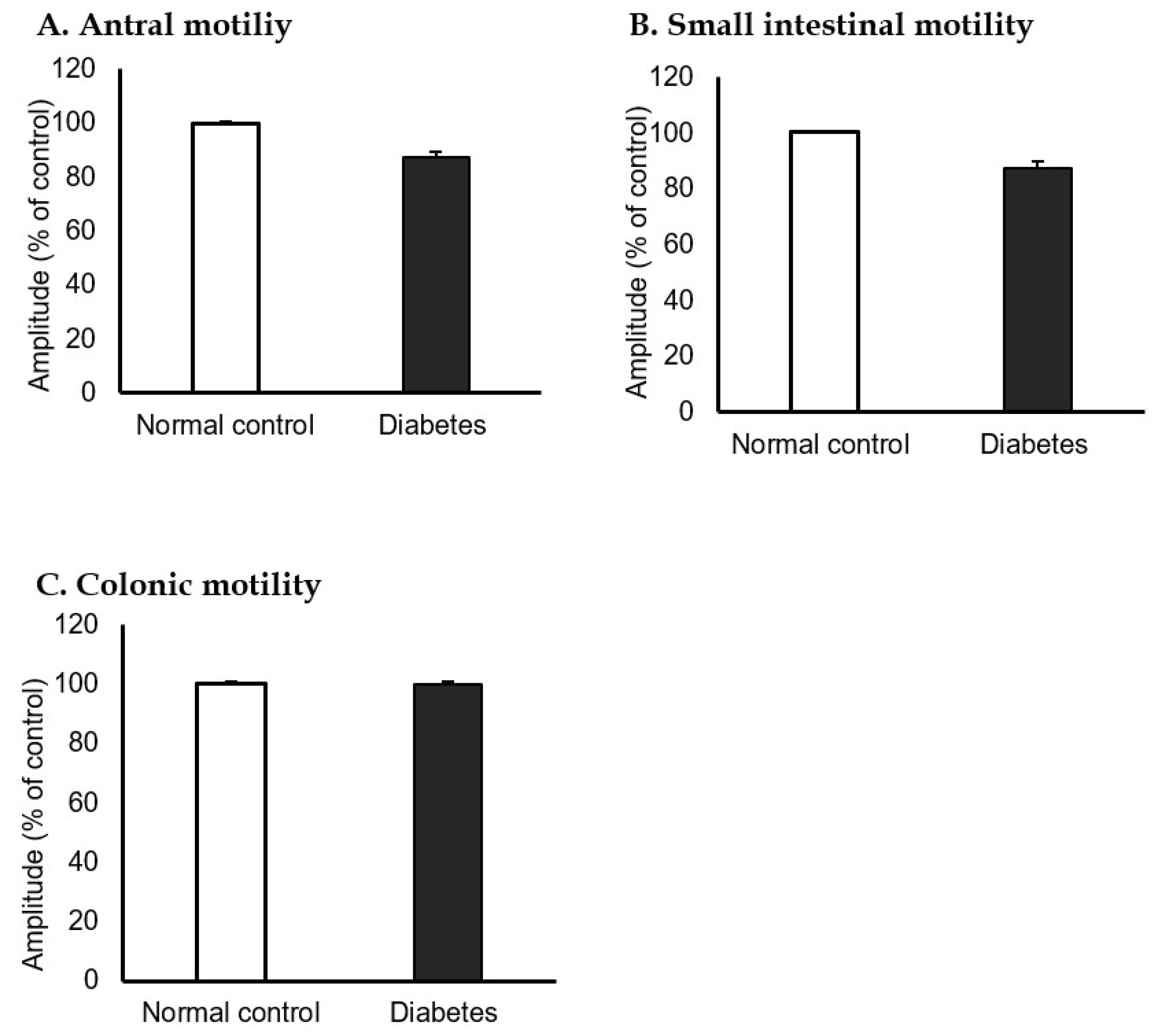
3.2. GI Motility in the STZ-Induced Diabetic Mice Model
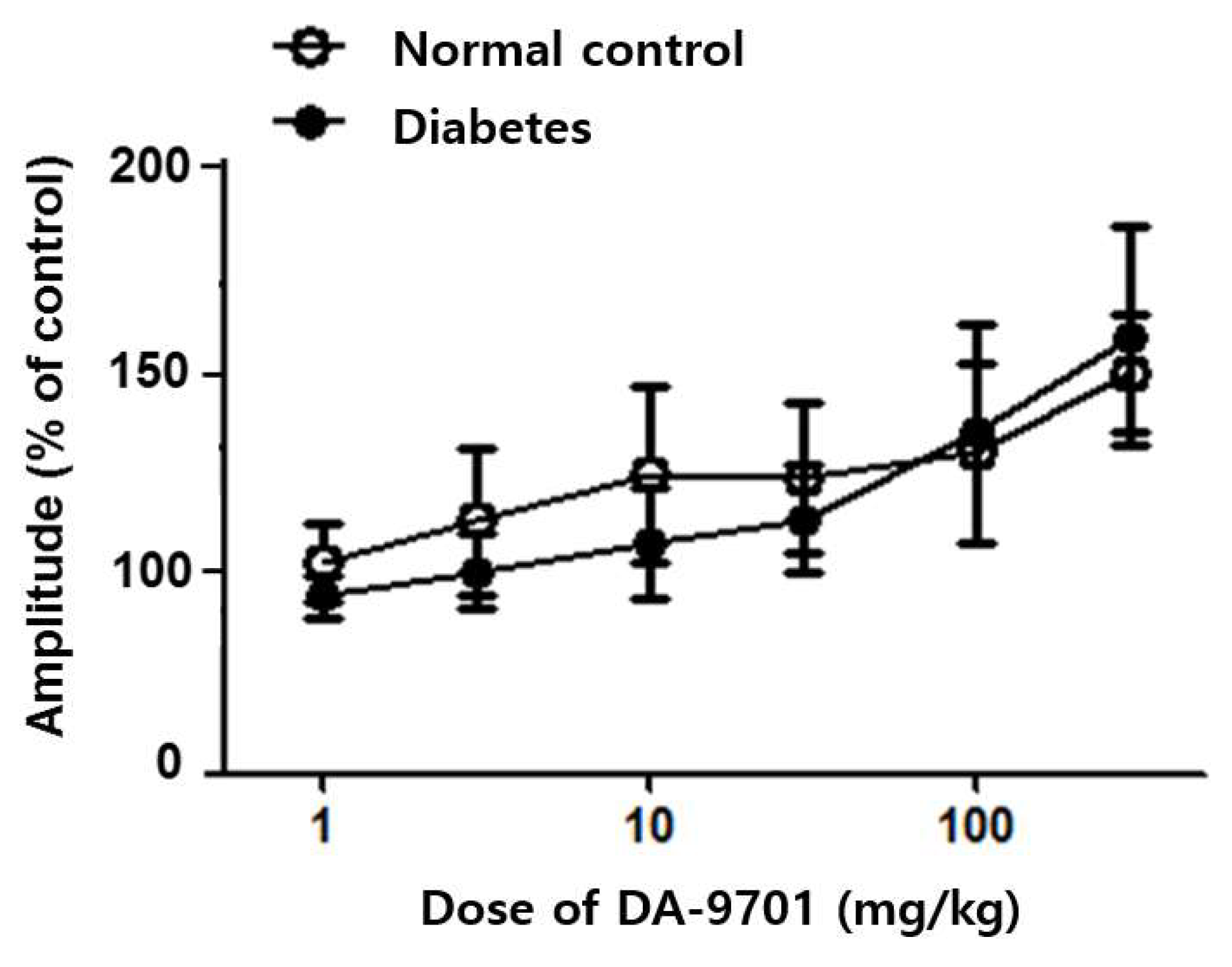
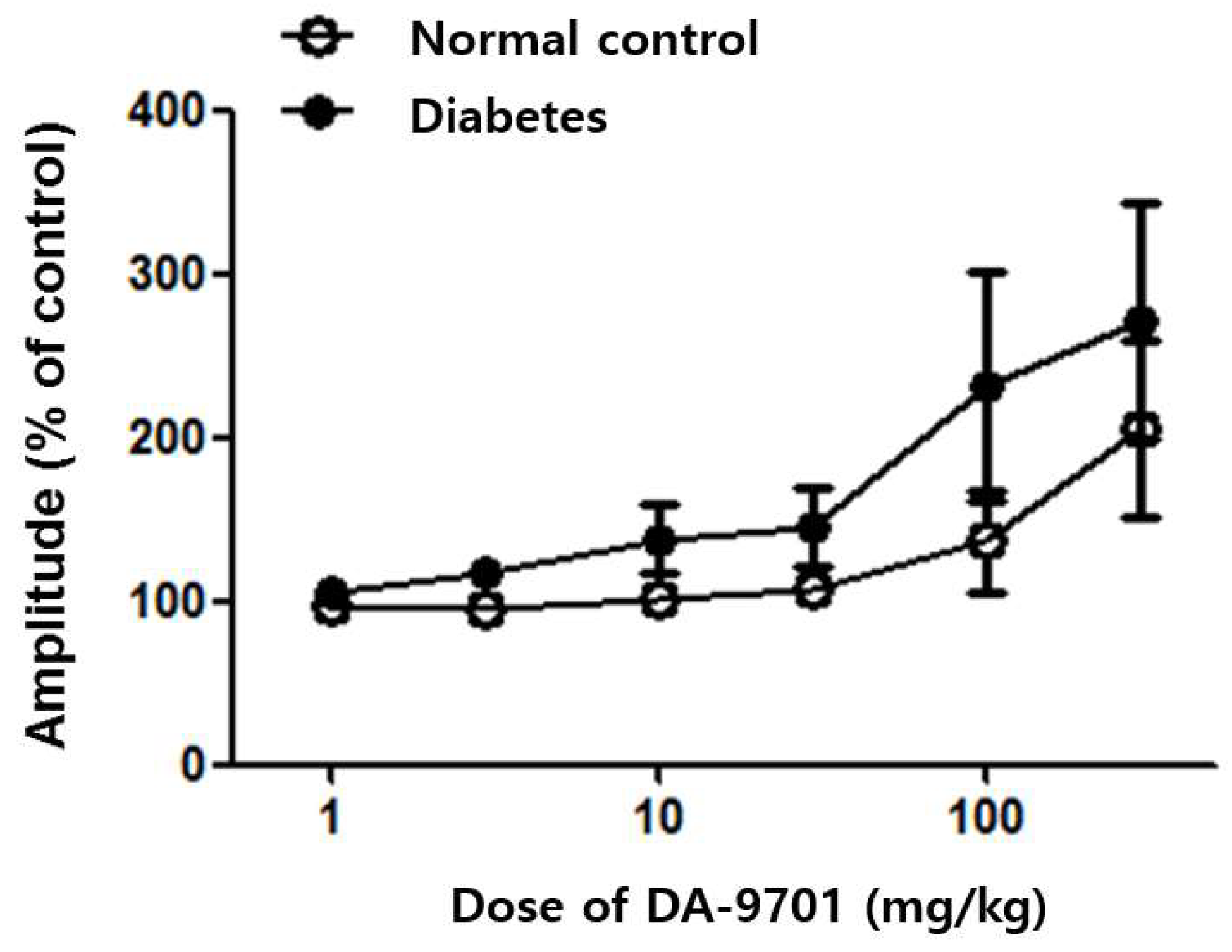
3.3. Effects of DA-9701 on GI Motility
3.4. Small Intestinal Transit and GE Rate in the STZ-Induced Diabetic Mice
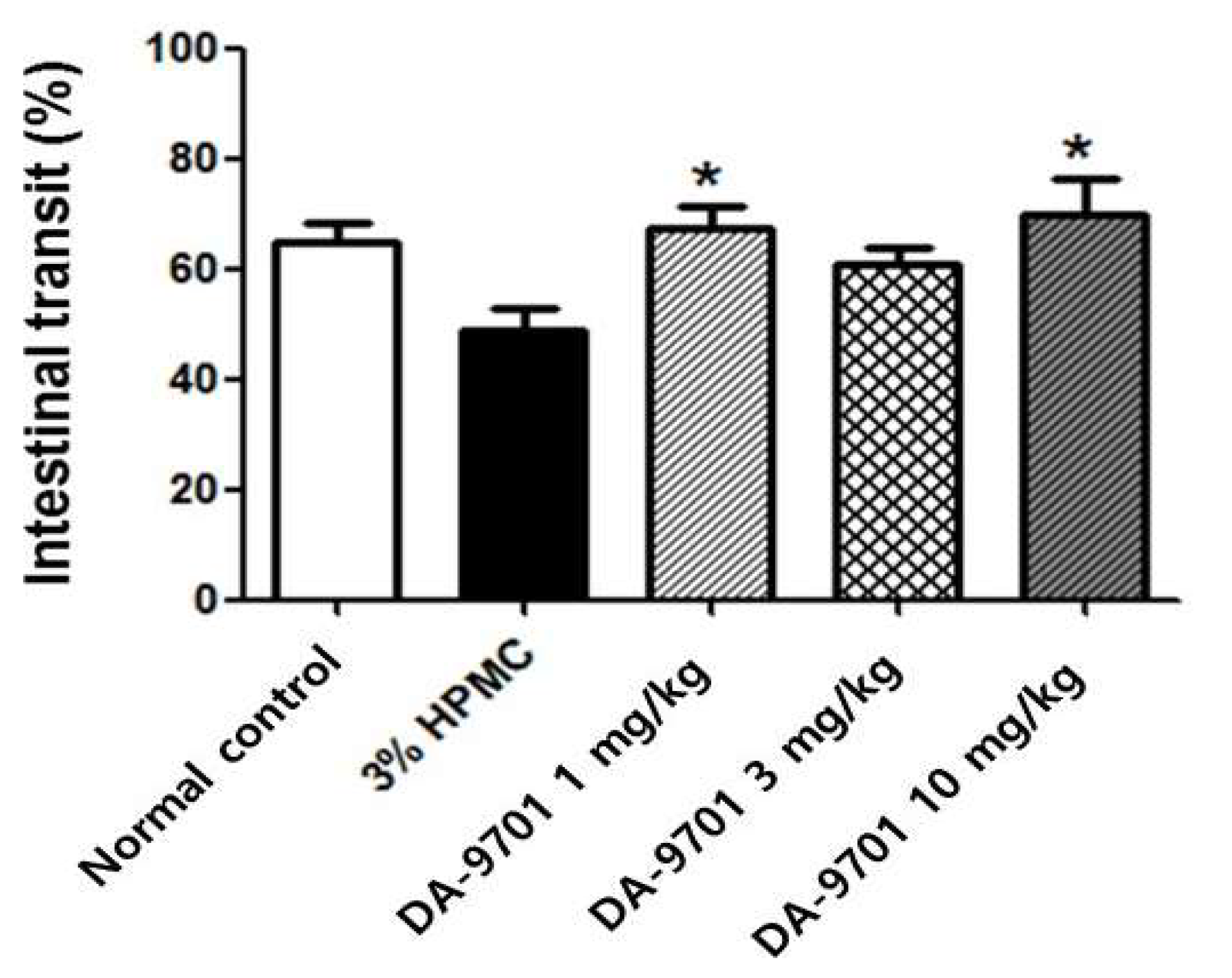
3.5. Effects of DA-9701 on Intestinal Transit
3.6. Effects of DA-9701 on GE Rate
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parkman, H.P.; Hasler, W.L.; Fisher, R.S. American Gastroenterological Association technical review on the diagnosis and treatment of gastroparesis. Gastroenterology 2004, 127, 1592–1622. [Google Scholar] [CrossRef] [Green Version]
- Krishnasamy, S.; Abell, T.L. Diabetic Gastroparesis: Principles and Current Trends in Management. Diabetes Ther. Res. Treat. Educ. Diabetes Relat. Disord. 2018, 9, 1–42. [Google Scholar] [CrossRef] [Green Version]
- Yagihashi, S.; Sima, A.A. Diabetic autonomic neuropathy in BB rat. Ultrastructural and morphometric changes in parasympathetic nerves. Diabetes 1986, 35, 733–743. [Google Scholar] [CrossRef]
- Kwon, Y.S.; Son, M. DA-9701: A New Multi-Acting Drug for the Treatment of Functional Dyspepsia. Biomol. Ther. 2013, 21, 181–189. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.R.; Min, B.H.; Lee, S.O.; Lee, T.H.; Son, M.; Rhee, P.L. Effects of DA-9701, a novel prokinetic agent, on gastric accommodation in conscious dogs. J. Gastroenterol. Hepatol. 2012, 27, 766–772. [Google Scholar] [CrossRef]
- Lee, T.H.; Choi, J.J.; Kim, D.H.; Choi, S.; Lee, K.R.; Son, M.; Jin, M. Gastroprokinetic effects of DA-9701, a new prokinetic agent formulated with Pharbitis Semen and Corydalis Tuber. Phytomed. Int. J. Phytother. Phytopharm. 2008, 15, 836–843. [Google Scholar] [CrossRef]
- Lee, S.P.; Lee, K.N.; Lee, O.Y.; Lee, H.L.; Jun, D.W.; Yoon, B.C.; Choi, H.S.; Hwang, S.J.; Lee, S.E. Effects of DA-9701, a novel prokinetic agent, on phosphorylated extracellular signal-regulated kinase expression in the dorsal root ganglion and spinal cord induced by colorectal distension in rats. Gut Liver 2014, 8, 140–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanders, K.M.; Ordog, T.; Koh, S.D.; Ward, S.M. A Novel Pacemaker Mechanism Drives Gastrointestinal Rhythmicity. News Physiol. Sci. Int. J. Physiol. Prod. Jt. By Int. Union Physiol. Sci. Am. Physiol. Soc. 2000, 15, 291–298. [Google Scholar] [CrossRef] [Green Version]
- Waseem, S.; Moshiree, B.; Draganov, P.V. Gastroparesis: Current diagnostic challenges and management considerations. World J. Gastroenterol. 2009, 15, 25–37. [Google Scholar] [CrossRef]
- Choi, K.M.; Gibbons, S.J.; Nguyen, T.V.; Stoltz, G.J.; Lurken, M.S.; Ordog, T.; Szurszewski, J.H.; Farrugia, G. Heme Oxygenase-1 Protects Interstitial Cells of Cajal From Oxidative Stress and Reverses Diabetic Gastroparesis. Gastroenterology 2008, 135, 2055–2064.e2. [Google Scholar] [CrossRef] [Green Version]
- Kniel, P.C.; Junker, U.; Perrin, I.V.; Bestetti, G.E.; Rossi, G.L. Varied effects of experimental diabetes on the autonomic nervous system of the rat. Lab. Investig. A J. Tech. Methods Pathol. 1986, 54, 523–530. [Google Scholar]
- Duchen, L.W.; Anjorin, A.; Watkins, P.J.; Mackay, J.D. Pathology of autonomic neuropathy in diabetes mellitus. Ann. Intern. Med. 1980, 92, 301–303. [Google Scholar] [CrossRef]
- Iwasaki, H.; Kajimura, M.; Osawa, S.; Kanaoka, S.; Furuta, T.; Ikuma, M.; Hishida, A. A deficiency of gastric interstitial cells of Cajal accompanied by decreased expression of neuronal nitric oxide synthase and substance P in patients with type 2 diabetes mellitus. J. Gastroenterol. 2006, 41, 1076–1087. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Sarosiek, I.; Forster, J.; Damjanov, I.; Hou, Q.; McCallum, R.W. Association of the status of interstitial cells of Cajal and electrogastrogram parameters, gastric emptying and symptoms in patients with gastroparesis. Neurogastroenterol. Motil. 2010, 22, 56–61.e10. [Google Scholar] [PubMed]
- Horváth, V.J.; Vittal, H.; Lörincz, A.; Chen, H.; Almeida–Porada, G.; Redelman, D.; Ördög, T. Reduced Stem Cell Factor Links Smooth Myopathy and Loss of Interstitial Cells of Cajal in Murine Diabetic Gastroparesis. Gastroenterology 2006, 130, 759–770. [Google Scholar] [CrossRef]
- Kindt, S.; Dubois, D.; Van Oudenhove, L.; Caenepeel, P.; Arts, J.; Bisschops, R.; Tack, J. Relationship between symptom pattern, assessed by the PAGI-SYM questionnaire, and gastric sensorimotor dysfunction in functional dyspepsia. Neurogastroenterol. Motil. 2009, 21, 1183-e1105. [Google Scholar] [CrossRef]
- Kim, B.J.; Kuo, B. Gastroparesis and Functional Dyspepsia: A Blurring Distinction of Pathophysiology and Treatment. J. Neurogastroenterol. Motil. 2019, 25, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Phillips, L.K.; Rayner, C.K.; Jones, K.L.; Horowitz, M. An update on autonomic neuropathy affecting the gastrointestinal tract. Curr. Diabetes Rep. 2006, 6, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekharan, B.; Anitha, M.; Blatt, R.; Shahnavaz, N.; Kooby, D.; Staley, C.; Mwangi, S.; Jones, D.P.; Sitaraman, S.V.; Srinivasan, S. Colonic motor dysfunction in human diabetes is associated with enteric neuronal loss and increased oxidative stress. Neurogastroenterol. Motil. 2011, 23, 131–138. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.Y.; Woo, H.S.; Kim, K.O.; Choi, S.H.; Kwon, K.A.; Chung, J.; Kim, Y.J.; Kim, J.H.; Park, D.K. DA-9701 improves colonic transit time and symptoms in patients with functional constipation: A prospective study. J. Gastroenterol. Hepatol. 2017, 32, 1943–1948. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Lee, J.Y.; Cho, J.W.; Koh, S.B.; Yang, Y.S.; Yoo, D.; Shin, C.M.; Kim, H.T. Double-Blind, Randomized, Placebo-Controlled Trial of DA-9701 in Parkinson’s Disease: PASS-GI Study. Mov. Disord. Off. J. Mov. Disord. Soc. 2020, 35, 1966–1976. [Google Scholar] [CrossRef] [PubMed]
- Ramsbottom, N.; Hunt, J.N. Studies of the effect of metoclopramide and apomorphine on gastric emptying and secretion in man. Gut 1970, 11, 989–993. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.; Choi, J.J.; Jun, J.Y.; Koh, J.W.; Kim, S.H.; Kim, D.H.; Pyo, M.Y.; Choi, S.; Son, J.P.; Lee, I.; et al. Induction of pacemaker currents by DA-9701, a prokinetic agent, in interstitial cells of Cajal from murine small intestine. Mol. Cells 2009, 27, 307–312. [Google Scholar] [CrossRef]
- Choi, M.-G.; Rhee, P.-L.; Park, H.; Lee, O.Y.; Lee, K.J.; Choi, S.C.; Seol, S.Y.; Chun, H.J.; Rew, J.-S.; Lee, N.H.; et al. Randomized, Controlled, Multi-center Trial: Comparing the Safety and Efficacy of DA-9701 and Itopride Hydrochloride in Patients With Functional Dyspepsia. J. Neurogastroenterol. Motil. 2015, 21, 414–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, C.-H.; Choi, M.-G.; Park, H.; Baeg, M.K.; Park, J.M. Effect of DA-9701 on gastric emptying in a mouse model: Assessment by 13C-octanoic acid breath test. World J. Gastroenterol. 2013, 19, 4380–4385. [Google Scholar] [CrossRef]
- Jung, Y.S.; Kim, M.Y.; Lee, H.S.; Park, S.L.; Lee, K.J. Effect of DA-9701, a novel prokinetic agent, on stress-induced delayed gastric emptying and hormonal changes in rats. Neurogastroenterol. Motil. 2013, 25, 254–259.e166. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ha, C.; Kim, H.; Cha, R.; Lee, J.; Lee, S.; Ryu, J.-H.; Kim, H.; Lee, O.-J. Effect of DA-9701 on the Gastrointestinal Motility in the Streptozotocin-Induced Diabetic Mice. J. Clin. Med. 2021, 10, 5282. https://doi.org/10.3390/jcm10225282
Ha C, Kim H, Cha R, Lee J, Lee S, Ryu J-H, Kim H, Lee O-J. Effect of DA-9701 on the Gastrointestinal Motility in the Streptozotocin-Induced Diabetic Mice. Journal of Clinical Medicine. 2021; 10(22):5282. https://doi.org/10.3390/jcm10225282
Chicago/Turabian StyleHa, Changyoon, Heejin Kim, Rari Cha, Jaemin Lee, Sangsoo Lee, Jung-Hwa Ryu, Hyunjin Kim, and Ok-Jae Lee. 2021. "Effect of DA-9701 on the Gastrointestinal Motility in the Streptozotocin-Induced Diabetic Mice" Journal of Clinical Medicine 10, no. 22: 5282. https://doi.org/10.3390/jcm10225282
APA StyleHa, C., Kim, H., Cha, R., Lee, J., Lee, S., Ryu, J.-H., Kim, H., & Lee, O.-J. (2021). Effect of DA-9701 on the Gastrointestinal Motility in the Streptozotocin-Induced Diabetic Mice. Journal of Clinical Medicine, 10(22), 5282. https://doi.org/10.3390/jcm10225282





