Early Surgery with Neuraxial Anaesthesia in Patients on Chronic Antiplatelet Therapy with a Proximal Femur Fracture: Multicentric Randomised Clinical Trial
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Methods
2.2. Study Design
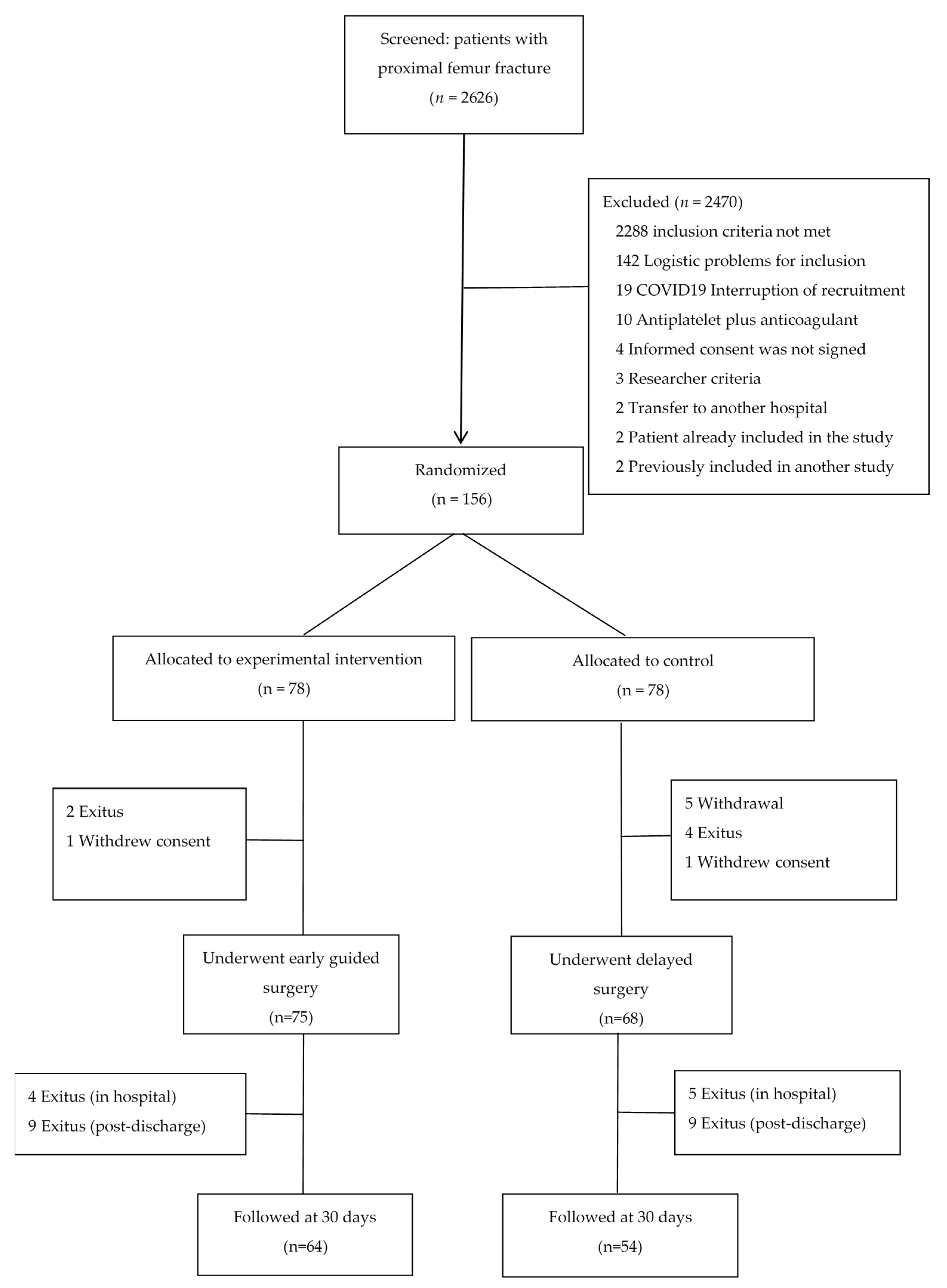
2.3. Participants
- Experimental group. Platelet function was measured on emergency room admission. The threshold indicating candidacy for early surgery was set to a minimum of 80 × 109/L to ensure anaesthetic and surgical safety [27]. In patients with >80 × 109/L functional platelets, surgery was scheduled for within 24 h. In patients with ≤80 × 109/L functional platelets, platelet function was measured daily to check if the minimum threshold count was reached; if platelet count had not normalised by the third day, surgery with neuraxial anaesthesia was scheduled following the margin of safety established for each specific antiplatelet drug (as specified for the control group).
- Control group. A platelet function test was performed 24 h prior to surgery, blinded so as not to influence clinical decision making regarding the control patients. Surgery with neuraxial anaesthesia was performed following the established margin of safety for each specific antiplatelet drug: 3 days for ASA > 100 mg/day and triflusal > 300 mg/day, 5 days for clopidogrel and ticagrelor, 7 days for prasugrel, and 10 days for ticlopidine [20,21,22,23].
2.4. Outcomes
2.5. Sample Size
2.6. Random Sequence Generation and Allocation Concealment
2.7. Implementation
2.8. Blinding
2.9. Statistical Analysis
3. Results
3.1. Platelet Function
3.2. Primary Outcome
3.3. Secondary Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Clement, N.D.; Aitken, S.A.; Duckworth, A.D.; McQueen, M.M.; Court-Brown, C.M. The outcome of fractures in very elderly patients. J. Bone Jt. Surg. Br. 2011, 93, 806–810. [Google Scholar] [CrossRef] [Green Version]
- Brauer, C.A.; Coca-Perraillon, M.; Cutler, D.M.; Rosen, A.B. Incidence and mortality of hip fractures in the United States. JAMA 2009, 302, 1573–1579. [Google Scholar] [CrossRef] [Green Version]
- Moran, C.G.; Wenn, R.T.; Sikand, M.; Taylor, A.M. Early mortality after hip fracture: Is delay before surgery important? J. Bone Jt. Surg. Am. 2005, 87, 483–489. [Google Scholar]
- Franzo, A.; Francescutti, C.; Simon, G. Risk factors correlated with postoperative mortality for hip fracture surgery in the elderly: A population-based approach. Eur. J. Epidemiol. 2005, 20, 985–991. [Google Scholar] [CrossRef]
- Bentler, S.E.; Liu, L.; Obrizan, M.; Cook, E.A.; Wright, K.B.; Geweke, J.F.; Chrischilles, E.A.; Pavlik, C.E.; Wallace, R.B.; Ohsfeldt, R.L.; et al. The aftermath of hip fracture: Discharge placement, functional status change, and mortality. Am. J. Epidemiol. 2009, 170, 1290–1299. [Google Scholar] [CrossRef] [Green Version]
- Institute of Health Information. Annotated Statistics: Attention to Hip Fracture in SNS Hospitals; Ministry of Health and Social Policy: Madrid, Spain, 2010. Available online: https://www.msssi.gob.es/estadEstudios/estadisticas/docs/Estadisticas_comentadas_01.pdf (accessed on 2 July 2018).
- Sahota, O.; Morgan, N.; Moran, C.G. The direct cost of acute hip fracture care in care home residents in the UK. Osteoporos. Int. 2012, 23, 917–920. [Google Scholar] [CrossRef]
- AAOS. Management of Hip Fractures in the Elderly Evidence-Based Clinical Practice Guideline. Adopted by the American Academy of Orthopaedic Surgeons Board of Directors. 2014. Available online: https://www.aaos.org/research/guidelines/hipfxguideline.pdf (accessed on 2 July 2018).
- National Institute for Health and Clinical Excellence. Hip Fracture: Management; Clinical guideline [CG124]; National Institute for Health and Care Excellence: London, UK, May 2017. [Google Scholar]
- Yang, Z.; Ni, J.; Long, Z.; Kuang, L.; Gao, Y.; Tao, S. Is hip fracture surgery safe for patients on antiplatelet drugs and is it necessary to delay surgery? A systematic review and meta-analysis. J. Orthop. Surg. Res. 2020, 15, 105. [Google Scholar] [CrossRef] [PubMed]
- Soo, C.G.; Della Torre, P.K.; Yolland, T.J.; Shatwell, M.A. Clopidogrel and hip fractures, is it safe? A systematic review and meta-analysis. BMC Musculoskelet. Disord. 2016, 17, 136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doleman, B.; Moppett, I.K. Is early hip fracture surgery safe for patients on clopidogrel? Systematic review, meta-analysis, and meta-regression. Injury 2015, 46, 954–962. [Google Scholar] [CrossRef] [PubMed]
- McIsaac, D.I.; Wijeysundera, D.N.; Huang, A.; Bryson, G.L.; van Walraven, C. Association of hospital- level neuraxial anesthesia use for hip fracture surgery with outcomes: A population-based cohort study. Anesthesiology 2018, 128, 480–491. [Google Scholar] [CrossRef]
- Van Waesberghe, J.; Stevanovic, A.; Rossaint, R.; Coburn, M. General vs. neuraxial anesthesia in hip fracture patients: A systematic review and meta-analysis. BMC Anesthesiol. 2017, 17, 87. [Google Scholar] [CrossRef] [Green Version]
- Neuman, M.D.; Ellenberg, S.S.; Sieber, F.E.; Magaziner, J.S.; Feng, R.; Carson, J.L. Regional versus General Anesthesia for Promoting Independence after Hip Fracture (REGAIN): Protocol for a pragmatic, international multicenter trial. BMJ Open 2016, 6, e013473. [Google Scholar] [CrossRef] [Green Version]
- Kowark, A.; Adam, C.; Ahrens, J.; Bajbouj, M.; Bollheimer, C.; Borowski, M.; Dodel, R.; Dolch, M.; Hachenberg, T.; Henzler, D.; et al. Improve hip fracture outcome in the elderly patient (iHOPE): A study protocol for a pragmatic, multicentre randomised controlled trial to test the efficacy of spinal versus general anesthesia. BMJ Open 2018, 8, e023609. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Yeung, J.; Li, J.; Zhang, Y.; Melody, T.; Gao, Y.; Wang, Y.; Lian, Q.; Gao, F. Comparison of regional with general anaesthesia on postoperative delirium (RAGA-delirium) in the older patients undergoing hip fracture surgery: Study protocol for a multicentre randomised controlled trial. BMJ Open 2017, 7, e016937. [Google Scholar] [CrossRef] [Green Version]
- Sanders, R.A.; Bendel, M.A.; Moeschler, S.M.; Mauck, W.D. Epidural Hematoma Following Interlaminar Epidural Injection in Patient Taking Aspirin. Reg. Anesth. Pain Med. 2018, 43, 310–312. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, J.; Hua, B.; Kahana, M.; Shaparin, N.; Yu, S.; Davila-Velazquez, J. Neuraxial Anesthesia in Parturients with Low Platelet Counts. Anesth. Analg. 2016, 123, 165–167. [Google Scholar] [CrossRef]
- Keeling, D.; Tait, R.C.; Watson, H. British Committee of Standards for Haematology. Perioperative management of anticoagulation and antiplatelet therapy. Br. J. Haematol. 2016, 175, 602–613. [Google Scholar] [CrossRef] [PubMed]
- Vivas, D.; Roldán, I.; Ferrandis, R.; Marin, F.; Roldan, V.; Tello-Montoliu, A.; Ruiz-Nodar, J.M.; Gómez-Doblas, J.J.; Martín, A.; Llau, J.V.; et al. Perioperative and Periprocedural Management of Antithrombotic Therapy: Consensus Document of SEC, SEDAR, SEACV, SECTCV, AEC, SECPRE, SEPD, SEGO, SEHH, SETH, SEMERGEN, SEMFYC, SEMG, SEMICYUC, SEMI, SEMES, SEPAR, SENEC, SEO, SEPA, SERVEI, SECOT and AEU. Rev. Esp. Cardiol. 2018, 71, 553–564. [Google Scholar] [CrossRef]
- Sierra, P.; Gómez-Luque, A.; Llau, J.V. Recommendations for perioperative antiplatelet treatment in non-cardiac surgery. Working Group of the Spanish Society of Anaesthesiology-Resuscitation and Pain Therapy, Division of Haemostasis, Transfusion Medicine, and Perioperative Fluid Therapy Update of the Clinical practice guide 2018. Rev. Esp. Anestesiol. Reanim. 2019, 66, 18–36. [Google Scholar]
- Narouze, S.; Benzon, H.T.; Provenzano, D.; Buvanendran, A.; de Andres, J.; Deer, T.; Rauck, R.; Huntoon, M.A. Interventional Spine and Pain Procedures in Patients on Antiplatelet and Anticoagulant Medications (Second Edition): Guidelines from the American Society of Regional Anesthesia and Pain Medicine, the European Society of Regional Anaesthesia and Pain Therapy, the American Academy of Pain Medicine, the International Neuromodulation Society, the North American Neuromodulation Society, and the World Institute of Pain. Reg. Anesth. Pain Med. 2018, 43, 225–262. [Google Scholar]
- Ferraris, V.A.; Saha, S.P.; Oestreich, J.; Song, H.K.; Rosengart, T.; Reece, T.B.; Mazer, C.D.; Bridges, C.R.; Despotis, G.J.; Jointer, K.; et al. 2012 update to the Society of Thoracic Surgeons guideline on use of antiplatelet drugs in patients having cardiac and noncardiac operations. Ann. Thorac. Surg. 2012, 94, 1761–1781. [Google Scholar] [CrossRef]
- Mahla, E.; Suarez, T.A.; Bliden, K.P.; Sequeira, A.J.; Cho, P.; Sell, J.; Fan, J.; Antonino, M.J.; Tantry, U.S.; Gurbel, P.A.; et al. Platelet function measurement- based strategy to reduce bleeding and waiting time in clopidogrel-treated patients undergoing coronary artery bypass graft surgery: The timing based on platelet function strategy to reduce clopidogrel-associated bleeding related to CABG (TARGET-CABG) study. Circ. Cardiovasc. Interv. 2012, 5, 261–269. [Google Scholar] [PubMed] [Green Version]
- Rafael, A.; Mireia, R.; María, G.J.; Victoria, M.; Angélica, M.; Noèlia, V.; Erica, C.; María, A.R.; Francesca, R.; Patricia, G.; et al. Evaluation of a strategy to shorten the time to surgery in patients on antiplatelet therapy with a proximal femur fracture (AFFEcT Study). Medicine 2019, 98, e15514. [Google Scholar] [CrossRef]
- Van Veen, J.J.; Nokes, T.J.; Makris, M. The risk of spinal haematoma following neuraxial anaesthesia or lumbar puncture in thrombocytopenic individuals. Br. J. Haematol. 2010, 148, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.; Ridgway, H.; Carville, D. Plateletworks: A novel point of care platelet function screen. Mol. Diagn. Ther. 2008, 12, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Nadler, S.B.; Hidalgo, J.U.; Bloch, T. Prediction of blood volume in normal human adults. Surgery 1962, 51, 224–232. [Google Scholar]

| Early Surgery N = 75 n (%) | Delayed Surgery N = 68 n (%) | p | |
|---|---|---|---|
| Age [m(SD)] | 85.44 (8.7) | 86.53 (6.8) | 0.4 |
| Female | 55 (73.3) | 42 (61.8) | 0.13 |
| Medical history | |||
| Arterial hypertension | 61 (81.3) | 57 (83.8) | 0.69 |
| Surgery | 55 (68.0) | 40 (52.9) | 0.06 |
| Ischaemic neurological disease | 34 (45.3) | 34 (50.0) | 0.57 |
| Delirium and cognitive impairment | 19 (25.3) | 25 (36.7) | 0.14 |
| Diabetes | 28 (37.3) | 21 (30.9) | 0.42 |
| Coronary disease | 31 (41.3) | 20 (29.4) | 0.14 |
| Chronic renal insufficiency | 24 (32.0) | 17 (25.0) | 0.35 |
| Pulmonary disease | 15 (20.0) | 13 (19.1) | 0.89 |
| Oncological disease | 9 (12.0) | 9 (13.2) | 0.824 |
| Auricular fibrillation | 11 (14.6) | 7 (10.3) | 0.43 |
| Other | 27 (36.5) | 25 (36.8) | 0.97 |
| American Society of Anesthesiologists score | |||
| 1 | 1 (1.3) | 0 (0) | 0.37 |
| 2 | 3 (4.0) | 6 (8.8) | |
| 3 | 67 (89.3) | 57 (83.8) | |
| 4 | 4 (5.3) | 5 (7.3) | |
| Type of femur fracture | |||
| Intracapsular | 34 (45.3) | 37 (54.4) | 0.28 |
| Extracapsular | 41 (54.7) | 31 (45.6) | |
| Antiplatelet drug: | |||
| Clopidogrel | 59 (78.7) | 54 (79.4) | 0.87 |
| ASA > 100 mg | 13 (17.3) | 10 (14.7) | |
| Clopidogrel/ASA | 2 (2.7) | 3 (4.4) | |
| Cilostazol/triflusal > 300 mg | 0 (0) | 1 (1.5) | |
| Ticagrelor | 1 (1.3) | 0 (0) | |
| Antiplatelet drug based on action mechanism | |||
| Cyclooxygenase inhibitors | 13 (17.3) | 11 (16.2) | 0.85 |
| (AAS > 100 and triflusal > 300) | |||
| P2Y12 receptor inhibitors | 62 (82.7) | 57 (83.8) | |
| (clopidogrel/ticagrelor) | |||
| Functional platelet count * (×109/L) | N = 67 | N = 66 | |
| All patients | 118 (90–144) | 136.5 (98–176) | 0.13 |
| On cyclooxygenase inhibitors | 117 (88–144) | 108 (68–166) | 0.92 |
| (11/11) † | |||
| On P2Y12 receptor inhibitors | 118 (91.5–146) | 140 (99–192) | 0.08 |
| (56/55) † | |||
| Early Surgery N = 75 | Last Antiplatelet Dose to Platelet Count > 80 × 109/L | Admission to Platelet Count > 80 × 109/L | |
|---|---|---|---|
| n (%) | Days, Median (IQR) | Days, Median (IQR) | |
| All patients | 67 (89.3) | 1.9 (0.9–2.9) | 0.8 (0.6–1.6) |
| On cyclooxygenase inhibitors | 11 (14.66) | 1.97 (1.89–2.04) | 0.70 (0.65–1.11) |
| On P2Y12 receptor inhibitors | 56 (74.66) | 1.92 (0.96–2.17) | 0.88 (0.61–1.60) |
| Platelet function tests (n) | |||
| 1 | 49 (65.3) | 1.9 (0.9–2.0) | 0.7 (0.4–0.9) |
| 2 | 13 (17.3) | 1.9 (1.9–2.9) | 1.6 (1.40–1.8) |
| 3 | 5 (6.7) | 2.9 (2.9–3.0) | 2.7 (2.7–3.3) |
| Referred for delayed surgery * | 8 (10.7) | - | - |
| Early Surgery N = 75 n (%) | Delayed Surgery N = 68 n (%) | p | |
|---|---|---|---|
| Orthopaedic treatment | 0.31 | ||
| Arthroplasty | 29 (38.7) | 32 (47.1) | |
| Osteosynthesis | 46 (62.2) | 36 (52.9) | |
| Anaesthesia type | 0.17 | ||
| General +/− peripheral nerve block | 5 (6.7) | 10 (14.7) | |
| Neuraxial +/− peripheral nerve block | 70 (93.3) | 58 (85.3) | |
| Tranexamic acid used | 3 (4.0) | 0 (0) | 0.09 |
| Surgery duration (median (IQR) mins; t-test) | 70 (46–87) | 64 (52–95.5) | 0.77 |
| Haemoglobin values (mean (SD) g/L) | |||
| On admission (n = 143) | 117.2 (18.7) | 119.5 (16.0) | 0.42 |
| 12 h pre-surgery (n = 57/60) | 109.9 (14.3) | 108.7 (15.7) | 0.67 |
| 2 h post-surgery (n = 45/40) | 104.8 (14.2) | 114.0 (14.7) | 0.004 |
| 24 h post-surgery (n = 71/63) | 96.1 (11.7) | 97.2 (15.1) | 0.64 |
| 5 d post-surgery (n = 67/61) | 98.3 (12.8) | 98.7 (12.6) | 0.85 |
| Perioperative blood loss | 577.3 | 604.2 | 0.94 |
| (median (IQR) mL) (n = 73/67) | (1.2–1061.5) | (1.7–1020.0) | |
| Hospital stay | 9.7 | 12.6 | <0.001 |
| (median (IQR) days) (n = 71/63) | (8.2–13.4) | (10.1–16.5) | |
| Early Surgery N = 75 n (%) | Delayed Surgery N = 68 n (%) | p | |
|---|---|---|---|
| Transfusions | |||
| Patients with at least 1 transfusion | 53 (70.7) | 48 (70.6) | 0.99 0.45 |
| Patients by number of transfusions | |||
| 1 | 35 (46.7) | 39 (57.3) | |
| 2 | 11 (14.7) | 7 (10.3) | |
| 3 | 5 (6.7) | 2 (2.9) | |
| 4 | 2 (2.7) | 0 (0) | |
| Units transfused post-surgery | |||
| Mean (SD) | 1.68 (1.6) | 1.46 (1.3) | 0.37 0.13 |
| Units transfused | |||
| 1 | 17 (22.7) | 14 (20.6) | |
| 2 | 19 (25.3) | 27 (39.7) | |
| 3–6 | 17 (22.7) | 7 (10.3) | |
| Complications in hospital | N = 75 | N = 68 | |
| Wound: all patients | 6 (8.0) | 8 (11.8) | 0.58 |
| Infection | 0 (0) | 1 (1.5) | - |
| Hematoma | 6 (8.0) | 8 (11.8) | 0.58 |
| Re-intervention | 0 (0) | 1 (1.5) | - |
| Medical: all patients | 41 (54.7) | 39 (57.3) | 0.87 |
| Urinary tract infection | 16 (21.3) | 12 (17.6) | 0.58 |
| Delirium | 10 (13.3) | 11 (16.2) | 0.63 |
| Acute renal insufficiency | 4 (5.3) | 9 (13.2) | 0.14 |
| Symptomatic hypotension | 3 (4.0) | 7 (10.3) | 0.19 |
| Pressure ulcer | 2 (2.7) | 6 (8.8) | 0.15 |
| Cardiac insufficiency | 3 (4.0) | 4 (5.9) | 0.71 |
| Death | 4 (5.3) | 5 (7.3) | 0.74 |
| Pneumonia | 1 (1.3) | 3 (4.4) | 0.35 |
| Ictus | 2 (2.7) | 1 (1.5) | 1 |
| Spinal haematoma | 0 | 0 | - |
| Other | 19 (25.3) | 18 (26.5) | 0.88 |
| Complications 30 ± 15 d post-discharge | N = 71 | N = 63 | |
| Wound: all patients | 2 (2.8) | 2 (3.2) | - |
| Haematoma | 1 | 0 | - |
| Dislocation | 1 | 2 | - |
| Medical: all patients | 19 (25.3) | 21 (34.9) | 0.23 |
| Mortality | 9 (12.7) | 9(14.3) | 0.8 |
| Urinary tract infection | 4 (5.6) | 3 (4.8) | 0.82 |
| Pneumonia | 1 (1.4) | 3 (4.8) | 0.34 |
| Delirium | 0 (0) | 2 (3.2) | 0.22 |
| Myocardial infarction | 2 (2.8) | 0 (0) | 0.5 |
| Gastrointestinal bleeding | 3 (4.2) | 1 (1.6) | 0.62 |
| COVID-19 | 3 (4.2) | 0 (0) | 0.25 |
| Other | 9 (12.7) | 16 (25.4) | 0.08 |
| Perioperative mortality (from randomisation to 30 ± 15 d post-discharge) | |||
| ITT analysis * | 15 (19.2) | 18 (23.1) | 0.56 |
| PP analysis * | 13 (17.3) | 14 (20.6) | 0.62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anaya, R.; Rodriguez, M.; Millan, A.; Reguant, F.; Llorca, J.; Guilabert, P.; Ruiz, A.; Pantoja, P.-E.; Gil, J.M.; Moral, V.; et al. Early Surgery with Neuraxial Anaesthesia in Patients on Chronic Antiplatelet Therapy with a Proximal Femur Fracture: Multicentric Randomised Clinical Trial. J. Clin. Med. 2021, 10, 5371. https://doi.org/10.3390/jcm10225371
Anaya R, Rodriguez M, Millan A, Reguant F, Llorca J, Guilabert P, Ruiz A, Pantoja P-E, Gil JM, Moral V, et al. Early Surgery with Neuraxial Anaesthesia in Patients on Chronic Antiplatelet Therapy with a Proximal Femur Fracture: Multicentric Randomised Clinical Trial. Journal of Clinical Medicine. 2021; 10(22):5371. https://doi.org/10.3390/jcm10225371
Chicago/Turabian StyleAnaya, Rafael, Mireia Rodriguez, Angélica Millan, Francesca Reguant, Jordi Llorca, Patricia Guilabert, Ana Ruiz, Percy-Efrain Pantoja, José María Gil, Victoria Moral, and et al. 2021. "Early Surgery with Neuraxial Anaesthesia in Patients on Chronic Antiplatelet Therapy with a Proximal Femur Fracture: Multicentric Randomised Clinical Trial" Journal of Clinical Medicine 10, no. 22: 5371. https://doi.org/10.3390/jcm10225371






