Prognostic Impact of Sleep Patterns and Related-Drugs in Patients with Heart Failure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Measures
- Pittsburgh Sleep Quality Index—PSQI [16]
- 2.
- Epworth Sleepiness Scale-ESS [17]
- 3.
- Chalder Fatigue scale [18]
- 4.
- Insomnia Severity Scale-ISI [19]
- 5.
- Beck Depression Inventory-BDI [20]
- 6.
- Berlin Questionnaire [21]
2.3. CVD Outcome
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Groenewegen, A.; Rutten, F.H.; Mosterd, A.; Hoes, A.W. Epidemiology of heart failure. Eur. J. Heart Fail. 2020, 22, 1342–1356. [Google Scholar] [CrossRef]
- Shah, K.S.; Xu, H.; Matsouaka, R.A.; Bhatt, D.L.; Heidenreich, P.A.; Hernandez, A.F.; Devore, A.D.; Yancy, C.W.; Fonarow, G.C. Heart failure with preserved, borderline, and reduced ejection fraction: 5-Year outcomes. J. Am. Coll. Cardiol. 2017, 70, 2476–2486. [Google Scholar] [CrossRef]
- Malik, A.; Gill, G.S.; Lodhi, F.K.; Tummala, L.S.; Singh, S.N.; Morgan, C.J.; Allman, R.M.; Fonarow, G.C.; Ahmed, A. Prior heart failure hospitalization and outcomes in patients with heart failure with preserved and reduced ejection fraction. Am. J. Med. 2020, 133, 84–94. [Google Scholar] [CrossRef]
- Bhatt, A.S.; Ambrosy, A.P.; Dunning, A.; DeVore, A.D.; Butler, J.; Reed, S.; Voors, A.; Starling, R.; Armstrong, P.W.; Ezekowitz, J.A.; et al. The burden of non-cardiac comorbidities and association with clinical outcomes in an acute heart failure trial-insights from ASCEND-HF. Eur. J. Heart Fail. 2020, 22, 1022–1031. [Google Scholar] [CrossRef] [PubMed]
- Kanno, Y.; Yoshihisa, A.; Watanabe, S.; Takiguchi, M.; Yokokawa, T.; Sato, A.; Miura, S.; Shimizu, T.; Nakamura, Y.; Abe, S.; et al. Prognostic significance of insomnia in heart failure. Circ. J. 2016, 80, 1571–1577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, Y.; Yoshihisa, A.; Hotsuki, Y.; Watanabe, K.; Kimishima, Y.; Kiko, T.; Kanno, Y.; Yokokawa, T.; Abe, S.; Misaka, T.; et al. Associations of benzodiazepine with adverse prognosis in heart failure patients with insomnia. J. Am. Heart Assoc. 2020, 9, e013982. [Google Scholar] [CrossRef]
- Jorge-Samitier, P.; Durante, A.; Gea-Caballero, V.; Antón-Solanas, I.; Fernández-Rodrigo, M.T.; Juárez-Vela, R. Sleep quality in patients with heart failure in the spanish population: A cross-sectional study. Int. J. Environ. Res. Public Health 2020, 17, 7772. [Google Scholar] [CrossRef]
- Zilberman, M.; Silverberg, D.S.; Schwartz, D.; Oksenberg, A. Restless legs syndrome (RLS) in anemic patients with congestive heart failure and chronic renal failure: Lack of effect of anemia treatment. Int. J. Cardiol. 2010, 143, 205–207. [Google Scholar] [CrossRef] [PubMed]
- Príncipe-Rodríguez, K.; Strohl, K.P.; Hadziefendic, S.; Piña, I.L. Sleep symptoms and clinical markers of illness in patients with heart failure. Sleep Breath. 2005, 9, 127–133. [Google Scholar] [CrossRef]
- Javaheri, S.; Barbe, F.; Campos-Rodriguez, F.; Dempsey, J.A.; Khayat, R.; Javaheri, S.; Malhotra, A.; Martinez-Garcia, M.A.; Mehra, R.; Pack, A.I.; et al. Types, mechanisms, and clinical cardiovascular consequences. J. Am. Coll. Cardiol. 2017, 69, 841–858. [Google Scholar] [CrossRef] [PubMed]
- Zuurbier, L.A.; Luik, A.I.; Leening, M.J.G.; Hofman, A.; Freak-Poli, R.; Franco, O.H.; Stricker, B.H.; Tiemeier, H. Associations of heart failure with sleep quality: The Rotterdam Study. J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med. 2015, 11, 117–121. [Google Scholar] [CrossRef]
- Lee, K.S.; Lennie, T.A.; Heo, S.; Song, E.K.; Moser, D.K. Prognostic importance of sleep quality in patients with heart failure. Am. J. Crit. Care Off. Publ. Am. Assoc. Crit. Care Nurs. 2016, 25, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Damy, T.; Margarit, L.; Noroc, A.; Bodez, D.; Guendouz, S.; Boyer, L.; Drouot, X.; Lamine, A.; Paulino, A.; Rappeneau, S.; et al. Prognostic impact of sleep-disordered breathing and its treatment with nocturnal ventilation for chronic heart failure. Eur. J. Heart Fail. 2012, 14, 1009–1019. [Google Scholar] [CrossRef] [PubMed]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.-P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2016, 18, 891–975. [Google Scholar] [PubMed]
- Allen, R.P.; Picchietti, D.L.; Garcia-Borreguero, D.; Ondo, W.G.; Walters, A.S.; Winkelman, J.W.; Zucconi, M.; Ferri, R.; Trenkwalder, C.; Lee, H.B.; et al. Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: Updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria—History, rationale, description, and significance. Sleep Med. 2014, 15, 860–873. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatr. Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Johns, M.W. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef] [Green Version]
- Cella, M.; Chalder, T. Measuring fatigue in clinical and community settings. J. Psychosom. Res. 2010, 69, 17–22. [Google Scholar] [CrossRef]
- Bastien, C.H.; Vallières, A.; Morin, C.M. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001, 2, 297–307. [Google Scholar] [CrossRef]
- Beck, A.T.; Steer, R.A.; Ball, R.; Ranieri, W. Comparison of beck depression inventories-IA and -II in psychiatric outpatients. J. Pers. Assess. 1996, 67, 588–597. [Google Scholar] [CrossRef]
- Netzer, N.C.; Stoohs, R.A.; Netzer, C.M.; Clark, K.; Strohl, K.P. Using the berlin questionnaire to identify patients at risk for the sleep apnea syndrome. Ann. Intern. Med. 1999, 131, 485–491. [Google Scholar] [CrossRef]
- Jaussent, I.; Ancelin, M.-L.; Berr, C.; Pérès, K.; Scali, J.; Besset, A.; Ritchie, K.; Dauvilliers, Y. Hypnotics and mortality in an elderly general population: A 12-year prospective study. BMC Med. 2013, 11, 212. [Google Scholar] [CrossRef] [Green Version]
- Enomoto, M.; Kitamura, S.; Tachimori, H.; Takeshima, M.; Mishima, K. Long-term use of hypnotics: Analysis of trends and risk factors. Gen. Hosp. Psychiatr. 2020, 62, 49–55. [Google Scholar] [CrossRef]
- Kang, M.; Galuska, M.A.; Ghassemzadeh, S. Benzodiazepine Toxicity; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Depner, C.M.; Cheng, P.C.; Devine, J.K.; Khosla, S.; de Zambotti, M.; Robillard, R.; Vakulin, A.; Drummond, S.P.A. Wearable technologies for developing sleep and circadian biomarkers: A summary of workshop discussions. Sleep 2019, 43, zsz254. [Google Scholar] [CrossRef] [Green Version]
- Yin, J.; Jin, X.; Shan, Z.; Li, S.; Huang, H.; Li, P.; Peng, X.; Peng, Z.; Yu, K.; Bao, W.; et al. Relationship of sleep duration with all-cause mortality and cardiovascular events: A systematic review and dose-response meta-analysis of prospective cohort studies. J. Am. Heart Assoc. Cardiovasc. Cerebrovasc. Dis. 2017, 6, e005947. [Google Scholar] [CrossRef] [Green Version]
- Kwok, C.S.; Kontopantelis, E.; Kuligowski, G.; Gray, M.; Muhyaldeen, A.; Gale, C.P.; Peat, G.M.; Cleator, J.; Chew-Graham, C.; Loke, Y.K.; et al. Self-reported sleep duration and quality and cardiovascular disease and mortality: A dose-response meta-analysis. J. Am. Heart Assoc. 2018, 7, e008552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wannamethee, S.G.; Papacosta, O.; Lennon, L.; Whincup, P.H. Self-reported sleep duration, napping, and incident heart failure: Prospective associations in the british regional heart study. J. Am. Geriatr. Soc. 2016, 64, 1845–1850. [Google Scholar] [CrossRef] [PubMed]
- Ohayon, M.; Wickwire, E.M.; Hirshkowitz, M.; Albert, S.M.; Avidan, A.; Daly, F.J.; Dauvilliers, Y.; Ferri, R.; Fung, C.; Gozal, D.; et al. National sleep foundation’s sleep quality recommendations: First report. Sleep Health 2017, 3, 6–19. [Google Scholar] [CrossRef] [Green Version]
- Reinhard, W.; Plappert, N.; Zeman, F.; Hengstenberg, C.; Riegger, G.; Novack, V.; Maimon, N.; Pfeifer, M.; Arzt, M. Prognostic impact of sleep duration and sleep efficiency on mortality in patients with chronic heart failure. Sleep Med. 2013, 14, 502–509. [Google Scholar] [CrossRef]
- Johansson, P.; Broström, A.; Sanderman, R.; Jaarsma, T. The course of sleep problems in patients with heart failure and associations to rehospitalizations. J. Cardiovasc. Nurs. 2015, 30, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Empana, J.-P.; Dauvilliers, Y.; Dartigues, J.-F.; Ritchie, K.; Gariepy, J.; Jouven, X.; Tzourio, C.; Amouyel, P.; Besset, A.; Ducimetiere, P. Excessive daytime sleepiness is an independent risk indicator for cardiovascular mortality in community-dwelling elderly: The three city study. Stroke 2009, 40, 1219–1224. [Google Scholar] [CrossRef] [Green Version]
- Blachier, M.; Dauvilliers, Y.; Jaussent, I.; Helmer, C.; Ritchie, K.; Jouven, X.; Tzourio, C.; Amouyel, P.; Besset, A.; Ducimetiere, P.; et al. Excessive daytime sleepiness and vascular events: The Three city study. Ann. Neurol. 2012, 71, 661–667. [Google Scholar] [CrossRef]
- Yoshihisa, A.; Suzuki, S.; Kanno, Y.; Takiguchi, M.; Sato, A.; Miura, S.; Masuda, A.; Yokokawa, T.; Shimizu, T.; Nakamura, Y.; et al. Prognostic significance of periodic leg movements during sleep in heart failure patients. Int. J. Cardiol. 2016, 212, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Y.; Winkelman, J.W.; Walters, A.S.; Han, J.; Hu, F.B.; Gao, X. Prospective study of restless legs syndrome and total and cardiovascular mortality among women. Neurology 2018, 90, e135–e141. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Ba, D.M.; Bagai, K.; Liu, G.; Ma, C.; Walters, A.S. Treating restless legs syndrome was associated with low risk of cardiovascular disease: A cohort study with 3.4 years of follow-up. J. Am. Heart Assoc. 2021, 10, e018674. [Google Scholar] [CrossRef] [PubMed]
- Reuter, H.; Herkenrath, S.; Treml, M.; Halbach, M.; Steven, D.; Frank, K.; Castrogiovanni, A.; Kietzmann, I.; Baldus, S.; Randerath, W.J. Sleep-disordered breathing in patients with cardiovascular diseases cannot be detected by ESS, STOP-BANG, and Berlin questionnaires. Clin. Res. Cardiol. Off. J. Geriatr. Cardiol. Soc. 2018, 107, 1071–1078. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Kapelios, C.J.; Laroche, C.; Crespo-Leiro, M.G.; Anker, S.D.; Coats, A.J.S.; Díaz-Molina, B.; Filippatos, G.; Lainscak, M.; Maggioni, A.P.; McDonagh, T.; et al. Association between loop diuretic dose changes and outcomes in chronic heart failure: Observations from the ESC-EORP Heart Failure Long-Term Registry. Eur. J. Heart Fail. 2020, 22, 1424–1437. [Google Scholar] [CrossRef] [Green Version]
| Whole Sample | Occurrence of CV Events | |||||||
|---|---|---|---|---|---|---|---|---|
| n = 119 | No n = 82 | Yes n = 37 | Model 0 | |||||
| Variable | n | % | n | % | n | % | HR (95% CI) | p |
| Sex, Female % | 35 | 29.41 | 25 | 30.49 | 10 | 27.03 | 0.79 (0.38; 1.64) | 0.531 |
| BMI, kg/m2 a | 117; 26.56 (18.21; 50.24) | 81; 26.17 (18.21; 50.24) | 36; 27.43 (18.36; 49.44) | 1.03 (0.97; 1.09) | 0.352 | |||
| LVEF, % a HR for 10% increase | 116; 40.00 (15.00; 70.00) | 79; 43.00 (18.00; 70.00) | 37; 30.00 (15.00; 60.00) | 0.74 (0.57; 0.96) | 0.021 * | |||
| Hypertension, Yes | 59 | 50.00 | 39 | 48.15 | 20 | 54.05 | 1.08 (0.57; 2.07) | 0.807 |
| Diabetes mellitus, Yes | 43 | 36.75 | 26 | 32.10 | 17 | 47.22 | 1.60 (0.83; 3.09) | 0.159 |
| Hypercholesterolemia, Yes | 72 | 60.50 | 43 | 52.44 | 29 | 78.38 | 2.79 (1.28; 6.11) | 0.010 * |
| Current smoker, Yes | 43 | 36.44 | 33 | 40.74 | 10 | 27.03 | 0.57 (0.28; 1.19) | 0.133 |
| BDI-score total a | 112; 10.50 (0.00; 39.00) | 77; 9.00 (0.00; 38.00) | 35; 13.00 (3.00; 39.00) | 1.03 (0.99; 1.06) | 0.139 | |||
| CFS—Total score a | 115; 8.00 (0.00; 14.00) | 80; 7.00 (0.00; 14.00) | 35; 9.00 (2.00; 14.00) | 1.09 (0.99; 1.20) | 0.078 | |||
| Whole Sample | Occurrence of CV Events | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No n = 82 | Yes n = 37 | Model 0 | Model 1 | Model 2 | ||||||||
| Variable | n | % | n | % | n | % | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p |
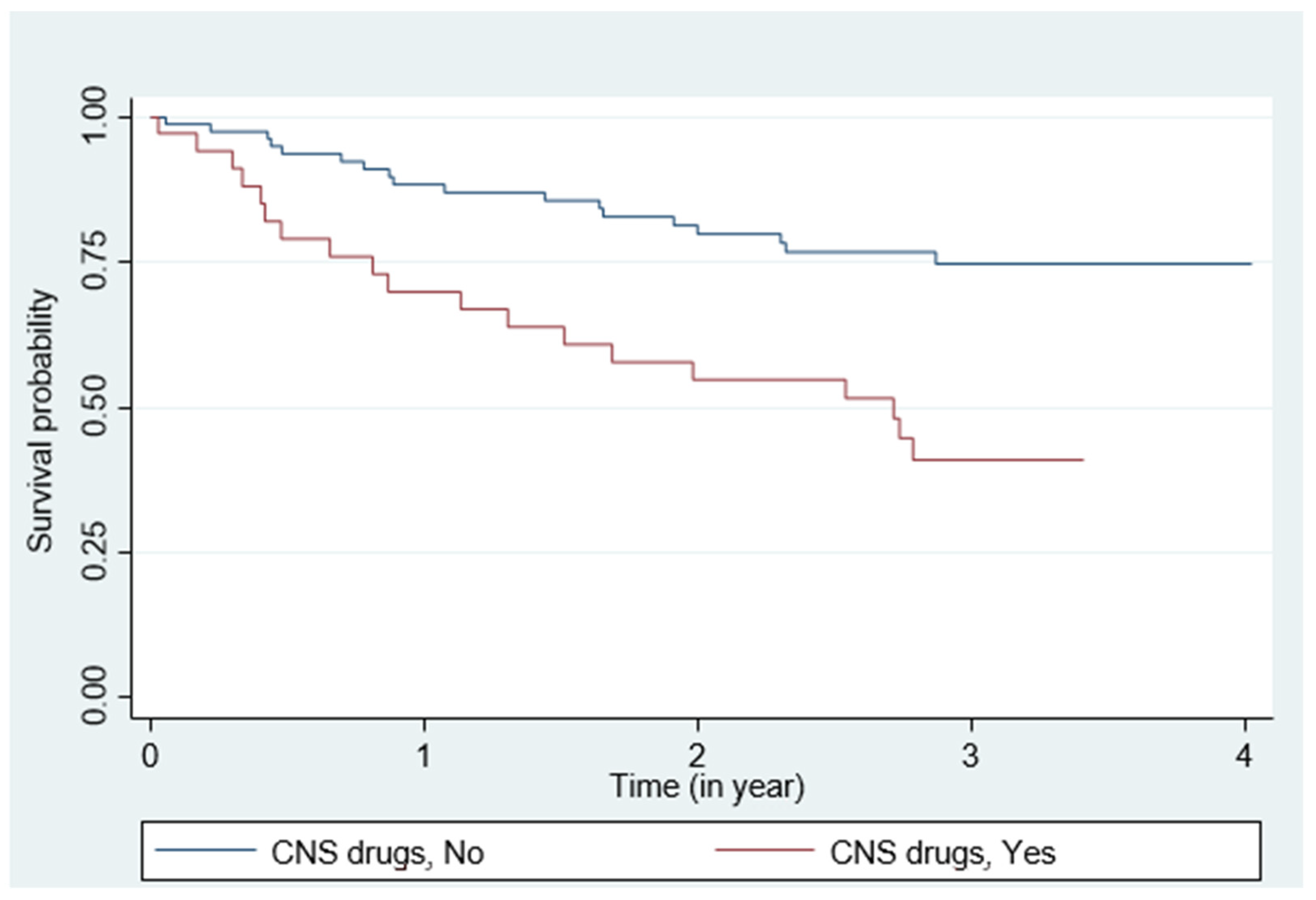
| CNS drugs, Yes | 36 | 30.25 | 17 | 20.73 | 19 | 51.35 | 3.03 (1.59; 5.79) | <0.001 * | 2.37 (1.22;4.59) | 0.010 * | ||
| ESS score a | 115; 7.00 (0.00; 22.00) | 80; 6.00 (0.00; 22.00) | 35; 9.00 (0.00; 22.00) | 1.03 (0.97;1.09) | 0.303 | 1.04 (0.98; 1.11) | 0.187 | 1.04 (0.98; 1.11) | 0.234 | |||
| ESS score, >10 | 33 | 28.70 | 21 | 26.25 | 12 | 34.29 | 1.31 (0.65; 2.64) | 0.448 | 1.62 (0.80; 3.27) | 0.178 | 1.45 (0.71; 2.93) | 0.307 |
| ISI score a | 115; 8.00 (0.00; 28.00) | 79; 7.00 (0.00; 21.00) | 36; 11.00 (1.00; 28.00) | 1.07 (1.01; 1.12) | 0.012 * | 1.05 (1.00; 1.10) | 0.048 * | 1.03 (0.98; 1.09) | 0.197 | |||
| ISI score, >14 | 24 | 20.87 | 14 | 17.72 | 10 | 27.78 | 1.52 (0.73; 3.15) | 0.261 | 1.22 (0.58; 2.56) | 0.593 | 0.96 (0.45; 2.07) | 0.921 |
| BQ score, ≥2 | 40 | 35.09 | 25 | 32.05 | 15 | 41.67 | 1.29 (0.67; 2.51) | 0.449 | 1.41 (0.72; 2.74) | 0.313 | 1.05 (0.51; 2.13) | 0.903 |
| RLS, ≥4 | 6 | 5.56 | 4 | 5.56 | 2 | 5.56 | 1.20 (0.29; 5.03) | 0.798 | 1.22 (0.29; 5.09) | 0.785 | 1.43 (0.34; 6.03) | 0.630 |
| PSQI score a | 104; 6.00 (0.00; 16.00) | 71; 6.00 (0.00; 14.00) | 33; 7.00 (1.00; 16.00) | 1.10 (1.01; 1.20) | 0.028 * | 1.09 (1.00; 1.19) | 0.059 | 1.05 (0.95; 1.15) | 0.362 | |||
| PSQI score, ≥5 | 66 | 63.46 | 42 | 59.15 | 24 | 72.73 | 1.29 (0.67; 2.51) | 0.268 | 1.31 (0.60; 2.84) | 0.496 | 0.94 (0.40; 2.18) | 0.882 |
| Sleep latency, min a HR For 15 min Increase | 117; 15.00 (0.00; 150.00) | 80; 15.00 (0.00; 150.00) | 37; 30.00 (0.00; 90.00) | 1.11 (0.97; 1.28) | 0.127 | 1.09 (0.94; 1.25) | 0.255 | 1.10 (0.95; 1.28) | 0.201 | |||
| Sleep latency, min | ||||||||||||
| <30 | 70 | 59.83 | 54 | 67.50 | 16 | 43.24 | 1 | 0.033 * | 1 | 0.091 | 1 | 0.071 |
| (30–60) | 25 | 21.37 | 16 | 20.00 | 9 | 24.32 | 1.54 (0.68; 3.48) | 1.47 (0.65; 3.36) | 1.13 (0.48; 2.65) | |||
| >60 | 22 | 18.80 | 10 | 12.50 | 12 | 32.43 | 2.72 (1.28; 5.74) | 2.34 (1.09; 5.01) | 2.38 (1.10; 5.14) | |||
| TST, hour a HR For 1 h decrease | 119; 7:30 (3:00; 11:45) | 82; 7:52 (3:00; 12:00) | 37; 7:00 (3:15; 10:15) | 0.79 (0.65; 0.98) | 0.030 * | 0.79 (0.64; 0.97) | 0.037 * | 0.81 (0.65; 1.01) | 0.092 | |||
| TST, ≤7 h | 57 | 47.90 | 34 | 41.46 | 23 | 62.16 | 1.84 (0.95; 3.58) | 0.072 | 1.72 (0.88; 3.36) | 0.111 | 1.41 (0.70; 2.84) | 0.333 |
| Efficiency, % a HR for 10% increase | 118; 87.50 (35.29; 100.00) | 81; 88.57 (35.29; 100.00) | 37; 82.35 (38.24; 100.00) | 0.79 (0.65; 0.97) | 0.022 * | 0.85 (0.69; 1.05) | 0.135 | 0.99 (0.97; 1.01) | 0.353 | |||
| Efficiency ≥85% | 63 | 53.39 | 45 | 55.56 | 18 | 48.65 | 0.82 (0.43; 1.57) | 0.551 | 1.02 (0.53; 1.97) | 0.947 | 1.31 (0.66; 2.62) | 0.440 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bughin, F.; Jaussent, I.; Ayoub, B.; Aguilhon, S.; Chapet, N.; Soltani, S.; Mercier, J.; Dauvilliers, Y.; Roubille, F. Prognostic Impact of Sleep Patterns and Related-Drugs in Patients with Heart Failure. J. Clin. Med. 2021, 10, 5387. https://doi.org/10.3390/jcm10225387
Bughin F, Jaussent I, Ayoub B, Aguilhon S, Chapet N, Soltani S, Mercier J, Dauvilliers Y, Roubille F. Prognostic Impact of Sleep Patterns and Related-Drugs in Patients with Heart Failure. Journal of Clinical Medicine. 2021; 10(22):5387. https://doi.org/10.3390/jcm10225387
Chicago/Turabian StyleBughin, François, Isabelle Jaussent, Bronia Ayoub, Sylvain Aguilhon, Nicolas Chapet, Sonia Soltani, Jacques Mercier, Yves Dauvilliers, and François Roubille. 2021. "Prognostic Impact of Sleep Patterns and Related-Drugs in Patients with Heart Failure" Journal of Clinical Medicine 10, no. 22: 5387. https://doi.org/10.3390/jcm10225387
APA StyleBughin, F., Jaussent, I., Ayoub, B., Aguilhon, S., Chapet, N., Soltani, S., Mercier, J., Dauvilliers, Y., & Roubille, F. (2021). Prognostic Impact of Sleep Patterns and Related-Drugs in Patients with Heart Failure. Journal of Clinical Medicine, 10(22), 5387. https://doi.org/10.3390/jcm10225387







