Subclinical Signs of Retinal Involvement in Hereditary Angioedema
Abstract
:1. Introduction
2. Materials and Methods
2.1. Immunologic Assessment
2.2. Ophthalmological Assessment
2.3. Statistical Analysis
3. Results
3.1. Ophthalmological Examinations in HAE Patients
3.2. Concomitant Therapies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Zuraw, B.L. Clinical practice. Hereditary angioedema. N. Engl. J. Med. 2008, 359, 1027–1036. [Google Scholar] [CrossRef]
- Zuraw, B.L.; Christiansen, S.C. HAE Pathophysiology and Underlying Mechanisms. Clin. Rev. Allergy Immunol. 2016, 51, 216–229. [Google Scholar] [CrossRef]
- Guarino, M.D.; Perricone, C.; Guarino, S.; Gambardella, S.; D’Apice, M.R.; Fontana, L.; Novelli, G.; Perricone, R. Denaturing HPLC in laboratory diagnosis of hereditary angioedema. J. Allergy Clin. Immunol. 2007, 120, 962. [Google Scholar] [CrossRef]
- Haslund, D.; Ryø, L.B.; Majidi, S.S.; Rose, I.K.; Skipper, K.A.; Fryland, T.; Bohn, A.B.; Koch, C.; Thomsen, M.K.; Palarasah, Y.; et al. Dominant negative SERPING1 variants cause intracellular retention of C1-inhibitor in hereditary angioedema. J. Clin. Invest. 2018, 129, 388–405. [Google Scholar] [CrossRef]
- Longhurst, H.; Cicardi, M. Hereditary angio-oedema. Lancet 2012, 379, 474–481. [Google Scholar] [CrossRef]
- Triggianese, P.; Guarino, M.D.; Pellicano, C.; Borzi, M.; Greco, E.; Modica, S.; De Carolis, C.; Perricone, R. Recurrent Angioedema: Occurrence, Features, and Concomitant Diseases in an Italian Single-Center Study. Int. Arch. Allergy Immunol. 2017, 172, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Hofman, Z.L.M.; de Maat, S.; Suffritti, C.; Zanichelli, A.; van Doorn, C.; Sebastian, S.A.E.; Veszeli, N.; Csuka, D.; Renné, T.; Pasterkamp, G.; et al. Cleaved kininogen as a biomarker for bradykinin release in hereditary angioedema. J. Allergy Clin. Immunol. 2017, 140, 1700–1703. [Google Scholar] [CrossRef]
- Banerji, A.; Busse, P.; Shennak, M.; Lumry, W.; Davis-Lorton, M.; Wedner, H.J.; Jacobs, J.; Baker, J.; Bernstein, J.A.; Lockey, R.; et al. Inhibiting Plasma Kallikrein for Hereditary Angioedema Prophylaxis. N. Engl. J. Med. 2017, 376, 717–728. [Google Scholar] [CrossRef]
- Loffredo, S.; Ferrara, A.L.; Bova, M.; Borriello, F.; Suffritti, C.; Veszeli, N.; Petraroli, A.; Galdiero, M.R.; Varricchi, G.; Granata, F.; et al. Secreted Phospholipases A2 in Hereditary Angioedema with C1-Inhibitor Deficiency. Front. Immunol. 2018, 9, 1721. [Google Scholar] [CrossRef]
- Zuraw, B.L. Hereditary angioedema with normal C1 inhibitor: Four types and counting. J. Allergy Clin. Immunol. 2018, 141, 884–885. [Google Scholar] [CrossRef] [PubMed]
- Bork, K.; Barnstedt, S.E.; Koch, P.; Traupe, H. Hereditary angioedema with normal C1-inhibitor activity in women. Lancet 2000, 356, 213–217. [Google Scholar] [CrossRef]
- Bouillet, L.; Mannic, T.; Arboleas, M.; Subileau, M.; Massot, C.; Drouet, C.; Huber, P.; Vilgrain, I. Hereditary angioedema: Key role for kallikrein and bradykinin in vascular endothelial-cadherin cleavage and edema formation. J. Allergy Clin. Immunol. 2011, 128, 232–234. [Google Scholar] [CrossRef]
- De Maat, S.; Björkqvist, J.; Suffritti, C.; Wiesenekker, C.P.; Nagtegaal, W.; Koekman, A.; van Dooremalen, S.; Pasterkamp, G.; de Groot, P.G.; Cicardi, M.; et al. Plasmin is a natural trigger for bradykinin production in patients with hereditary angioedema with factor XII mutations. J. Allergy Clin. Immunol. 2016, 138, 1414–1423. [Google Scholar] [CrossRef] [PubMed]
- Charest-Morin, X.; Hébert, J.; Rivard, G.É.; Bonnefoy, A.; Wagner, E.; Marceau, F. Comparing Pathways of Bradykinin Formation in Whole Blood from Healthy Volunteers and Patients with Hereditary Angioedema Due to C1 Inhibitor Deficiency. Front. Immunol. 2018, 9, 2183. [Google Scholar] [CrossRef]
- Aygören-Pürsün, E.; Bygum, A.; Grivcheva-Panovska, V.; Magerl, M.; Graff, J.; Steiner, U.C.; Fain, O.; Huissoon, A.; Kinaciyan, T.; Farkas, H.; et al. Oral Plasma Kallikrein Inhibitor for Prophylaxis in Hereditary Angioedema. N. Engl. J. Med. 2018, 379, 352–362. [Google Scholar] [CrossRef]
- Loffredo, S.; Bova, M.; Suffritti, C.; Borriello, F.; Zanichelli, A.; Petraroli, A.; Varricchi, G.; Triggiani, M.; Cicardi, M.; Marone, G.; et al. Elevated plasma levels of vascular permeability factors in C1 inhibitor-deficient hereditary angioedema. Allergy 2016, 71, 989–996. [Google Scholar] [CrossRef]
- Castellano, G.; Divella, C.; Sallustio, F.; Montinaro, V.; Curci, C.; Zanichelli, A.; Bonanni, E.; Suffritti, C.; Caccia, S.; Bossi, F.; et al. A transcriptomics study of hereditary angioedema attacks. J. Allergy Clin. Immunol. 2018, 142, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Colombo, R.; Wu, M.A.; Catena, E.; Perotti, A.; Fossali, T.; Cioffi, F.; Rech, R.; Castelli, A.; Cicardi, M. The Role of Failing Autonomic Nervous System on Life-Threatening Idiopathic Systemic Capillary Leak Syndrome. Front. Med. 2018, 5, 111. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Ghosh, C.C.; Patel, R.; Iwaki, S.; Gaskins, D.; Nelson, C.; Jones, N.; Greipp, P.R.; Parikh, S.M.; Druey, K.M. Vascular endothelial hyperpermeability induces the clinical symptoms of Clarkson disease (the systemic capillary leak syndrome). Blood 2012, 119, 4321–4332. [Google Scholar] [CrossRef]
- Abdulaal, M.; Haddad, N.M.; Sun, J.K.; Silva, P.S. The Role of Plasma Kallikrein-Kinin Pathway in the Development of Diabetic Retinopathy: Pathophysiology and Therapeutic Approaches. Semin. Ophthalmol. 2016, 31, 19–24. [Google Scholar] [CrossRef]
- Edalati, K.; Roesch, M.T.; Buchanan, M.L.; Teeter, M.; Maberley, D.A. Central serous chorioretinopathy and idiopathic nonhistaminergic angioedema. Can. J. Ophthalmol. 2009, 44, 606–607. [Google Scholar] [CrossRef]
- Catalioto, R.M.; Valenti, C.; Maggi, C.A.; Giuliani, S. Enhanced Ca(2+) response and stimulation of prostaglandin release by the bradykinin B2 receptor in human retinal pigment epithelial cells primed with proinflammatory cytokines. Biochem. Pharmacol. 2015, 97, 189–202. [Google Scholar] [CrossRef]
- Cai, W.; Wei, Q.; Liu, Q.; Ren, C.; Liu, J.; Zhang, R.; He, M.; Wang, Q.; Du, Y.; Yu, J. Effect of bradykinin on TGF-β1-induced retinal pigment epithelial cell proliferation and extracellular matrix secretion. BMC Ophthalmol. 2016, 16, 199. [Google Scholar] [CrossRef]
- Kawa, M.P.; Machalinska, A.; Roginska, D.; Machalinski, B. Complement system in pathogenesis of AMD: Dual player in degeneration and protection of retinal tissue. J. Immunol. Res. 2014, 2014, 483960. [Google Scholar] [CrossRef]
- Schick, T.; Steinhauer, M.; Aslanidis, A.; Altay, L.; Karlstetter, M.; Langmann, T.; Kirschfink, M.; Fauser, S. Local complement activation in aqueous humor in patients with age-related macular degeneration. Eye 2017, 31, 810–813. [Google Scholar] [CrossRef] [PubMed]
- Mukai, R.; Okunuki, Y.; Husain, D.; Kim, C.B.; Lambris, J.D.; Connor, K.M. The Complement System Is Critical in Maintaining Retinal Integrity during Aging. Front. Aging Neurosci. 2018, 10, 15. [Google Scholar] [CrossRef]
- Luo, C.; Zhao, J.; Chen, M.; Xu, H. The expression of C1 inhibitor (C1INH) in macrophages is upregulated by retinal pigment epithelial cells - implication in subretinal immune privilege in the aging eye. Aging 2018, 10, 1380–1389. [Google Scholar] [CrossRef] [PubMed]
- Dollin, M.; Srinivasan, S.; Leith, E.; Slomovic, A. Unilateral lacrimal gland atrophy in a patient with hereditary angioedema. Cornea 2007, 26, 505–506. [Google Scholar] [CrossRef]
- Caballero, T.; Baeza, M.L.; Cabañas, R.; Campos, A.; Cimbollek, S.; Gómez-Traseira, C.; González-Quevedo, T.; Guilarte, M.; Jurado-Palomo, J.; Larco, J.I.; et al. Consensus statement on the diagnosis, management, and treatment of angioedema mediated by bradykinin. Part II. Treatment, follow-up, and special situations. J. Investig. Allergol. Clin. Immunol. 2011, 21, 422–441. [Google Scholar] [PubMed]
- Triggianese, P.; Cesareo, M.; Guarino, M.D.; Conigliaro, P.; Chimenti, M.S.; Cedola, F.; Mazzeo, C.; Nucci, C.; Perricone, R. Evaluation of retinal microvascular perfusion in hereditary angioedema: A case-control study. Orphanet J. Rare Dis. 2020, 15, 20. [Google Scholar] [CrossRef]
- Samara, W.A.; Shahlaee, A.; Adam, M.K.; Khan, M.A.; Chiang, A.; Maguire, J.I.; Hsu, J.; Ho, A.C. Quantification of Diabetic Macular Ischemia Using Optical Coherence Tomography Angiography and Its Relationship with Visual Acuity. Ophthalmology 2017, 124, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Conigliaro, P.; Cesareo, M.; Chimenti, M.S.; Triggianese, P.; Canofari, C.; Aloe, G.; Nucci, C.; Perricone, R. Evaluation of retinal microvascular density in patients affected by systemic lupus erythematosus: An optical coherence tomography angiography study. Ann. Rheum. Dis. 2018, 78, 287–289. [Google Scholar] [CrossRef]
- Cesareo, M.; Ciuffoletti, E.; Martucci, A.; Sebastiani, J.; Sorge, R.P.; Lamantea, E.; Garavaglia, B.; Ricci, F.; Cusumano, A.; Nucci, C.; et al. Assessment of the retinal posterior pole in dominant optic atrophy by spectral-domain optical coherence tomography and microperimetry. PLoS ONE 2017, 12, e0174560. [Google Scholar]
- Conigliaro, P.; Triggianese, P.; Draghessi, G.; Canofari, C.; Aloe, G.; Chimenti, M.S.; Valeri, C.; Nucci, C.; Perricone, R.; Cesareo, M. Evidence for the Detection of Subclinical Retinal Involvement in Systemic Lupus Erythematosus and Sjögren Syndrome: A Potential Association with Therapies. Int. Arch. Allergy Immunol. 2018, 177, 45–56. [Google Scholar] [CrossRef]
- Triggianese, P.; Chimenti, M.S.; Toubi, E.; Ballanti, E.; Guarino, M.D.; Perricone, C.; Perricone, R. The autoimmune side of hereditary angioedema: Insights on the pathogenesis. Autoimmun. Rev. 2015, 14, 665–669. [Google Scholar] [CrossRef]
- Jonas, J.B.; Bergua, A.; Schmitz-Valckenberg, P.; Papastathopoulos, K.I.; Budde, W.M. Ranking of optic disc variables for detection of glaucomatous optic nerve damage. Invest. Ophthalmol. Vis. Sci. 2000, 41, 1764–1773. [Google Scholar] [PubMed]
- Birt, C.M.; Shin, D.H.; Samudrala, V.; Hughes, B.A.; Kim, C.; Lee, D. Analysis of reliability indices from Humphrey visual field tests in an urban glaucoma population. Ophthalmology 1997, 104, 1126–1130. [Google Scholar] [CrossRef]
- Cesareo, M.; Martucci, A.; Ciuffoletti, E.; Mancino, R.; Cerulli, A.; Sorge, R.P.; Martorana, A.; Sancesario, G.; Nucci, C. Association Between Alzheimer’s Disease and Glaucoma: A Study Based on Heidelberg Retinal Tomography and Frequency Doubling Technology Perimetry. Front. Neurosci. 2015, 9, 479. [Google Scholar] [CrossRef] [PubMed]
- Cesareo, M.; Giannini, C.; Di Marino, M.; Aloe, G.; Martucci, A.; Aiello, F.; Cusumano, A.; Mancino, R.; Ricci, F.; Sorge, R.P.; et al. Optical coherence tomography angiography in the multimodal assessment of the retinal posterior pole in autosomal dominant optic atrophy. Acta Ophthalmol. 2021, 10, 1111. [Google Scholar] [CrossRef]
- Di Marino, M.; Cesareo, M.; Aloe, G.; Nucci, C.; Giannini, C.; Martucci, A.; Aiello, F.; Pisano, C.; Ruvolo, G.; Mancino, R. Retinal and Choroidal Vasculature in Patients with Marfan Syndrome. Transl. Vis. Sci. Technol. 2020, 9, 5. [Google Scholar] [CrossRef]
- Druey, K.M.; Parikh, S.M. Idiopathic systemic capillary leak syndrome (Clarkson disease). J. Allergy Clin. Immunol. 2017, 140, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Bouillet, L.; Vilgrain, I. VE-cadherin, a potential marker for endothelial cell activation during hereditary angioedema attacks. J. Allergy Clin. Immunol. 2014, 134, 241. [Google Scholar] [CrossRef]
- Cunha-Vaz, J. Mechanisms of Retinal Fluid Accumulation and Blood-Retinal Barrier Breakdown. Dev. Ophthalmol. 2017, 58, 11–20. [Google Scholar]
- Martucci, A.; Toschi, N.; Cesareo, M.; Giannini, C.; Pocobelli, G.; Garaci, F.; Mancino, R.; Nucci, C. Spectral Domain Optical Coherence Tomography Assessment of Macular and Optic Nerve Alterations in Patients with Glaucoma and Correlation with Visual Field Index. J. Ophthalmol. 2018, 2018, 6581846. [Google Scholar] [CrossRef] [PubMed]
- Wheat, J.L.; Rangaswamy, N.V.; Harwerth, R.S. Correlating RNFL thickness by OCT with perimetric sensitivity in glaucoma patients. J. Glaucoma 2012, 21, 95–101. [Google Scholar] [CrossRef]
- Costello, F.; Burton, J.M. Retinal imaging with optical coherence tomography: A biomarker in multiple sclerosis? Eye Brain 2018, 10, 47–63. [Google Scholar] [CrossRef]
- Wang, Q.; Chan, S.; Yang, J.Y.; You, B.; Wang, Y.X.; Jonas, J.B.; Wei, W.B. Vascular Density in Retina and Choriocapillaris as Measured by Optical Coherence Tomography Angiography. Am. J. Ophthalmol. 2016, 168, 95–109. [Google Scholar] [CrossRef]
- Hamed, L.M.; Glaser, J.S.; Schatz, N.J.; Perez, T.H. Pseudotumor cerebri induced by danazol. Am. J. Ophthalmol. 1989, 107, 105–110. [Google Scholar] [CrossRef]
- Hornby, C.; Mollan, S.P.; Mitchell, J.; Markey, K.A.; Yangou, A.; Wright, B.L.C.; O’Reilly, M.W.; Sinclair, A.J. What Do Transgender Patients Teach Us About Idiopathic Intracranial Hypertension? Neuroophthalmology 2017, 41, 326–329. [Google Scholar] [CrossRef]
- Carnevali, A.; Sacconi, R.; Corbelli, E.; Tomasso, L.; Querques, L.; Zerbini, G.; Scorcia, V.; Bandello, F.; Querques, G. Optical coherence tomography angiography analysis of retinal vascular plexuses and choriocapillaris in patients with type 1 diabetes without diabetic retinopathy. Acta Diabetol. 2017, 54, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Kempen, J.H.; Sugar, E.A.; Jaffe, G.J.; Acharya, N.R.; Dunn, J.P.; Elner, S.G.; Lightman, S.L.; Thorne, J.E.; Vitale, A.T.; Altaweel, M.M.; et al. Fluorescein angiography versus optical coherence tomography for diagnosis of uveitic macular edema. Ophthalmology 2013, 120, 1852–1859. [Google Scholar] [CrossRef] [PubMed]
- Najeeb, B.H.; Simader, C.; Deak, G.; Vass, C.; Gamper, J.; Montuoro, A.; Gerendas, B.S.; Schmidt-Erfurth, U. The Distribution of Leakage on Fluorescein Angiography in Diabetic Macular Edema: A New Approach to Its Etiology. Invest. Ophthalmol. Vis. Sci. 2017, 58, 3986–3990. [Google Scholar] [CrossRef] [PubMed]
| HAE (n = 40) | HC (n = 40) | |
|---|---|---|
| Age (years) | 41.4 ± 14 | 42 ± 10.8 |
| Female sex (n/%) | 21/52 | 17/42 |
| Age at the onset (years) | 10.4 ± 8.6 | N/A |
| Age at the diagnosis (years) | 19.7 ± 16.4 | N/A |
| Disease duration (years) | 30.7 ± 14.3 | N/A |
| Number of attacks § | 18.6 ± 24.4 | N/A |
| Attack-free period (days) §§ | 50.1 ± 61.7 | N/A |
| Danazol long-term prophylaxis (n/%) | 7/17.5 | N/A |
| C4 (mg/dL) | 9.2 ± 3.5 | 20 ± 3 |
| C3 (mg/dL) | 118.5 ± 37.8 | 100 ± 42 |
| C1q (mg/L) | 144 ± 16.9 | 83 ± 20 |
| C1INH (mg/dL) | 6.5 ± 2.7 | 24 ± 4.5 |
| C1INH (%) | 31.7 ± 10.8 | N/A |
| Creatinine (mg/dL) | 0.8 ± 0.2 | N/A |
| Glucose (mg/dL) | 87.2 ± 4.2 | N/A |
| BCVA (logMAR) | 0.01 ± 0.1 (R) 0.01± 0.1 (L) | 0.013 ± 0.03 |
| IOP (mmHg) | 16.5 ± 3 (R) 16.7 ± 2.9 (L) | 16 ± 3 |
| MD (median, dB) | −2 ***(R) −2 ***(L) | 0.4 |
| PSD (median, dB) | 2.2 **(R) 2 *(L) | 1.7 |
| VFI (range %) | 94–98 (R, L) | 98–100 |
| Ophthalmological Scanning | HAE | HC | |||
|---|---|---|---|---|---|
| Right Eyes (n = 40) | Left Eyes (n = 40) | Control Eyes (n = 40) | |||
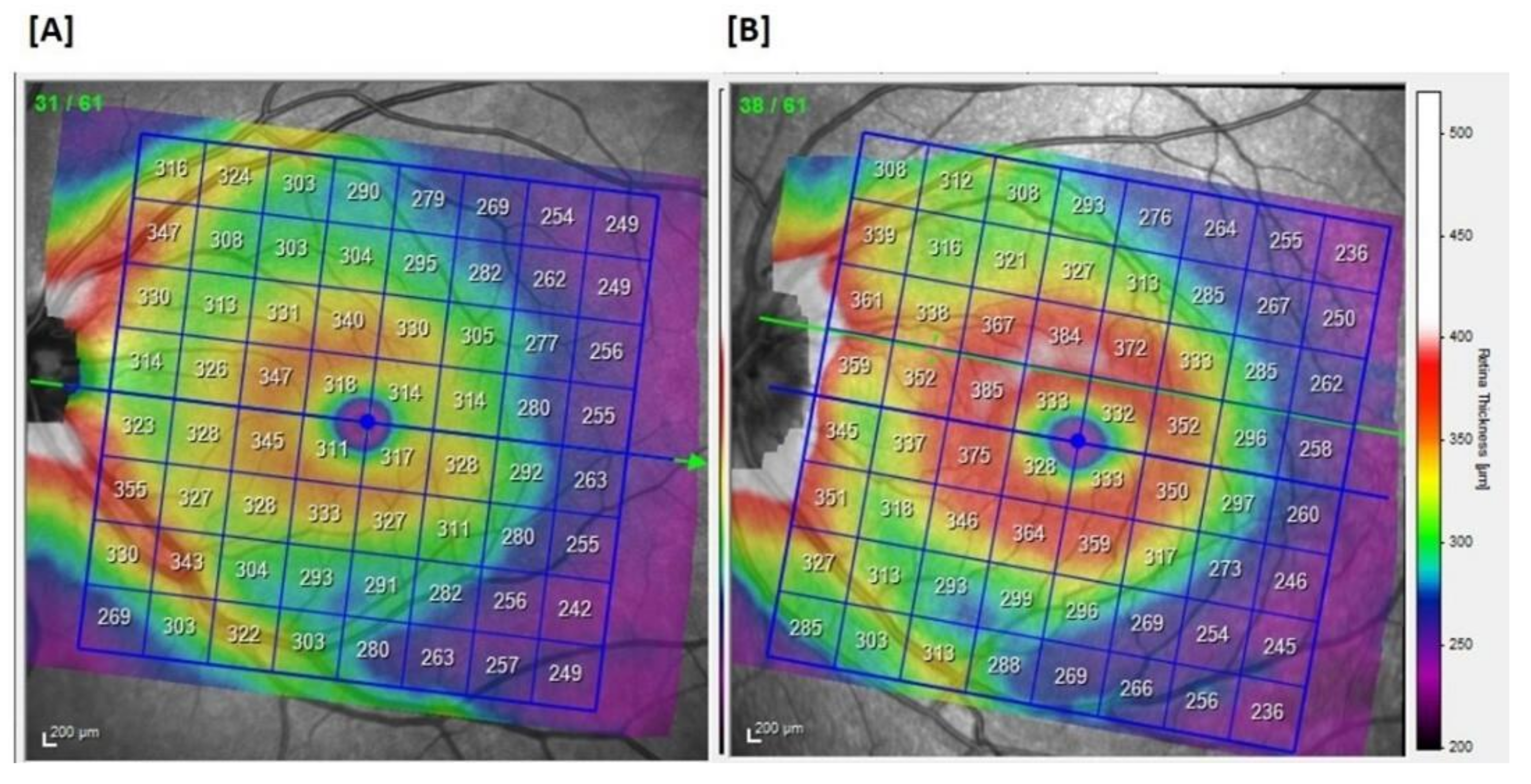
| SD-OCT | Posterior Pole (µm) | Superior Inferior Total | 291.5 ± 15.1 290.4 ± 14.4 290.9 ± 14.6 | 288.9 ± 15.5 288.9 ± 15.2 288.8 ± 15.3 | 292.9 ± 13.3 292.8 ± 12.8 292.9 ± 12.9 |
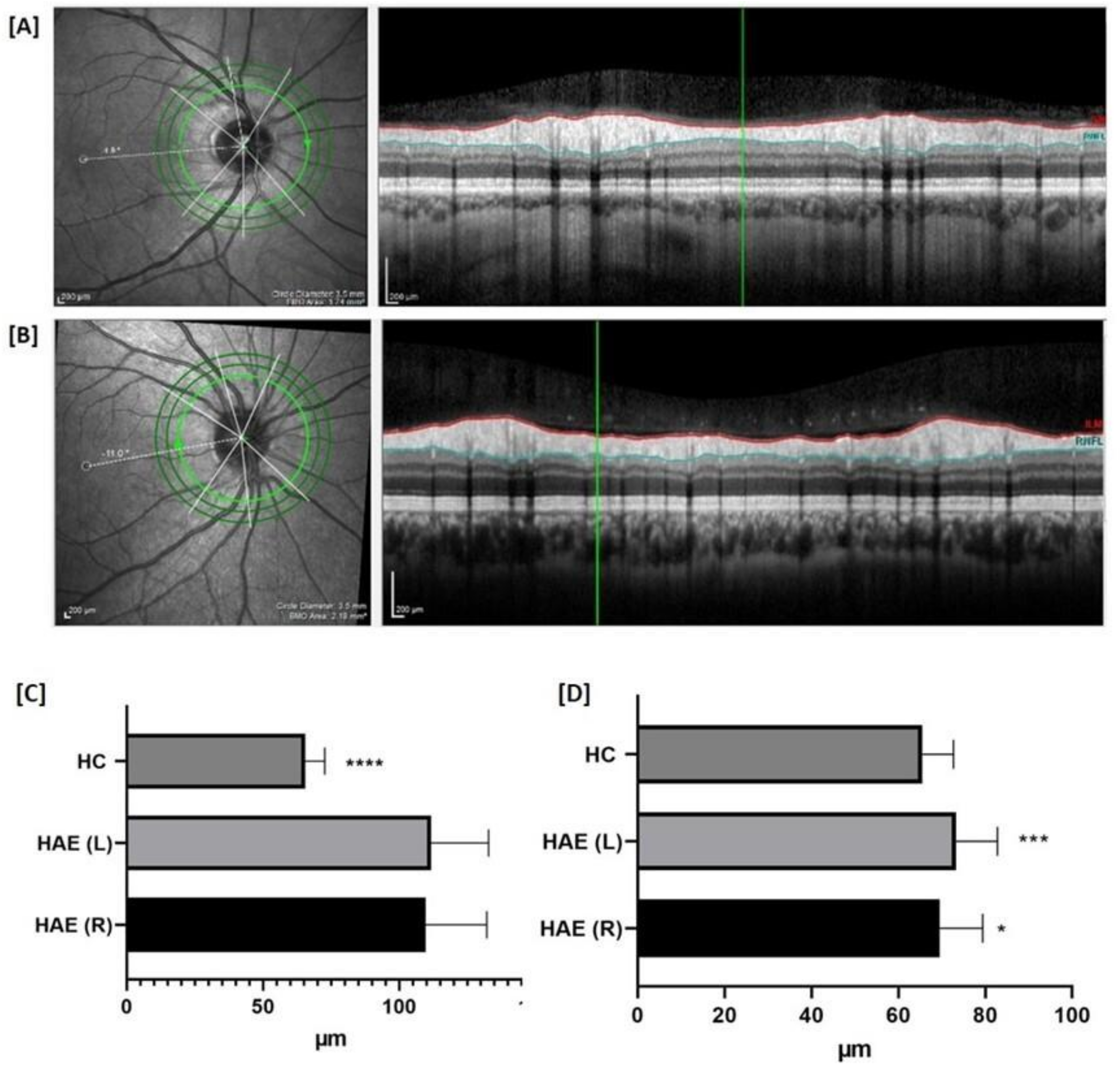
| ONH (µm) | Nasal superior Nasal Nasal Inferior Temporal superior Temporal Temporal inferior Global | 391 ± 102.7 402 ± 78.3 ** 420.2 ± 93.8 346 ± 78.6 267.2 ± 64.6 ** 391.4 ± 89.4 *** 362.5 ± 72.6 ** | 420 ± 118 411.2 ± 100 ** 426 ± 109 364 ± 110 * 282.7 ± 77.3 *** 381.2 ± 102.7 ** 373.6 ± 93 ** | 361.4 ± 96.7 344 ± 75.8 376.6 ± 73 305.8 ± 71.7 220.7 ± 43.7 318.2 ± 59.7 311.8 ± 59.5 | |
| RNFL (µm) | Nasal superior Nasal Nasal Inferior Temporal superior Temporal Temporal inferior Global | 109.7 ± 22.5 * 82.3 ± 12.5 101.3 ± 22.8 128.8 ± 17.8 73.3 ± 9.5 *** 146.7 ± 17.8 97.1 ± 8.6 | 111.7 ± 21.1 * 81.7 ± 14.5 101.8 ± 27.5 124.4 ± 22.4 69.5 ± 9.9 *** 138.3 ± 24.3 95.4 ± 11.8 | 102.7 ± 15.5 81.1 ± 10.3 113.1 ± 21.5 120.7 ± 20.4 65.5 ± 7.2 145.8 ± 16.1 94.6 ± 8.1 | |
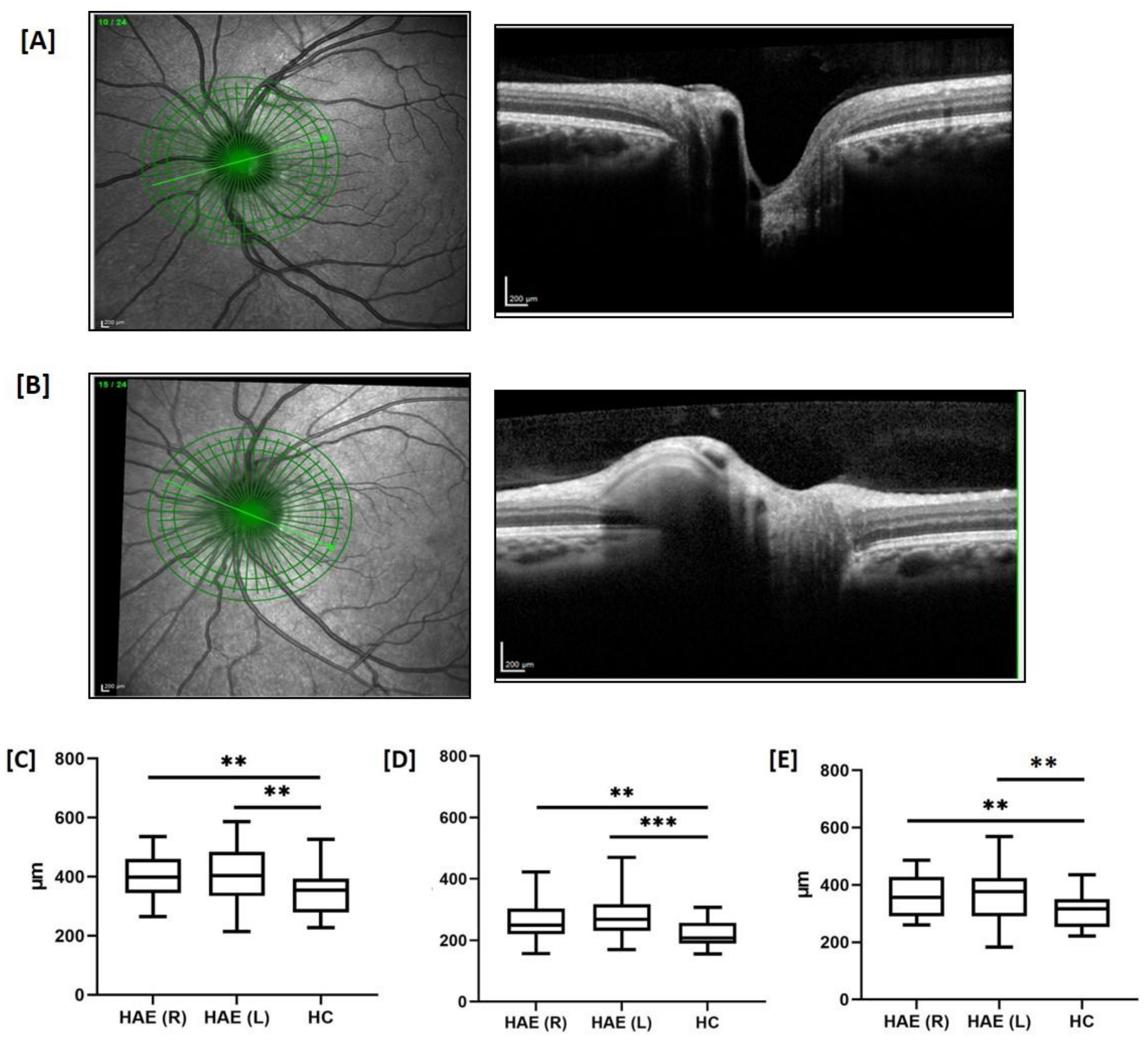
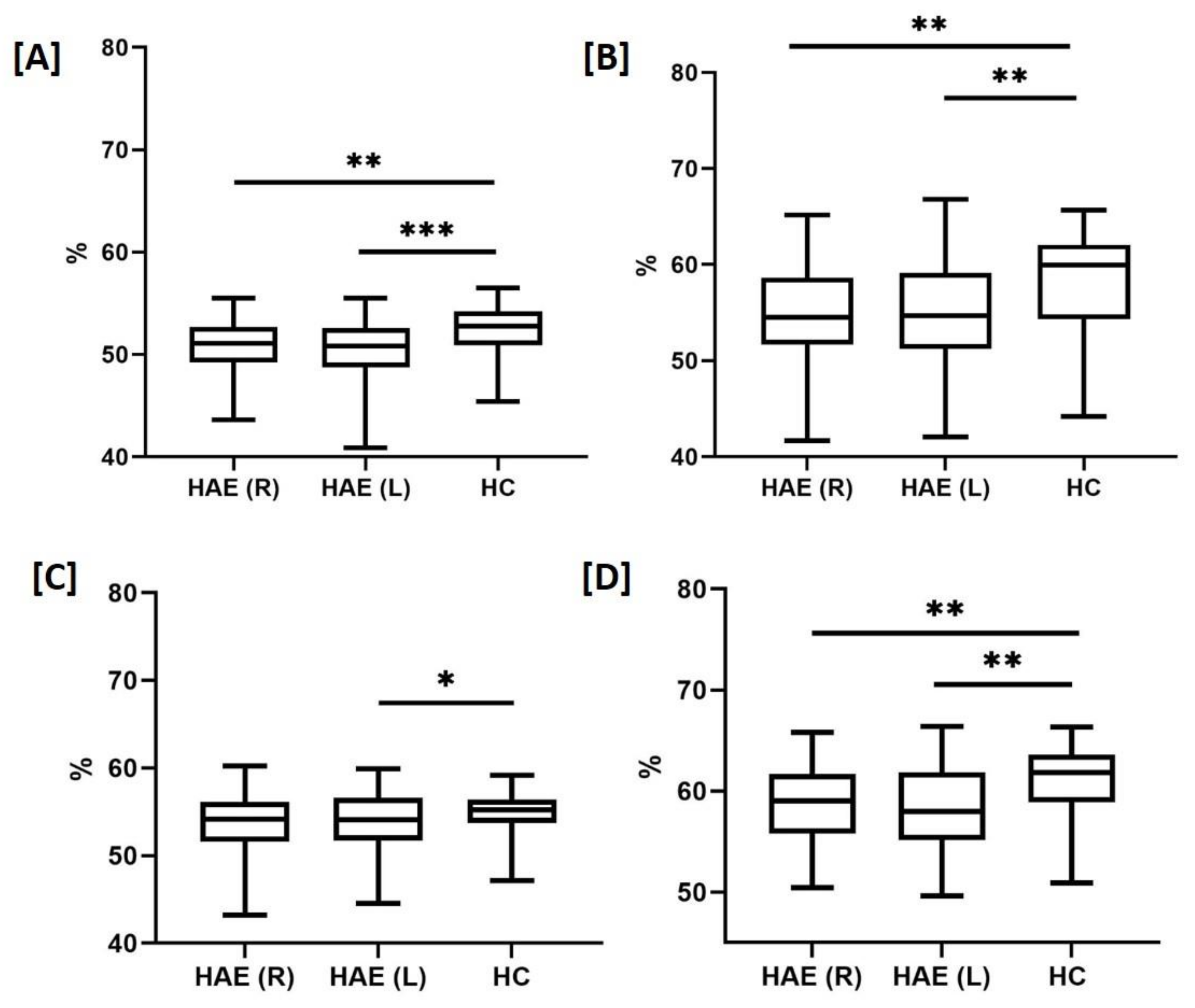
| OCT-A | Capillary density (%) | Deep scans Fovea Parafovea Whole image Superficial scans Fovea Parafovea Whole image | 41.7 ± 6.7 58.7 ± 3.6 ** 54.9 ± 4.9 ** 22.5 ± 6.1 *** 54.9 ± 3.4 50.6 ± 2.9 ** | 41.3 ± 6.6 58.4 ± 4.3 ** 54.9 ± 5.8 ** 22.7 ± 6.6 *** 53.1 ± 4.2 * 49.7 ± 3.4 ** | 41.8 ± 9.2 60.8 ± 3.4 58.1 ± 4.8 31.1 ± 8.7 55.2 ± 2.3 52.2 ± 2.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Triggianese, P.; Di Marino, M.; Nesi, C.; Greco, E.; Modica, S.; Chimenti, M.S.; Conigliaro, P.; Mancino, R.; Nucci, C.; Cesareo, M. Subclinical Signs of Retinal Involvement in Hereditary Angioedema. J. Clin. Med. 2021, 10, 5415. https://doi.org/10.3390/jcm10225415
Triggianese P, Di Marino M, Nesi C, Greco E, Modica S, Chimenti MS, Conigliaro P, Mancino R, Nucci C, Cesareo M. Subclinical Signs of Retinal Involvement in Hereditary Angioedema. Journal of Clinical Medicine. 2021; 10(22):5415. https://doi.org/10.3390/jcm10225415
Chicago/Turabian StyleTriggianese, Paola, Matteo Di Marino, Carolina Nesi, Elisabetta Greco, Stella Modica, Maria Sole Chimenti, Paola Conigliaro, Raffaele Mancino, Carlo Nucci, and Massimo Cesareo. 2021. "Subclinical Signs of Retinal Involvement in Hereditary Angioedema" Journal of Clinical Medicine 10, no. 22: 5415. https://doi.org/10.3390/jcm10225415
APA StyleTriggianese, P., Di Marino, M., Nesi, C., Greco, E., Modica, S., Chimenti, M. S., Conigliaro, P., Mancino, R., Nucci, C., & Cesareo, M. (2021). Subclinical Signs of Retinal Involvement in Hereditary Angioedema. Journal of Clinical Medicine, 10(22), 5415. https://doi.org/10.3390/jcm10225415






