Efficacy and Safety of Oral Ibandronate versus Intravenous Zoledronic Acid on Bone Metabolism and Bone Mineral Density in Postmenopausal Japanese Women with Osteoporosis
Abstract
:1. Introduction
2. Methods
2.1. Inclusion Criteria
2.2. Exclusion Criteria
2.3. Patients
2.4. Data Sources
2.5. Clinic Sites
2.6. Randomization Methods
2.7. Adverse Effects Assessment
2.8. Evaluation and Statistical Analysis
3. Results
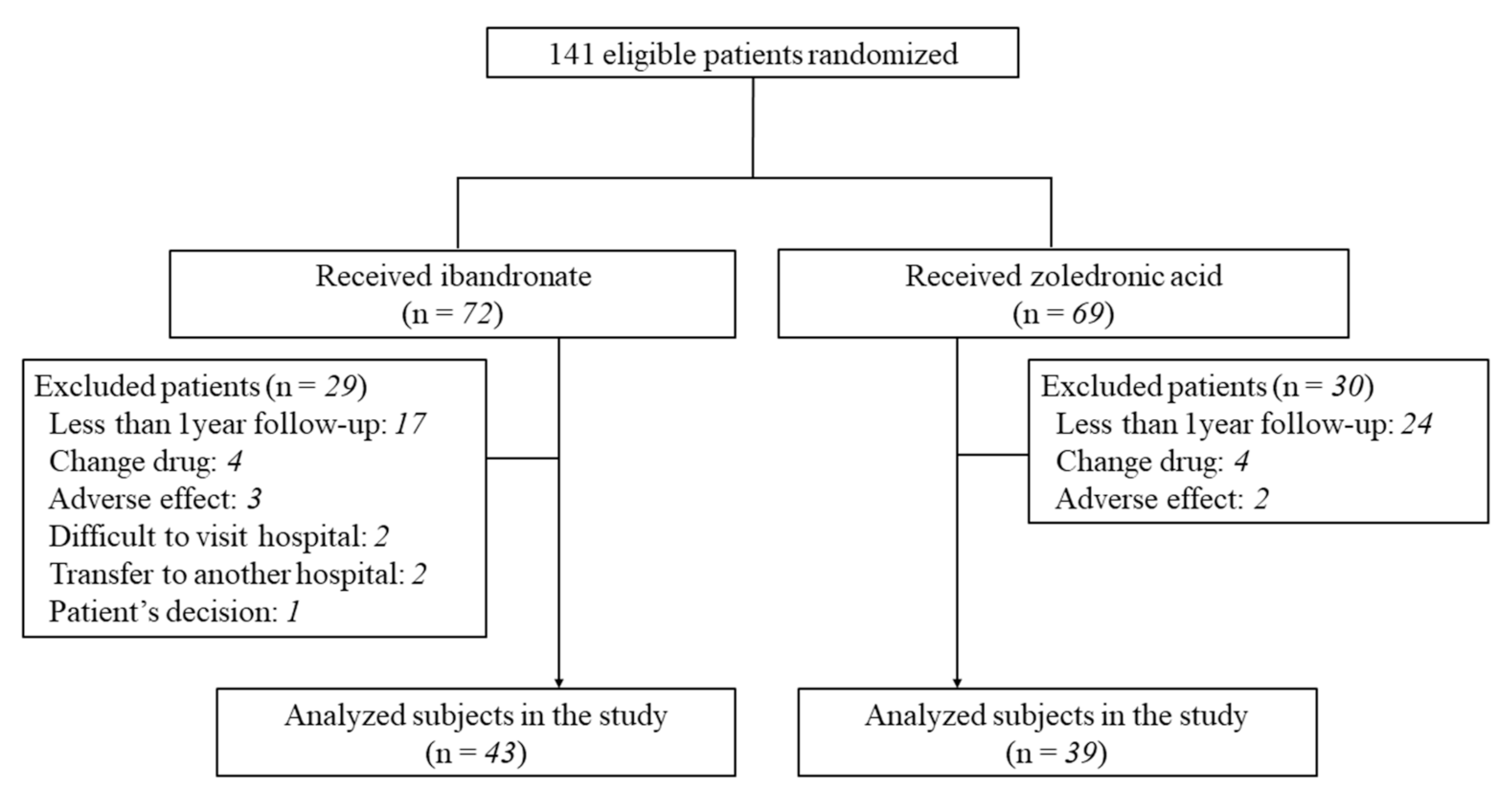
3.1. Patient Data
3.2. Levels of Serum Albumin-Corrected Calcium, Phosphorus, Whole PTH, 1,25(OH)2D3, and 25OHD
3.3. Bone Turnover Markers
3.3.1. Markers of Bone Formation
3.3.2. Markers of Bone Resorption
3.4. L-BMD and H-BMD
3.5. Safety Evaluations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lorentzon, M. Treating osteoporosis to prevent fractures: Current concepts and future developments. J. Intern. Med. 2019, 285, 381–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, P.D.; McClung, M.R.; Macovei, L.; Stakkestad, J.A.; Luckey, M.; Bonvoisin, B.; Reginster, J.Y.; Recker, R.R.; Hughes, C.; Lewiecki, E.M.; et al. Monthly oral ibandronate therapy in postmenopausal osteoporosis: 1-year results from the MOBILE study. J. Bone Miner. Res. 2005, 20, 1315–1322. [Google Scholar] [CrossRef] [Green Version]
- Inderjeeth, C.A.; Glendenning, P.; Ratnagobal, S.; Inderjeeth, D.C.; Ondhia, C. Long-term efficacy, safety, and patient acceptability of ibandronate in the treatment of postmenopausal osteoporosis. Int. J. Women’s Health 2014, 7, 7–17. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, T.; Nakano, T.; Ito, M.; Hagino, H.; Hashimoto, J.; Tobinai, M.; Mizunuma, H. For the MOVER Study Group: MOVER Study Group. Clinical efficacy on fracture risk and safety of 0.5 mg or 1 mg/month intravenous ibandronate versus 2.5 mg/day oral risedronate in patients with primary osteoporosis. Calcif. Tissue Int. 2013, 93, 137–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senn, C.; Günther, B.; Popp, A.W.; Perrelet, R.; Hans, D.; Lippuner, K. Comparative effects of teriparatide and ibandronate on spine bone mineral density (BMD) and microarchitecture (TBS) in postmenopausal women with osteoporosis: A 2-year open-label study. Osteoporos. Int. 2014, 25, 1945–1951. [Google Scholar] [CrossRef]
- Nakamura, T.; Ito, M.; Hashimoto, J.; Shinomiya, K.; Asao, Y.; Katsumata, K.; Hagino, H.; Inoue, T.; Nakano, T.; Mizunuma, H. MOVEST Study Group. Clinical efficacy and safety of monthly oral ibandronate 100 mg versus monthly intravenous ibandronate 1 mg in Japanese patients with primary osteoporosis. Osteoporos. Int. 2015, 26, 2685–2693. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, T.; Nakamura, Y.; Kato, H. Efficacy, safety, and compliance of ibandronate treatment for 3 years in postmenopausal Japanese women with primary osteoporosis. Osteoporos. Sarcopenia 2018, 4, 69–72. [Google Scholar] [CrossRef]
- Sooragonda, B.; Cherian, K.E.; Jebasingh, F.K.; Dasgupta, R.; Asha, H.S.; Kapoor, N.; Thomas, N.; Paul, T.V. Longitudinal changes in bone mineral density and trabecular bone score following yearly zoledronic acid infusion in postmenopausal osteoporosis-a retrospective-prospective study from southern India. Arch. Osteoporos. 2019, 14, 79. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Sone, T.; Shiraki, M.; Tanaka, S.; Irie, C.; Ota, Y.; Nakamura, T. The effect of once-yearly zoledronic acid on hip structural and biomechanical properties derived using computed tomography (CT) in Japanese women with osteoporosis. Bone 2018, 106, 179–186. [Google Scholar] [CrossRef] [Green Version]
- Soen, S.; Fukunaga, M.; Sugimoto, T.; Sone, T.; Fujiwara, S.; Endo, N.; Gorai, I.; Shiraki, M.; Hagino, H.; Hosoi, T.; et al. Japanese Society for Bone and Mineral Research and Japan Osteoporosis Society Joint Review Committee for the Revision of the Diagnostic Criteria for Primary Osteoporosis. Diagnostic criteria for primary osteoporosis: Year 2012 revision. J. Bone Miner. Metab. 2013, 31, 247–257. [Google Scholar] [CrossRef]
- Lee, D.R.; Lee, J. Comparison of the efficacy between once-monthly oral ibandronate and risedronate among Korean women with osteoporosis: A nationwide population-based study. Osteoporos. Int. 2019, 30, 659–666. [Google Scholar] [CrossRef]
- Kishimoto, H.; Maehara, M. Compliance and persistence with daily, weekly, and monthly bisphosphonates for osteoporosis in Japan: Analysis of data from the CISA. Arch. Osteoporos. 2015, 10, 231. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, Y.; Suzuki, T.; Kamimura, M.; Murakami, K.; Ikegami, S.; Uchiyama, S.; Kato, H. Vitamin D and calcium are required at the time of denosumab administration during osteoporosis treatment. Bone Res. 2017, 5, 17021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiraki, M.; Nakamura, T.; Fukunaga, M.; Sone, T.; Usami, A.; Inoue, T. A multicenter randomized double-masked comparative study of different preparations of alendronate in osteoporosis–monthly (four weeks) intravenous versus once weekly oral administrations. Curr. Med. Res. Opin. 2012, 28, 1357–1367. [Google Scholar] [CrossRef]
- Agarwala, S.; Vijayvargiya, M. Single Dose Therapy of Zoledronic Acid for the Treatment of Transient Osteoporosis of Hip. Ann. Rehabil. Med. 2019, 43, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Anastasilakis, A.D.; Papapoulos, S.E.; Polyzos, S.A.; Appelman-Dijkstra, N.M.; Makras, P. Zoledronate for the prevention of bone loss in women discontinuing denosumab treatment. A prospective 2-year clinical trial. J. Bone Miner. Res. 2019, 34, 2220–2228. [Google Scholar] [CrossRef]
- Okimoto, N.; Sakai, A.; Yoshioka, T.; Kobayashi, T.; Asano, K.; Akahoshi, S.; Ishikura, T.; Fukuhara, S.; Fuse, Y.; Mizuno, T.; et al. Efficacy of non-steroidal anti-inflammatory drugs on zoledronic acid-induced acute-phase reactions: Randomized, open-label, Japanese OZ study. J. Bone Miner. Metab. 2020, 38, 230–239. [Google Scholar] [CrossRef]
- Green, J.R.; Seltenmeyer, Y.; Jaeggi, K.A.; Widler, L. Renal tolerability profile of novel, potent bisphosphonates in two short-term rat models. Pharmacol. Toxicol. 1997, 80, 225–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reid, I.R.; Brown, J.P.; Burckhardt, P.; Horowitz, Z.; Richardson, P.; Trechsel, U.; Widmer, A.; Devogelaer, J.P.; Kaufman, J.M.; Jaeger, P.; et al. Intravenous zoledronic acid in postmenopausal women with low bone mineral density. N. Engl. J. Med. 2002, 346, 653–661. [Google Scholar] [CrossRef]
- Body, J.J.; Lichinitser, M.; Tjulandin, S.; Garnero, P.; Bergström, B. Oral ibandronate is as active as intravenous zoledronic acid for reducing bone turnover markers in women with breast cancer and bone metastases. Ann. Oncol. 2007, 18, 1165–1171. [Google Scholar] [CrossRef]
- Francini, F.; Pascucci, A.; Bargagli, G.; Francini, E.; Conca, R.; Miano, S.T.; Martellucci, I.; Migali, C.; Gotti, G.; Fiaschi, A.I.; et al. Effects of intravenous zoledronic acid and oral ibandronate on early changes in markers of bone turnover in patients with bone metastases from non-small cell lung cancer. Int. J. Clin. Oncol. 2011, 16, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Özsoy-Ünübol, T.; Akyüz, G.; Mirzayeva, S.; Güler, T. Evaluation of pain, quality of life, and patient satisfaction in parenterally treated patients with postmenopausal osteoporosis. Turk. J. Phys. Med. Rehabil. 2020, 66, 262–270. [Google Scholar] [CrossRef]
- Uchiyama, S.; Ikegami, S.; Kamimura, M.; Mukaiyama, K.; Nakamura, Y.; Nonaka, K.; Kato, H. The skeletal muscle cross sectional area in long-term bisphosphonate users is smaller than that of bone mineral density-matched controls with increased serum pentosidine concentrations. Bone 2015, 75, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Ikegami, S.; Uchiyama, S.; Nakamura, Y.; Mukaiyama, K.; Hirabayashi, H.; Kamimura, M.; Nonaka, K.; Kato, H. Factors that characterize bone health with aging in healthy postmenopausal women. J. Bone Miner. Metab. 2015, 33, 440–447. [Google Scholar] [CrossRef] [PubMed]



| Characteristic | IBN Group (n = 43) | ZOL Group (n = 39) | p-Value |
|---|---|---|---|
| Age (years) | 70.9 ± 11.5 | 67.8 ± 9.4 | 0.22 |
| BMI (kg/m2) | 21.9 ± 3.6 | 22.1 ± 3.7 | 0.77 |
| Previous history of lower limb fracture (n) | 2 | 7 | 0.08 |
| Previous history of lower limb fracture (SMD) | 0.000055 ± 1.0 | 0.000018 ± 1.0 | 1.00 |
| Previous history of upper limb fracture (n) | 3 | 1 | 0.62 |
| Previous history of vertebral fracture (n) | 12 | 7 | 0.31 |
| Previous history of pelvic fracture (n) | 2 | 1 | 1.00 |
| Serum albumin-corrected calcium (mg/dL) | 9.2 ± 0.9 | 9.3 ± 0.5 | 0.21 |
| Serum phosphorus (mg/dL) | 3.8 ± 0.5 | 3.7 ± 0.5 | 0.18 |
| Serum BAP (μg/L) | 14.8 ± 8.4 | 17.3 ± 10.5 | 0.13 |
| Serum PINP (ng/mL) | 61.1 ± 42.7 | 67.7 ± 45.5 | 0.54 |
| Serum TRACP-5b (mU/dL) | 465 ± 212 | 552 ± 273 | 0.12 |
| Urinary NTX (nmol BCE/mmoL CRE) | 61.2 ± 57.8 | 50.9 ± 31.3 | 0.24 |
| Serum whole PTH (pg/mL) | 29.4 ± 15.1 | 32.0 ± 12.5 | 0.40 |
| Serum 1,25(OH)2D3 (pg/mL) | 53.4 ± 18.8 | 60.5 ± 24.8 | 0.16 |
| Serum 25OHD (ng/mL) | 15.6 ± 6.3 | 16.2 ± 5.0 | 0.66 |
| eGFR (mL/min/1.73 m2) | 62.4 ± 20.6 | 69.8 ± 12.5 | 0.07 |
| Lumbar BMD (g/cm2) | 0.89 ± 0.18 | 0.87 ± 0.23 | 0.70 |
| T score | −1.64 ± 1.27 | −1.87 ± 1.68 | 0.38 |
| Total hip BMD (g/cm2) | 0.69 ± 0.13 | 0.69 ± 0.16 | 0.91 |
| T score | −1.98 ± 1.08 | −1.79 ± 1.26 | 0.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uehara, M.; Nakamura, Y.; Suzuki, T.; Nakano, M.; Takahashi, J. Efficacy and Safety of Oral Ibandronate versus Intravenous Zoledronic Acid on Bone Metabolism and Bone Mineral Density in Postmenopausal Japanese Women with Osteoporosis. J. Clin. Med. 2021, 10, 5420. https://doi.org/10.3390/jcm10225420
Uehara M, Nakamura Y, Suzuki T, Nakano M, Takahashi J. Efficacy and Safety of Oral Ibandronate versus Intravenous Zoledronic Acid on Bone Metabolism and Bone Mineral Density in Postmenopausal Japanese Women with Osteoporosis. Journal of Clinical Medicine. 2021; 10(22):5420. https://doi.org/10.3390/jcm10225420
Chicago/Turabian StyleUehara, Masashi, Yukio Nakamura, Takako Suzuki, Masaki Nakano, and Jun Takahashi. 2021. "Efficacy and Safety of Oral Ibandronate versus Intravenous Zoledronic Acid on Bone Metabolism and Bone Mineral Density in Postmenopausal Japanese Women with Osteoporosis" Journal of Clinical Medicine 10, no. 22: 5420. https://doi.org/10.3390/jcm10225420
APA StyleUehara, M., Nakamura, Y., Suzuki, T., Nakano, M., & Takahashi, J. (2021). Efficacy and Safety of Oral Ibandronate versus Intravenous Zoledronic Acid on Bone Metabolism and Bone Mineral Density in Postmenopausal Japanese Women with Osteoporosis. Journal of Clinical Medicine, 10(22), 5420. https://doi.org/10.3390/jcm10225420







