Comparison of Indexes to Measure Comorbidity Burden and Predict All-Cause Mortality in Rheumatoid Arthritis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Sources and Patient Cohort
2.3. Comparison of the Four Comorbidity Indexes
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics of RA Patients
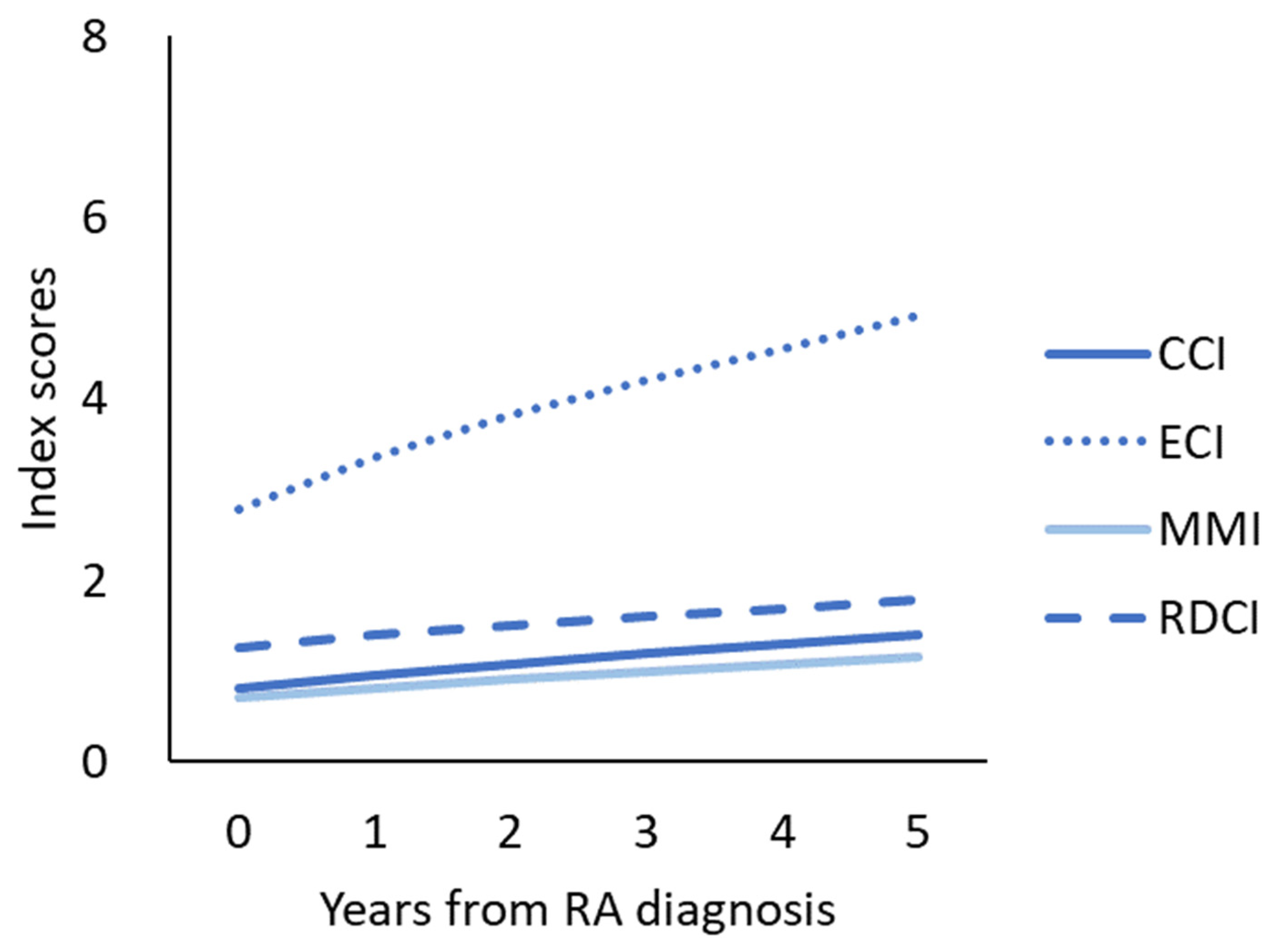
3.2. Four Comorbidity Index Increased after RA Was Diagnosed
3.3. Mortality Risk Associated with High Comorbidity Index
3.4. The Predictive Ability for Mortality among the Four Comorbidity Indexes
3.5. Comparison of Comorobidity Indexes in RA Patients and Control Group
4. Discussion
4.1. The Factors That Cause Comorbidity Indexes of RA Increase with Time
4.2. A High Comorbidity Index Predicts a High Mortality Rate
4.3. Comparison of the Four Comorbidity Indexes in RA Relevant to Mortality
4.4. Comorobidity Indexes in Control Group
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- An, J.; Nyarko, E.; Hamad, M.A. Prevalence of comorbidities and their associations with health-related quality of life and healthcare expenditures in patients with rheumatoid arthritis. Clin. Rheumatol. 2019, 38, 2717–2726. [Google Scholar] [CrossRef]
- Li, N.; Chan, E.; Peterson, S. The economic burden of depression among adults with rheumatoid arthritis in the United States. J. Med. Econ. 2019, 22, 372–378. [Google Scholar] [CrossRef]
- Uhlig, T.; Moe, R.H.; Kvien, T.K. The burden of disease in rheumatoid arthritis. PharmacoEconomics 2014, 32, 841–851. [Google Scholar] [CrossRef]
- Mikuls, T.R.; Saag, K.G. Comorbidity in rheumatoid arthritis. Rheum. Dis. Clin. N. Am. 2001, 27, 283–303. [Google Scholar] [CrossRef]
- Jonsen, A.; Clarke, A.E.; Joseph, L.; Belisle, P.; Bernatsky, S.; Nived, O.; Bengtsson, A.A.; Sturfelt, G.; Pineau, C.A. Association of the Charlson comorbidity index with mortality in systemic lupus erythematosus. Arthritis Care Res. 2011, 63, 1233–1237. [Google Scholar] [CrossRef]
- Soussan, M.; Abisror, N.; Abad, S.; Nunes, H.; Terrier, B.; Pop, G.; Eder, V.; Valeyre, D.; Sberro-Soussan, R.; Guillevin, L.; et al. FDG-PET/CT in patients with ANCA-associated vasculitis: Case-series and literature review. Autoimmun. Rev. 2014, 13, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Lionaki, S.; Blyth, E.R.; Hogan, S.L.; Hu, Y.; Senior, B.A.; Jennette, C.E.; Nachman, P.H.; Jennette, J.C.; Falk, R.J. Classification of antineutrophil cytoplasmic autoantibody vasculitides: The role of antineutrophil cytoplasmic autoantibody specificity for myeloperoxidase or proteinase 3 in disease recognition and prognosis. Arthritis Rheum. 2012, 64, 3452–3462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molto, A.; Dougados, M. Comorbidity indices. Clin. Exp. Rheumatol. 2014, 32 (Suppl. S85), S-131–S-134. [Google Scholar]
- Newschaffer, C.J.; Bush, T.L.; Penberthy, L.T. Comorbidity measurement in elderly female breast cancer patients with administrative and medical records data. J. Clin. Epidemiol. 1997, 50, 725–733. [Google Scholar] [CrossRef]
- Melfi, C.; Holleman, E.; Arthur, D.; Katz, B. Selecting a patient characteristics index for the prediction of medical outcomes using administrative claims data. J. Clin. Epidemiol. 1995, 48, 917–926. [Google Scholar] [CrossRef]
- Poses, R.M.; Smith, W.R.; McClish, D.K.; Anthony, M. Controlling for confounding by indication for treatment. Are administrative data equivalent to clinical data? Med. Care 1995, 33 (Suppl. S4), AS36–AS46. [Google Scholar] [PubMed]
- Ghali, W.A.; Hall, R.E.; Rosen, A.K.; Ash, A.S.; Moskowitz, M.A. Searching for an improved clinical comorbidity index for use with ICD-9-CM administrative data. J. Clin. Epidemiol. 1996, 49, 273–278. [Google Scholar] [CrossRef]
- Quan, H.; Sundararajan, V.; Halfon, P.; Fong, A.; Burnand, B.; Luthi, J.C.; Saunders, L.D.; Beck, C.A.; Feasby, T.E.; Ghali, W.A. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med. Care 2005, 43, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Hill, M.D. Stroke: The Elixhauser Index for comorbidity adjustment of in-hospital case fatality. Neurology 2008, 71, 283–287. [Google Scholar] [CrossRef] [PubMed]
- van Walraven, C.; Austin, P.C.; Jennings, A.; Quan, H.; Forster, A.J. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med. Care 2009, 47, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Condon, J.R.; You, J.; McDonnell, J. Performance of comorbidity indices in measuring outcomes after acute myocardial infarction in Australian indigenous and non-indigenous patients. Intern. Med. J. 2012, 42, e165–e173. [Google Scholar] [CrossRef] [PubMed]
- Menendez, M.E.; Neuhaus, V.; van Dijk, C.N.; Ring, D. The Elixhauser comorbidity method outperforms the Charlson index in predicting inpatient death after orthopaedic surgery. Clin. Orthop. Relat. Res. 2014, 472, 2878–2886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radner, H.; Yoshida, K.; Mjaavatten, M.D.; Aletaha, D.; Frits, M.; Lu, B.; Iannaccone, C.; Shadick, N.; Weinblatt, M.; Hmamouchi, I.; et al. Development of a multimorbidity index: Impact on quality of life using a rheumatoid arthritis cohort. Semin. Arthritis Rheum. 2015, 45, 167–173. [Google Scholar] [CrossRef]
- Wolfe, F.; Michaud, K.; Li, T.; Katz, R.S. Chronic conditions and health problems in rheumatic diseases: Comparisons with rheumatoid arthritis, noninflammatory rheumatic disorders, systemic lupus erythematosus, and fibromyalgia. J. Rheumatol. 2010, 37, 305–315. [Google Scholar] [CrossRef]
- England, B.R.; Sayles, H.; Mikuls, T.R.; Johnson, D.S.; Michaud, K. Validation of the rheumatic disease comorbidity index. Arthritis Care Res. 2015, 67, 865–872. [Google Scholar] [CrossRef]
- Arnett, F.C.; Edworthy, S.M.; Bloch, D.A.; McShane, D.J.; Fries, J.F.; Cooper, N.S.; Healey, L.A.; Kaplan, S.R.; Liang, M.H.; Luthra, H.S.; et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988, 31, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Deyo, R.A.; Cherkin, D.C.; Ciol, M.A. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J. Clin. Epidemiol. 1992, 45, 613–619. [Google Scholar] [CrossRef]
- Radner, H.; Yoshida, K.; Hmamouchi, I.; Dougados, M.; Smolen, J.S.; Solomon, D.H. Treatment Patterns of Multimorbid Patients with Rheumatoid Arthritis: Results from an International Cross-sectional Study. J. Rheumatol. 2015, 42, 1099–1104. [Google Scholar] [CrossRef]
- Shen, Q.; Lu, D.; Schelin, M.E.; Jöud, A.; Cao, Y.; Adami, H.O.; Cnattingius, S.; Fall, K.; Valdimarsdóttir, U.; Fang, F. Injuries before and after diagnosis of cancer: Nationwide register based study. BMJ 2016, 354, i4218. [Google Scholar] [CrossRef] [Green Version]
- Putrik, P.; Ramiro, S.; Lie, E.; Michaud, K.; Kvamme, M.K.; Keszei, A.P.; Kvien, T.K.; Uhlig, T.; Boonen, A. Deriving common comorbidity indices from the MedDRA classification and exploring their performance on key outcomes in patients with rheumatoid arthritis. Rheumatology 2018, 57, 548–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikiphorou, E.; de Lusignan, S.; Mallen, C.; Roberts, J.; Khavandi, K.; Bedarida, G.; Buckley, C.D.; Galloway, J.; Raza, K. Prognostic value of comorbidity indices and lung diseases in early rheumatoid arthritis: A UK population-based study. Rheumatology 2019, 59, 1296–1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- England, B.R.; Roul, P.; Yang, Y.; Sayles, H.; Yu, F.; Michaud, K.; Xie, F.; Curtis, J.R.; Mikuls, T.R. Burden and trajectory of multimorbidity in rheumatoid arthritis: A matched cohort study from 2006 to 2015. Ann. Rheum. Dis. 2020, 80, 286–292. [Google Scholar] [CrossRef]
- Gladman, D.D.; Hussain, F.; Ibanez, D.; Urowitz, M.B. The nature and outcome of infection in systemic lupus erythematosus. Lupus 2002, 11, 234–239. [Google Scholar] [CrossRef]
- Bosch, X.; Guilabert, A.; Pallares, L.; Cerveral, R.; Ramos-Casals, M.; Bove, A.; Ingelmo, M.; Font, J. Infections in systemic lupus erythematosus: A prospective and controlled study of 110 patients. Lupus 2006, 15, 584–589. [Google Scholar] [CrossRef]
- Bruce, I.N. ‘Not only...but also’: Factors that contribute to accelerated atherosclerosis and premature coronary heart disease in systemic lupus erythematosus. Rheumatology 2005, 44, 1492–1502. [Google Scholar] [CrossRef] [Green Version]
- Rahman, A. Management of cardiovascular risk factors in patients with systemic lupus erythematosus. Acta Reumatol. Port. 2008, 33, 13–15. [Google Scholar] [PubMed]
- Gordon, C. Long-term complications of systemic lupus erythematosus. Rheumatology 2002, 41, 1095–1100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Libby, P. Role of inflammation in atherosclerosis associated with rheumatoid arthritis. Am. J. Med. 2008, 121 (Suppl. S4), S21–S31. [Google Scholar] [CrossRef] [PubMed]
- Kapetanovic, M.C.; Lindqvist, E.; Geborek, P.; Saxne, T.; Eberhard, K. Long-term mortality rate in rheumatoid arthritis patients with disease onset in the 1980s. Scand. J. Rheumatol. 2011, 40, 433–438. [Google Scholar] [CrossRef]
- Yoshida, K.; Lin, T.C.; Wei, M.Y.; Malspeis, S.; Chu, S.H.; Camargo, C.A., Jr.; Raby, B.A.; Choi, H.K.; Tedeschi, S.K.; Barbhaiya, M.; et al. Roles of Postdiagnosis Accumulation of Morbidities and Lifestyle Changes in Excess Total and Cause-Specific Mortality Risk in Rheumatoid Arthritis. Arthritis Care Res. 2021, 73, 188–198. [Google Scholar] [CrossRef]
- Dadoun, S.; Zeboulon-Ktorza, N.; Combescure, C.; Elhai, M.; Rozenberg, S.; Gossec, L.; Fautrel, B. Mortality in rheumatoid arthritis over the last fifty years: Systematic review and meta-analysis. Jt. Bone Spine 2013, 80, 29–33. [Google Scholar] [CrossRef]
- Agca, R.; Heslinga, S.C.; Rollefstad, S.; Heslinga, M.; McInnes, I.B.; Peters, M.J.; Kvien, T.K.; Dougados, M.; Radner, H.; Atzeni, F.; et al. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann. Rheum. Dis. 2017, 76, 17–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panoulas, V.F.; Douglas, K.M.; Milionis, H.J.; Stavropoulos-Kalinglou, A.; Nightingale, P.; Kita, M.D.; Tselios, A.L.; Metsios, G.S.; Elisaf, M.S.; Kitas, G.D. Prevalence and associations of hypertension and its control in patients with rheumatoid arthritis. Rheumatology 2007, 46, 1477–1482. [Google Scholar] [CrossRef] [Green Version]
- Wilson, P.W. Established risk factors and coronary artery disease: The Framingham Study. Am. J. Hypertens. 1994, 7 Pt 2, 7s–12s. [Google Scholar] [CrossRef]
- Aslam, F.; Khan, N.A. Tools for the Assessment of Comorbidity Burden in Rheumatoid Arthritis. Front. Med. 2018, 5, 39. [Google Scholar] [CrossRef]
- Wei, M.Y.; Mukamal, K.J. Multimorbidity, Mortality, and Long-Term Physical Functioning in 3 Prospective Cohorts of Community-Dwelling Adults. Am. J. Epidemiol. 2018, 187, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Biggioggero, M.; Mesina, F.; Favalli, E.G. The Use of Rheumatic Disease Comorbidity Index for Predicting Clinical Response and Retention Rate in a Cohort of Rheumatoid Arthritis Patients Receiving Tumor Necrosis Factor Alpha Inhibitors. BioMed Res. Int. 2019, 2019, 6107217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pefoyo, A.J.; Bronskill, S.E.; Gruneir, A.; Calzavara, A.; Thavorn, K.; Petrosyan, Y.; Maxwell, C.J.; Bai, Y.; Wodchis, W.P. The increasing burden and complexity of multimorbidity. BMC Public Health 2015, 15, 415. [Google Scholar] [CrossRef] [Green Version]
- Fraccaro, P.; Kontopantelis, E.; Sperrin, M.; Peek, N.; Mallen, C.; Urban, P.; Buchan, I.E.; Mamas, M.A. Predicting mortality from change-over-time in the Charlson Comorbidity Index: A retrospective cohort study in a data-intensive UK health system. Medicine 2016, 95, e4973. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Value | ||||
|---|---|---|---|---|---|
| Male, n (%) | 5140 (20.8) | ||||
| Age years, mean ± SD | 50.2 ± 15.7 | ||||
| Comorbidity indexes, mean ± SD | |||||
| CCI | 0.8 ± 1.4 | ||||
| ECI | 2.8 ± 5.2 | ||||
| MMI | 0.7 ± 1.1 | ||||
| RDCI | 1.3 ± 1.5 | ||||
| Place of residence, n (%) | |||||
| Urban | 14,140 (57.1) | ||||
| Suburban | 7333 (29.6) | ||||
| Rural | 2279 (9.2) | ||||
| Unknown | 1015 (4.1) | ||||
| Income levels, n (%) | |||||
| Quintile 1 | 4548 (18.4) | ||||
| Quintile 2 | 4038 (16.3) | ||||
| Quintile 3 | 6926 (28.0) | ||||
| Quintile 4 | 4355 (17.6) | ||||
| Quintile 5 | 4758 (19.2) | ||||
| Unknown | 142 (0.6) | ||||
| Occupation, n (%) | |||||
| Dependents of the insured individuals | 6561 (26.5) | ||||
| Civil servants, teachers, military personnel and veterans | 1057 (4.3) | ||||
| Non-manual workers and professionals | 5688 (23.0) | ||||
| Manual workers | 9382 (37.9) | ||||
| Other | 2079 (8.4) | ||||
| Medications | |||||
| Hydroxychloroquine | 8001 (73.5) | ||||
| Azathioprine | 586 (5.4) | ||||
| Methotrexate | 2751 (25.3) | ||||
| Sulfasalazine | 4554 (41.8) | ||||
| Leflunomide | 105 (1.0) | ||||
| Cyclosporine | 165 (1.5) | ||||
| TNF inhibitor | 17 (0.2) | ||||
| Comorbidity Prevalence, % | CCI | ECI | MMI | RDCI | |
| Ulcer or stomach problem | 29.66% | V | |||
| Hypertension | 18.83% 15.61% | V | V | V | |
| Ulcer disease | 15.28% | V | |||
| Other cardiovascular | 12.46% | V | |||
| Peptic Ulcer Disease excluding bleeding | 11.12% | V | |||
| Liver Disease | 8.94% | V | |||
| Lung disease | 8.9% | V | |||
| Chronic Pulmonary Disease | 8.18% | V | |||
| Diabetes | 7.33% | V | V | ||
| Chronic pulmonary disease | 7.17% | V | |||
| Diabetes Uncomplicated (mild to moderate) | 6.27% | V | V | ||
| Coronary heart disease | 5.37% | V | |||
| Chronic obstructive pulmonary disease | 4.34% | V | |||
| Depression | 3.41% 3.22% | V | V | ||
| Asthma | 3.26% | V | |||
| Viral hepatitis | 3.19% | V | |||
| Diabetes with chronic complications | 2.92% | V | V | ||
| Cerebrovascular disease | 2.74% | V | |||
| Deficiency Anemia | 2.57% | V | |||
| Cardiac Arrhythmia | 2.51% | V | |||
| Cancer | 2.45% | V | V | ||
| Any tumor | 2.35% | V | |||
| Solid Tumor without Metastasis | 2.17% | V | |||
| Congestive heart failure | 2.11% | V | |||
| Mild liver disease | 1.98% | V | |||
| Congestive Heart Failure | 1.96% | V | |||
| Valvular Disease | 1.92% | V | |||
| Stroke | 1.75% 0.76% | V | V | ||
| Hypothyroidism | 1.49% | V | |||
| Renal Failure | 1.36% | V | |||
| Renal disease | 1.34% | V | |||
| Fluid and Electrolyte Disorders | 1.23% | V | |||
| Chronic Kidney Disease | 1.19% | V | |||
| Peripheral vascular disease | 1.07% 0.96% | V | V | ||
| Other Neurological Disorders | 1.05% | V | |||
| Fracture spine, hip, or leg | 0.98% | V | |||
| Myocardial infarction | 0.65% | V | |||
| Coagulopathy | 0.63% | V | |||
| Paralysis | 0.53% | V | |||
| Dementia | 0.5% | V | |||
| Hemiplegia | 0.49% | V | |||
| Pulmonary circulation disorders | 0.43% | V | |||
| Blood Loss Anemia | 0.4% | V | |||
| Weight Loss | 0.39% | V | |||
| Alcohol Abuse | 0.36% | V | |||
| Psychoses | 0.36% | V | |||
| Myocardial infarct | 0.27% | V | |||
| Metastatic solid tumor | 0.25% | V | |||
| Metastatic cancer | 0.25% | V | |||
| Lymphoma | 0.19% | V | |||
| Obesity | 0.12% | V | V | ||
| Moderate or severe liver disease | 0.11% | V | |||
| Drug Abuse | 0.1% | V | |||
| Diverticulitis | 0.04% | V | |||
| Acquired immune deficiency syndrome | 0.02% | V | V |
| Comorbidity Indexes | Before the Diagnostic Period | During the Diagnostic Period | After the Diagnostic Period | IRR (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|
| No. of Events | Crude IR | No. of Events | Crude IR | No. of Events | Crude IR | During Vs. Before the Diagnostic Period | After Vs. Before the Diagnostic Period | |
| CCI | 1311 | 0.007 | 2353 | 0.013 | 2062 | 0.012 | 1.80 (1.68 to 1.92) | 1.57 (1.47 to 1.69) |
| ECI | 4099 | 0.023 | 11,333 | 0.064 | 10,011 | 0.057 | 2.77 (2.67 to 2.87) | 2.44 (2.36 to 2.53) |
| MMI | 2249 | 0.013 | 2985 | 0.017 | 2828 | 0.016 | 1.33 (1.26 to 1.40) | 1.26 (1.19 to 1.33) |
| RDCI | 3601 | 0.020 | 5309 | 0.030 | 4728 | 0.027 | 1.47 (1.41 to 1.54) | 1.31 (1.26 to 1.37) |
| Comorbidity Indexes | Patient Number (%) | Mortality Rate (Per 1000 People) | Crude HR (95% CI) for Death | Age- and Sex-Adjusted HR (95% CI) for Death | |||
|---|---|---|---|---|---|---|---|
| 1-Year | 5-Year | 1-Year | 5-Year | 1-Year | 5-Year | ||
| CCI | |||||||
| Low score (0–1) | 20,244 (81.7) | 3.2 | 41 | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) |
| High score (≥2) | 4523 (18.2) | 22.8 | 175.8 | 7.3 (5.3–9.9) | 4.6 (4.2–5.1) | 4.3 (3.1–6.0) | 2.4 (2.1–2.6) |
| ECI | |||||||
| Low score (0–3) | 16,720 (67.5) | 3.2 | 38.4 | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) |
| High Score (≥3) | 8047 (32.5) | 14.2 | 122.3 | 4.5 (3.2–6.2) | 3.3 (3.0–3.7) | 2.9 (2.1–4.1) | 2.1 (1.9–2.3) |
| MMI | |||||||
| Low score (0–1) | 14,679 (59.3) | 2.4 | 30 | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) |
| High score (≥1) | 10,088 (40.7) | 13.1 | 117.1 | 5.5 (3.8–8.0) | 4.0 (3.6–4.5) | 3.0 (2.0–4.5) | 1.9 (1.7–2.2) |
| RDCI | |||||||
| Low score (0–2) | 16,132 (65.1) | 3 | 33 | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) |
| High score (≥2) | 8635 (34.9) | 13.7 | 126.6 | 4.5 (3.2–6.3) | 4.0 (3.6–4.5) | 2.5 (1.7–3.5) | 2.0 (1.8–2.2) |
| Models | 1-Year Mortality | 5-Year Mortality | ||
|---|---|---|---|---|
| Harrell’s C-Statistics | AIC | Harrell’s C-Statistics | AIC | |
| Base model | 0.744 | 1868 | 0.777 | 10,281 |
| Base model + CCI | 0.796 | 1783 | 0.802 | 9879 |
| Base model + ECI | 0.772 | 1829 | 0.793 | 10,024 |
| Base model + MMI | 0.779 | 1821 | 0.792 | 10,038 |
| Base model + RDCI | 0.773 | 1817 | 0.791 | 10,048 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.-J.; Chen, J.-S.; Luo, S.-F.; Kuo, C.-F. Comparison of Indexes to Measure Comorbidity Burden and Predict All-Cause Mortality in Rheumatoid Arthritis. J. Clin. Med. 2021, 10, 5460. https://doi.org/10.3390/jcm10225460
Huang Y-J, Chen J-S, Luo S-F, Kuo C-F. Comparison of Indexes to Measure Comorbidity Burden and Predict All-Cause Mortality in Rheumatoid Arthritis. Journal of Clinical Medicine. 2021; 10(22):5460. https://doi.org/10.3390/jcm10225460
Chicago/Turabian StyleHuang, Yun-Ju, Jung-Sheng Chen, Shue-Fen Luo, and Chang-Fu Kuo. 2021. "Comparison of Indexes to Measure Comorbidity Burden and Predict All-Cause Mortality in Rheumatoid Arthritis" Journal of Clinical Medicine 10, no. 22: 5460. https://doi.org/10.3390/jcm10225460
APA StyleHuang, Y.-J., Chen, J.-S., Luo, S.-F., & Kuo, C.-F. (2021). Comparison of Indexes to Measure Comorbidity Burden and Predict All-Cause Mortality in Rheumatoid Arthritis. Journal of Clinical Medicine, 10(22), 5460. https://doi.org/10.3390/jcm10225460







