Unbiased Metabolomics Links Fatty Acid Pathways to Psychiatric Symptoms in People Living with HIV
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design
2.2. Participants
2.3. Instruments
2.3.1. Clinical Measures
2.3.2. Mass Spectrometry and Spectral Data Processing
2.4. Statistical Analyses
2.5. Data Availability
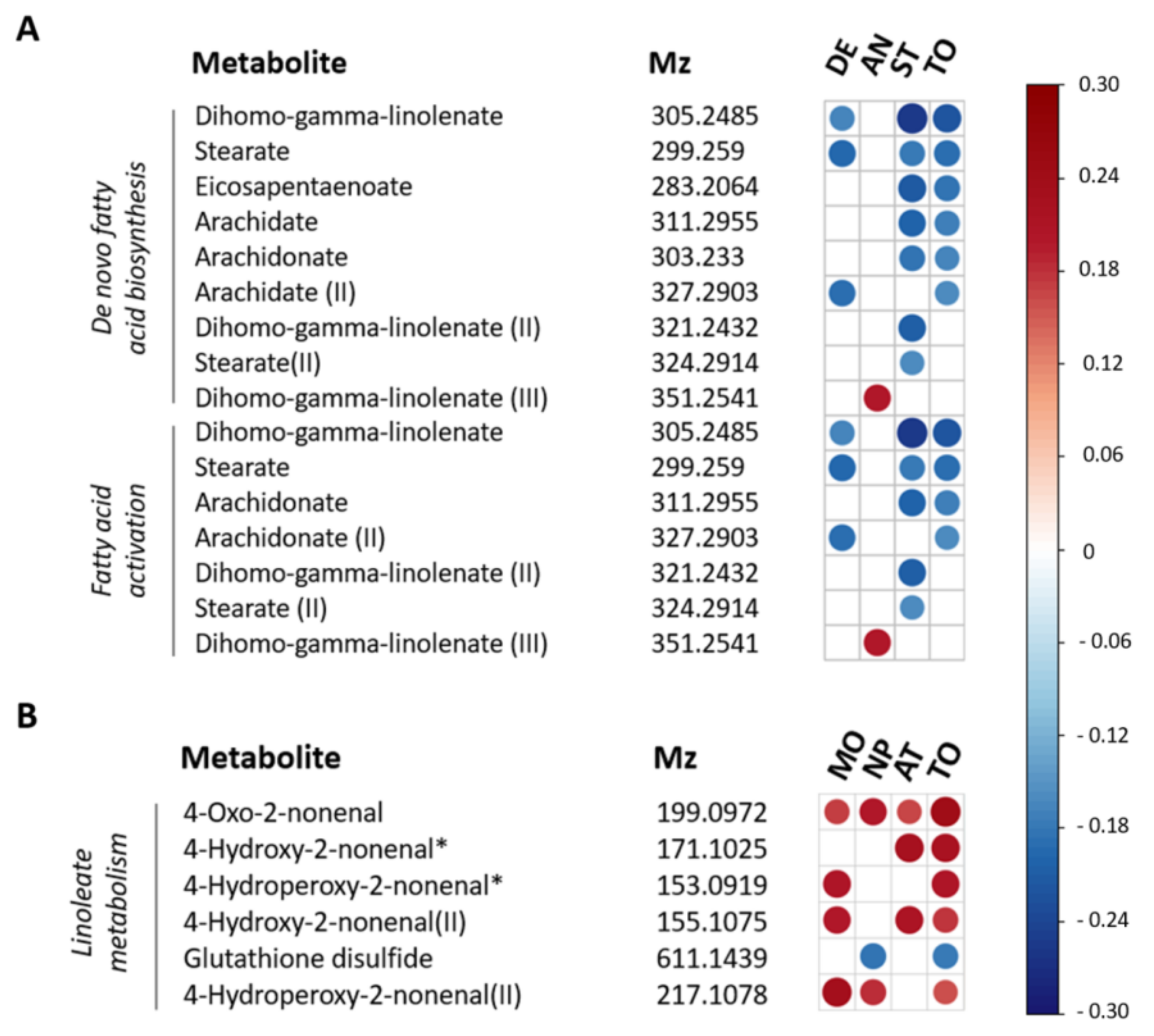
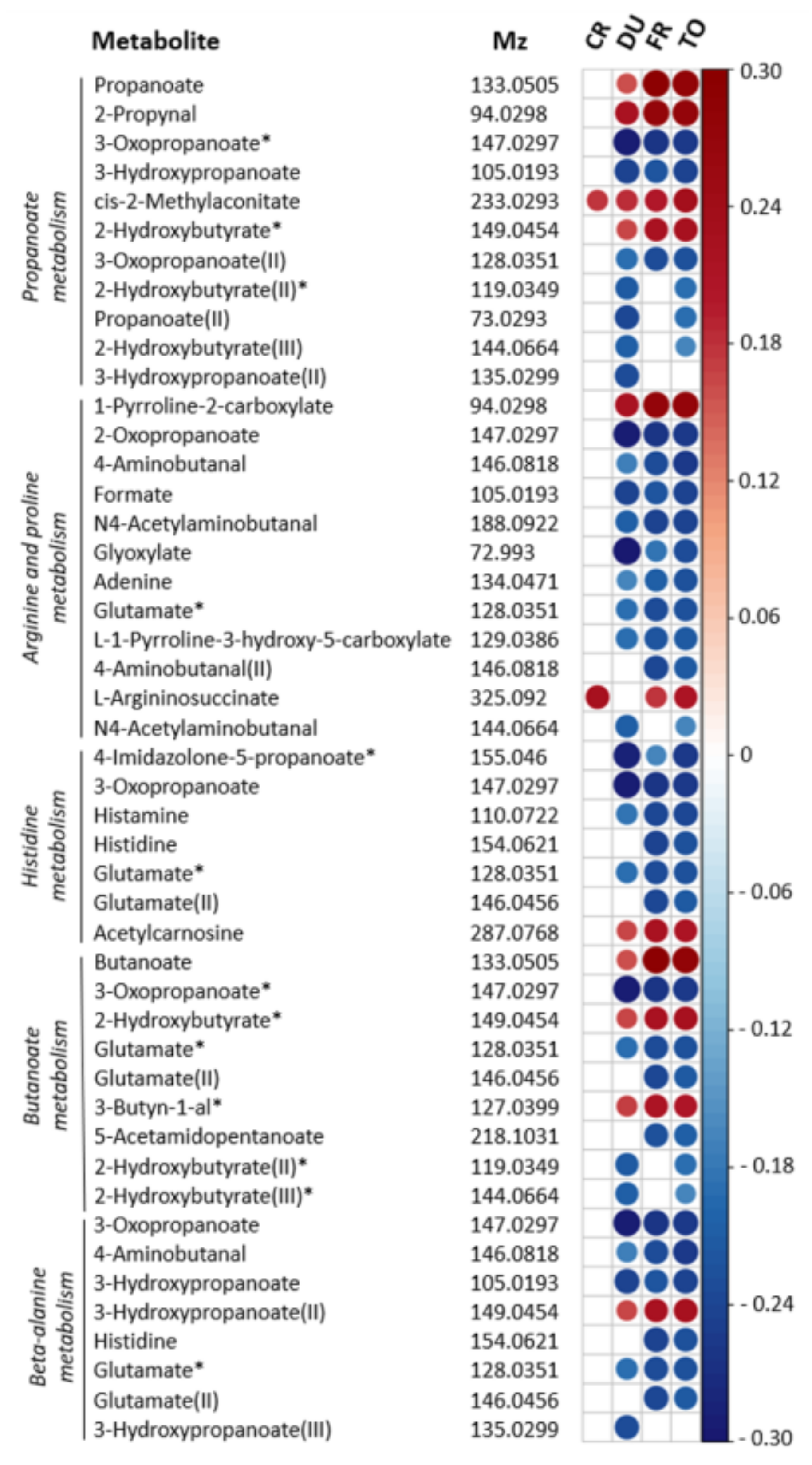
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UNAIDS Global AIDS. Update 2020. Seizing Moment–Tackling Entren. Inequalities End Epidemic 2020. [Google Scholar] [CrossRef]
- Deeks, S.G.; Lewin, S.R.; Havlir, D.V. The end of AIDS: HIV infection as a chronic disease. Lancet 2013, 382, 1525–1533. [Google Scholar] [CrossRef] [Green Version]
- Himelhoch, S.; Josephs, J.S.; Chander, G.; Korthuis, P.T.; Gebo, K.A. Use of outpatient mental health services and psychotropic medications among HIV-infected patients in a multisite, multistate study. Gen. Hosp. Psychiatry 2009, 31, 538–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catalan, J.; Tuffrey, V.; Ridge, D.; Rosenfeld, D. What influences quality of life in older people living with HIV? AIDS Res. Ther. 2017, 14, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Samet, J.H.; Phillips, S.J.; Horton, N.J.; Traphagen, E.T.; Freedberg, K.A. Detecting Alcohol Problems in HIV-Infected Patients: Use of the CAGE Questionnaire. AIDS Res. Hum. Retrovir. 2004, 20, 151–155. [Google Scholar] [CrossRef]
- Nanni, M.G.; Caruso, R.; Mitchell, A.J.; Meggiolaro, E.; Grassi, L. Depression in HIV Infected Patients: A Review. Curr. Psychiatry Rep. 2015, 17, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Carrico, A.W.; Riley, E.D.; Johnson, M.O.; Charlebois, E.D.; Neilands, T.B.; Remien, R.H.; Lightfoot, M.A.; Steward, W.T.; Weinhardt, L.S.; Kelly, J.A.; et al. Psychiatric risk factors for HIV disease progression: The role of inconsistent patterns of antiretroviral therapy utilization. J. Acquir. Immune Defic. Syndr. 2011, 56, 146–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arends, R.M.; Nelwan, E.J.; Soediro, R.; van Crevel, R.; Alisjahbana, B.; Pohan, H.T.; von Borries, A.K.L.; Schene, A.H.; van der Ven, A.J.A.M.; Schellekens, A.F.A. Associations between impulsivity, risk behavior and HIV, HBV, HCV and syphilis seroprevalence among female prisoners in Indonesia: A cross-sectional study. BioRxiv 2018, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Golub, S.A.; Gamarel, K.E.; Rendina, H.J. Loss and growth: Identity processes with distinct and complementary impacts on well-being among those living with chronic illness. Psychol. Health. Med. 2014, 19, 572–579. [Google Scholar] [CrossRef] [Green Version]
- Chambers, L.A.; Rueda, S.; Baker, D.N.; Wilson, M.G.; Deutsch, R.; Raeifar, E.; Rourke, S.B.; Adam, B.; Bacon, J.; Cairney, J.; et al. Stigma, HIV and health: A qualitative synthesis. BMC Public Health 2015, 15, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.M.; Fernandez, J.B.; Singer, E.J.; Commins, D.; Waldrop-Valverde, D.; Ownby, R.L.; Kumar, M. Human immunodeficiency virus type 1 in the central nervous system leads to decreased dopamine in different regions of postmortem human brains. J. Neurovirol. 2009, 15, 257–274. [Google Scholar] [CrossRef] [PubMed]
- Nutt, D.J.; Lingford-Hughes, A.; Erritzoe, D.; Stokes, P.R.A. The dopamine theory of addiction: 40 years of highs and lows. Nat. Rev. Neurosci. 2015, 16, 305–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riviera-Riviera, Y.; Garcia, Y.; Toro, V.; Cappas, N.; Lopez, P.; Yamamura, Y.; Rivera-Amill, V. Depression Correlates with Increased Plasma Levels of Inflammatory Cytokines and a Dysregulated Oxidant/Antioxidant Balance in HIV-1-Infected Subjects Undergoing Antiretroviral Therapy. J. Clin. Cell Immunol. 2014, 5, 1000276. [Google Scholar] [CrossRef] [Green Version]
- Bekhbat, M.; Mehta, C.C.; Kelly, S.D.; Vester, A.; Ofotokun, I.; Felger, J.; Wingood, G.; Anastos, K.; Gustafson, D.R.; Kassaye, S.; et al. HIV and symptoms of depression are independently associated with impaired glucocorticoid signaling. Psychoneuroendocrinology 2018, 96, 118–125. [Google Scholar] [CrossRef]
- Avdoshina, V.; Bachis, A.; Mocchetti, I. Synaptic dysfunction in human immunodeficiency virus type-1-positive subjects: Inflammation or impaired neuronal plasticity? J. Intern. Med. 2013, 273, 454–465. [Google Scholar] [CrossRef] [Green Version]
- Kesby, J.P.; Markou, A.; Semenova, S. The effects of HIV-1 regulatory TAT protein expression on brain reward function, response to psychostimulants and delay-dependent memory in mice. Neuropharmacology 2016, 109, 205–215. [Google Scholar] [CrossRef] [Green Version]
- Murrough, J.W.; Abdallah, C.G.; Mathew, S.J. Targeting glutamate signalling in depression: Progress and prospects. Nat. Rev. Drug Discov. 2017, 16, 472–486. [Google Scholar] [CrossRef] [PubMed]
- Amiel, J.M.; Mathew, S.J. Glutamate and anxiety disorders. Curr. Psychiatry Rep. 2007, 9, 278–283. [Google Scholar] [CrossRef]
- Ende, G.; Cackowski, S.; Van Eijk, J.; Sack, M.; Demirakca, T.; Kleindienst, N.; Bohus, M.; Sobanski, E.; Krause-Utz, A.; Schmahl, C. Impulsivity and Aggression in Female BPD and ADHD Patients: Association with ACC Glutamate and GABA Concentrations. Neuropsychopharmacology 2016, 41, 410–418. [Google Scholar] [CrossRef] [Green Version]
- Kalivas, P.W. The glutamate homeostasis hypothesis of addiction. Nat. Rev. Neurosci. 2009, 10, 561–572. [Google Scholar] [CrossRef]
- Johnson, C.H.; Ivanisevic, J.; Siuzdak, G. Metabolomics: Beyond biomarkers and towards mechanisms. Nat. Rev. Mol. Cell Biol. 2016, 17, 451–459. [Google Scholar] [CrossRef] [Green Version]
- Cassol, E.; Misra, V.; Morgello, S.; Kirk, G.D.; Mehta, S.H.; Gabuzda, D. Altered monoamine and acylcarnitine metabolites in HIV-positive and HIV-negative subjects with depression. J. Acquir. Immune Defic. Syndr. 2015, 69, 18–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGill, M.R.; Li, F.; Sharpe, M.R.; Williams, C.D.; Curry, S.C.; Ma, X.; Jaeschke, H. Circulating acylcarnitines as biomarkers of mitochondrial dysfunction after acetaminophen overdose in mice and humans. Arch. Toxicol. 2014, 88, 391–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukerji, S.S.; Misra, V.; Lorenz, D.R.; Chettimada, S.; Keller, K.; Letendre, S.; Ellis, R.J.; Morgello, S.; Parker, R.A.; Gabuzda, D. Low Neuroactive Steroids Identifies a Biological Subtype of Depression in Adults with Human Immunodeficiency Virus on Suppressive Antiretroviral Therapy. J. Infect. Dis. 2020, 223, 1601–1611. [Google Scholar] [CrossRef] [Green Version]
- Netea, M.G.; Joosten, L.A.B.; Li, Y.; Kumar, V.; Oosting, M.; Smeekens, S.; Jaeger, M.; ter Horst, R.; Schirmer, M.; Vlamakis, H.; et al. Understanding human immune function using the resources from the Human Functional Genomics Project. Nat. Med. 2016, 22, 831–833. [Google Scholar] [CrossRef]
- Van der Heijden, W.A.; Van de Wijer, L.; Keramati, F.; Trypsteen, W.; Rutsaert, S.; ter Horst, R.; Jaeger, M.; Koenen, H.J.P.M.; Stunnenberg, H.G.; Joosten, I.; et al. Chronic HIV infection induces transcriptional and functional reprogramming of innate immune cells. JCI Insight 2021, 6, e145928. [Google Scholar] [CrossRef] [PubMed]
- Lovibond, P.F.; Lovibond, S.H. The structure of negative emotional states: Comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behav. Res. Ther. 1995, 33, 335–343. [Google Scholar] [CrossRef]
- Brown, T.A.; Chorpita, B.F.; Korotitsch, W.; Barlow, D.H. Psychometric properties of the Depression Anxiety Stress Scales (DASS) in clinical samples. Behav. Res. Ther. 1997, 35, 79–89. [Google Scholar] [CrossRef]
- Crawford, J.R.; Henry, J.D. The Depression Anxiety Stress Scales (DASS): Normative data and latent structure in a large non-clinical sample. Br. J. Clin. Psychol. 2003, 42, 111–131. [Google Scholar] [CrossRef] [Green Version]
- Antony, M.M.; Cox, B.J.; Enns, M.W.; Bieling, P.J.; Swinson, R.P. Psychometric properties of the 42-item and 21-item versions of the Depression Anxiety Stress Scales in clinical groups and a community sample. Psychol. Assess. 1998, 10, 176–181. [Google Scholar] [CrossRef]
- Asante, K. Social support and the psychological wellbeing of people living with HIV/AIDS in Ghana. Afr. J. Psychiatry 2012, 15, 340–345. [Google Scholar] [CrossRef] [Green Version]
- Nüesch, R.; Gayet-Ageron, A.; Chetchotisakd, P.; Prasithsirikul, W.; Kiertiburanakul, S.; Munsakul, W.; Raksakulkarn, P.; Tansuphasawasdikul, S.; Chautrakarn, S.; Ruxrungtham, K.; et al. The Impact of Combination Antiretroviral Therapy and its Interruption on Anxiety, Stress, Depression and Quality of Life in Thai Patients. Open AIDS J. 2009, 3, 38–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barratt, E.S. Impulsiveness Subtraits: Arousal and Information Processing; Spence, C.E., Izard, C.E., Eds.; Elsevier: New York, NY, USA, 1985; pp. 137–146. [Google Scholar]
- Patton, J.H.; Stanford, M.S.; Barratt, E.S. Factor structure of the barratt impulsiveness scale. J. Clin. Psychol. 1995, 51, 768–774. [Google Scholar] [CrossRef]
- Carlson, S.R.; Johnson, S.C.; Jacobs, P.C. Disinhibited characteristics and binge drinking among university student drinkers. Addict. Behav. 2010, 35, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Kjome, K.; Lane, S.; Schmitz, J.; Green, C.; Ma, L.; Prasla, I.; Swann, A.; Moeller, G. Relationship between impulsivity and decision-making in cocaine dependence. Psychiatry Res. 2010, 178, 299–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, L.; Lim, A.; Lau, E.; Alicata, D. Chronic Tobacco-Smoking on Psychopathological Symptoms, Impulsivity and Cognitive Deficits in HIV-Infected Individuals. J. Neuroimmune Pharmacol. 2017, 12, 389–401. [Google Scholar] [CrossRef]
- Schippers, G.M.; Broekman, T.G.; Buchholz, A.; Koeter, M.W.J.; Van Den Brink, W. Measurements in the Addictions for Triage and Evaluation (MATE): An instrument based on the World Health Organization family of international classifications. Addiction 2010, 105, 862–871. [Google Scholar] [CrossRef] [PubMed]
- De Wildt, W.A.J.M.; Lehert, P.; Schippers, G.M.; Nakovics, H.; Mann, K.; Van Den Brink, W. Investigating the structure of craving using structural equation modeling in analysis of the obsessive-compulsive drinking scale: A multinational study. Alcohol. Clin. Exp. Res. 2005, 29, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Anton, R.F. The Obsessive Compulsive Drinking Scale. Arch. Gen. Psychiatry 1996, 53, 225. [Google Scholar] [CrossRef]
- Buchholz, A.; Broekman, T.; Schippers, G. Anwendung der ICF in der Suchthilfe am Beispiel des MATE-ICN. Suchttherapie 2011, 12, 14–19. [Google Scholar] [CrossRef]
- Hell, M.E.; Andersen, K.; Nielsen, A.S. Is the Danish Version of MATE Feasible? A Pilot Study on Feasibility and Adequacy. J. Dual Diagn. 2018, 14, 14–20. [Google Scholar] [CrossRef]
- Galland, D.; Simioni, N.; Schippers, G.; Broekman, T.; Alaux-Cantin, S.; Houchi, H.; Naassila, M.; Rolland, B. La MATE-Fr: Introduction à la version française d’un instrument d’évaluation globale en addictologie. Alcool. Addictol. 2018, 40, 140–148. [Google Scholar]
- Oudejans, S.; De Weert-Van Oene, G.; Spits, M.; De Wildt, W.; Merkx, M.; Dekker, J.; Visch, I.; Goudriaan, A. A Self-Reported Version of the Measurements in the Addictions for Triage and Evaluation-Q: Concurrent Validity with the MATE 2.1. Eur. Addict. Res. 2020, 26, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Fuhrer, T.; Heer, D.; Begemann, B.; Zamboni, N. High-throughput, accurate mass metabolome profiling of cellular extracts by flow injection-time-of-flight mass spectrometry. Anal. Chem. 2011, 83, 7074–7080. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for Comprehensive and Integrative Metabolomics Data Analysis. Curr. Protoc. Bioinform. 2019, 68, 1–128. [Google Scholar] [CrossRef]
- Li, S.; Park, Y.; Duraisingham, S.; Strobel, F.H.; Khan, N.; Soltow, Q.A.; Jones, D.P.; Pulendran, B. Predicting Network Activity from High Throughput Metabolomics. PLoS Comput. Biol. 2013, 9, e1003123. [Google Scholar] [CrossRef] [Green Version]
- Grosso, G.; Galvano, F.; Marventano, S.; Malaguarnera, M.; Bucolo, C.; Drago, F.; Caraci, F. Omega-3 fatty acids and depression: Scientific evidence and biological mechanisms. Oxid. Med. Cell. Longev. 2014, 2014, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Grosso, G.; Micek, A.; Marventano, S.; Castellano, S.; Mistretta, A.; Pajak, A.; Galvano, F. Dietary n-3 PUFA, fish consumption and depression: A systematic review and meta-analysis of observational studies. J. Affect. Disord. 2016, 205, 269–281. [Google Scholar] [CrossRef]
- Appleton, K.M.; Sallis, H.M.; Perry, R.; Ness, A.R.; Churchill, R. Omega-3 fatty acids for depression in adults (Intervention Review). Cochrane Collab. 2015, 11, 1–135. [Google Scholar] [CrossRef]
- Mocking, R.J.T.; Assies, J.; Ruhé, H.G.; Schene, A.H. Focus on fatty acids in the neurometabolic pathophysiology of psychiatric disorders. J. Inherit. Metab. Dis. 2018, 41, 597–611. [Google Scholar] [CrossRef] [Green Version]
- Lin, P.Y.; Huang, S.Y.; Su, K.P. A meta-analytic review of polyunsaturated fatty acid compositions in patients with depression. Biol. Psychiatry 2010, 68, 140–147. [Google Scholar] [CrossRef]
- Pu, J.; Liu, Y.; Zhang, H.; Tian, L.; Gui, S.; Yu, Y.; Chen, X.; Chen, Y.; Yang, L.; Ran, Y.; et al. An integrated meta-analysis of peripheral blood metabolites and biological functions in major depressive disorder. Mol. Psychiatry 2020, 26, 4265–4276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weidner, G.; Connor, S.L.; Hollis, J.F.; Connor, W.E. Improvements in hostility and depression in relation to dietary change and cholesterol lowering. The Family Heart Study. Ann. Intern. Med. 1992, 117, 820–823. [Google Scholar] [CrossRef]
- Bowman, E.R.; Kulkarni, M.; Gabriel, J.; Cichon, M.J.; Riedl, K.; Belury, M.A.; Lake, J.E.; Richardson, B.; Cameron, C.; Cameron, M.; et al. Altered Lipidome Composition Is Related to Markers of Monocyte and Immune Activation in Antiretroviral Therapy Treated Human Immunodeficiency Virus (HIV) Infection and in Uninfected Persons. Front. Immunol. 2019, 10, 785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hallahan, B.; Garland, M.R. Essential fatty acids and their role in the treatment of impulsivity disorders. Prostaglandins Leukot. Essent. Fat. Acids 2004, 71, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.P.C.; Su, K.P.; Mondelli, V.; Pariante, C.M. Omega-3 Polyunsaturated Fatty Acids in Youths with Attention Deficit Hyperactivity Disorder: A Systematic Review and Meta-Analysis of Clinical Trials and Biological Studies. Neuropsychopharmacology 2018, 43, 534–545. [Google Scholar] [CrossRef] [Green Version]
- Bazinet, R.P.; Layé, S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat. Rev. Neurosci. 2014, 15, 771–785. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef] [Green Version]
- Stilling, R.M.; van de Wouw, M.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. The neuropharmacology of butyrate: The bread and butter of the microbiota-gut-brain axis? Neurochem. Int. 2016, 99, 110–132. [Google Scholar] [CrossRef]
- Meckel, K.R.; Kiraly, D.D. A potential role for the gut microbiome in substance use disorders. Psychopharmacology 2019, 236, 1513–1530. [Google Scholar] [CrossRef] [PubMed]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota–gut–brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- Kiraly, D.D.; Walker, D.M.; Calipari, E.S.; Labonte, B.; Issler, O.; Pena, C.J.; Ribeiro, E.A.; Russo, S.J.; Nestler, E.J. Alterations of the host microbiome affect behavioral responses to cocaine. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef]
- Bandera, A.; De Benedetto, I.; Bozzi, G.; Gori, A. Altered gut microbiome composition in HIV infection: Causes, effects and potential intervention. Curr. Opin. HIV AIDS 2018, 13, 73–80. [Google Scholar] [CrossRef]
- González-Hernández, L.A.; Ruiz-Briseño, M.D.R.; Sánchez-Reyes, K.; Alvarez-Zavala, M.; Vega-Magaña, N.; López-Iñiguez, A.; Díaz-Ramos, J.A.; Martínez-Ayala, P.; Soria-Rodriguez, R.; Ramos-Solano, M.; et al. Alterations in bacterial communities, SCFA and biomarkers in an elderly HIV-positive and HIV-negative population in western Mexico. BMC Infect. Dis. 2019, 19, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.; Kim, M.; Kang, S.G.; Jannasch, A.H.; Cooper, B.; Patterson, J.; Kim, C.H. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol. 2015, 8, 80–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawkins, R.A. The blood-brain barrier and glutamate. Am. J. Clin. Nutr. 2009, 90, 867S–874S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alfredsson, G.; Wiesel, F.A.; Tylec, A. Relationships between glutamate and monoamine metabolites in cerebrospinal fluid and serum in healthy volunteers. Biol. Psychiatry 1988, 23, 689–697. [Google Scholar] [CrossRef]
- Koob, G.F.; Volkow, N.D. Neurocircuitry of addiction. Neuropsychopharmacology 2010, 35, 217–238. [Google Scholar] [CrossRef] [Green Version]
- Melendez, R.I.; Roman, C.; Capo-Velez, C.M.; Lasalde-Dominicci, J.A. Decreased glial and synaptic glutamate uptake in the striatum of HIV-1 gp120 transgenic mice. J. Neurovirol. 2016, 22, 358–365. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Bae, M.; Tovar-y-Romo, L.B.; Patel, N.; Bandaru, V.V.R.; Pomerantz, D.; Steiner, J.P.; Haughey, N.J. The human immunodeficiency virus coat protein gp120 promotes forward trafficking and surface clustering of NMDA receptors in membrane microdomains. J. Neurosci. 2011, 31, 17074–17090. [Google Scholar] [CrossRef] [Green Version]
- Vázquez-Santiago, F.J.; Noel, R.J.; Porter, J.T.; Rivera-Amill, V. Glutamate metabolism and HIV-associated neurocognitive disorders. J. Neurovirol. 2014, 20, 315–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gertsman, I.; Barshop, B.A. Promises and pitfalls of untargeted metabolomics. J. Inherit. Metab. Dis. 2018, 41, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Demetriou, C.; Ozer, B.U.; Essau, C.A. Self-Report Questionnaires. In The Encyclopedia of Clinical Psychology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 1–6. [Google Scholar]
- Insel, T.; Cuthbert, B.; Garvey, M.; Heinssen, R.; Pine, D.; Quinn, K.; Sanislow, C.; Wang, P. Research Domain Criteria (RDoC): Toward a new classification framework for research on mental disorders. Am. J. Psychiatry Online 2010, 748–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Demographic Characteristics | Psychiatric Characteristics | ||
|---|---|---|---|
| Age, mean (SD), y | 51.5 (10.7) | DASS-42 | |
| Females, No. (%) | 14 (8.9) | Total score, median (IQR) | 13 (24.6) |
| BMI, mean (SD), kg/m2 | 24.6 (3.8) | Total score > 60, No. (%) | 6 (3.8) |
| Civil status | Depression, median (IQR) | 4 (10.0) | |
| Living alone, No. (%) | 56 (25.7) | Anxiety, median (IQR) | 2 (5.0) |
| Living with partner/family, No. (%) | 95 (60.5) | Stress, median (IQR) | 7 (9.0) |
| Other, No. (%) | 6 (3.8) | BIS-11 | |
| Educational level achieved | Total score, mean (SD) | 60.0 (8.8) | |
| No certificate, No. (%) | 18 (11.5) | Motor impulsivity, mean (SD) | 20.4 (3.4) |
| Secondary school, No. (%) | 25 (15.9) | Nonplanning impulsivity, mean (SD) | 24.0 (4.9) |
| Vocational training, No. (%) | 60 (38.2) | Attentional impulsivity, mean (SD) | 15.6 (3.1) |
| Higher education, No. (%) | 54 (34.4) | MATE-Q | |
| HIV-related characteristics | Alcohol use, No. (%) | 109 (69.4) | |
| Time since HIV diagnosis, mean (SD), y | 9.9 (6.6) | Smoking, No. (%) | 46 (29.3) |
| Way of transmission | Cannabis use, No. (%) | 31 (19.7) | |
| MSM, No. (%) | 114 (72.6) | XTC use, No. (%) | 25 (15.9) |
| Heterosexual contact, No. (%) | 7 (4.5) | Cocaïne use, No. (%) | 5 (3.2) |
| Intravenous drug use, No. (%) | 3 (1.9) | OCDS-5, median (IQR) | 0 (4.0) |
| Other, No. (%) | 33 (21.0) | Use of psychiatric medication | |
| Nadir CD4+ count, mean (SD), cells/μL | 272.5 (170.6) | Benzodiazepines, No. (%) | 12 (7.6) |
| Current CD4+ count, mean (SD), cells/μL | 692.6 (258.6) | SSRI’s, No. (%) | 8 (5.1) |
| Time on cART, mean (SD), y | 8.6 (6.2) | TCA’s, No. (%) | 2 (1.3) |
| ARV classes | Antipsychotics, No. (%) | 6 (3.8) | |
| NRTI, No. (%) | 149 (94.9) | ||
| INSTI, No. (%) | 102 (65.0) | ||
| NtRTI, No. (%) | 70 (44.6) | ||
| NNRTI, No. (%) | 48 (30.6) | ||
| PI, No. (%) | 25 (15.9) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meeder, E.; Matzaraki, V.; Vadaq, N.; van de Wijer, L.; van der Ven, A.; Schellekens, A. Unbiased Metabolomics Links Fatty Acid Pathways to Psychiatric Symptoms in People Living with HIV. J. Clin. Med. 2021, 10, 5466. https://doi.org/10.3390/jcm10235466
Meeder E, Matzaraki V, Vadaq N, van de Wijer L, van der Ven A, Schellekens A. Unbiased Metabolomics Links Fatty Acid Pathways to Psychiatric Symptoms in People Living with HIV. Journal of Clinical Medicine. 2021; 10(23):5466. https://doi.org/10.3390/jcm10235466
Chicago/Turabian StyleMeeder, Elise, Vasiliki Matzaraki, Nadira Vadaq, Lisa van de Wijer, André van der Ven, and Arnt Schellekens. 2021. "Unbiased Metabolomics Links Fatty Acid Pathways to Psychiatric Symptoms in People Living with HIV" Journal of Clinical Medicine 10, no. 23: 5466. https://doi.org/10.3390/jcm10235466






