Multiple Approaches at Admission Based on Lung Ultrasound and Biomarkers Improves Risk Identification in COVID-19 Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Risk Prediction through Basic Clinical and Analytical Parameters
2.3. Point-of-Care Lung Ultrasound and Biomarkers
2.4. Primary and Secondary Outcomes
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Characteristics according to the PANDEMYC Score at Admission
3.3. Outcomes and Multivariable Logistic Regression Model
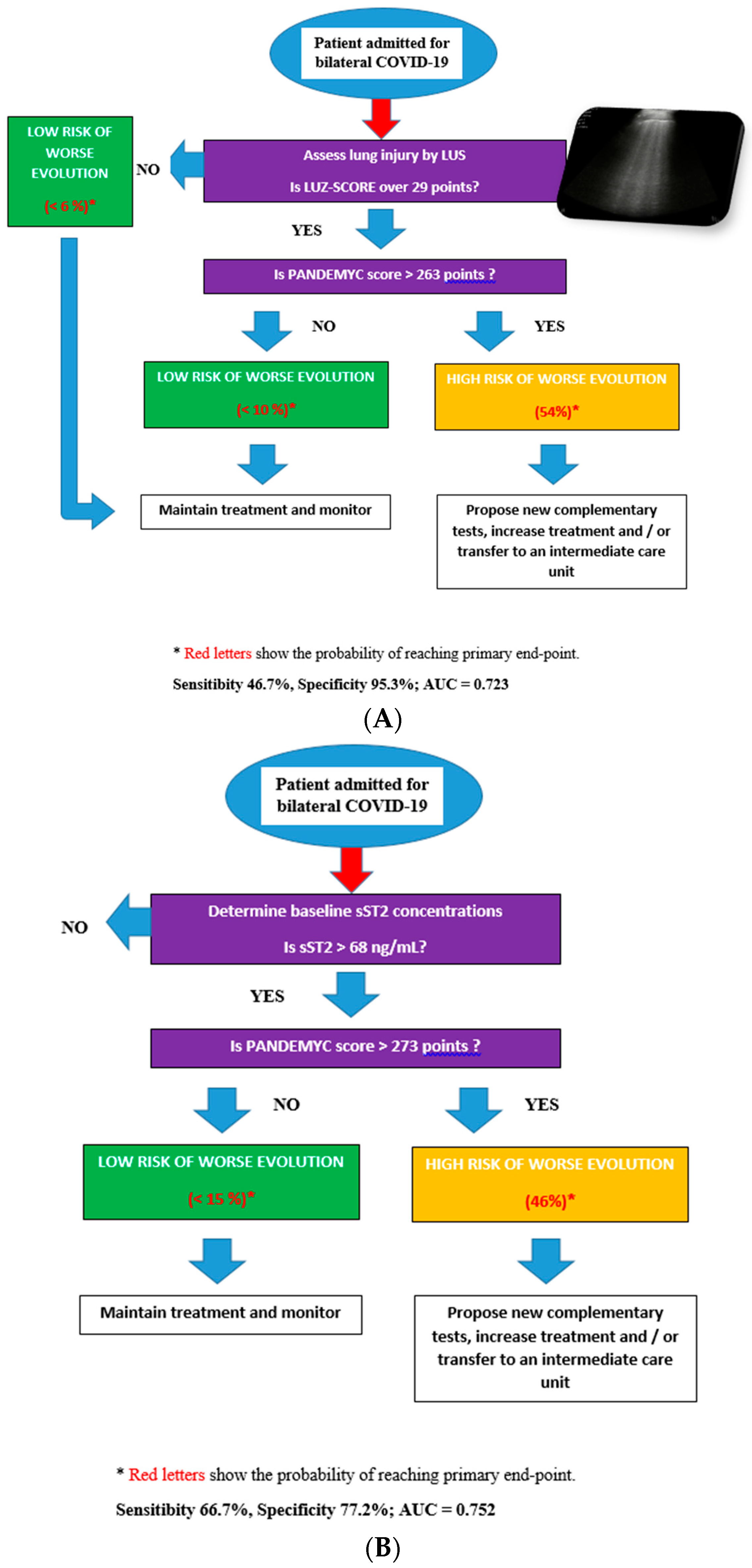
3.4. Decision Diagrams Based on Classification Trees
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ramanathan, K.; Antognini, D.; Combes, A.; Paden, M.; Zakhary, B.; Ogino, M.; Maclaren, G.; Brodie, D. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar]
- Guan, W.; Ni, Z.; Hu, Y.; Liang, W.; Ou, C.; He, J.; Liu, L.; Shan, H.; Lei, C.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Chen, R.; Lan, Z.; Ye, J.; Pang, L.; Liu, Y.; Wu, W.; Qin, X.; Guo, Y.; Zhang, P. Cytokine Storm: The Primary Determinant for the Pathophysiological Evolution of COVID-19 Deterioration. Front. Immunol. 2021, 12, 589095. [Google Scholar] [CrossRef]
- Darif, D.; Hammi, I.; Kihel, A.; El Idrissi Saik, I.; Guessous, F.; Akarid, K. The pro-inflammatory cytokines in COVID-19 pathogenesis: What goes wrong? Microb. Pathog. 2021, 153, 104799. [Google Scholar] [CrossRef]
- Stenmark, K.R.; Frid, M.G.; Gerasimovskaya, E.; Zhang, H.; McCarthy, M.K.; Thurman, J.M.; Morrison, T.E. Mechanisms of SARS-CoV-2-induced lung vascular disease: Potential role of complement. Pulm. Circ. 2021, 11, 20458940211015799. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, O.H.; Daas, F.M.; Salunkhe, V.; Petrey, J.L.; Cosar, E.F.; Ramirez, J.; Akca, O. Is Microthrombosis the Main Pathology in Coronavirus Disease 2019 Severity?—A Systematic Review of the Postmortem Pathologic Findings. Crit. Care Explor. 2021, 3, e0427. [Google Scholar] [CrossRef] [PubMed]
- Carod-Artal, F.J. Post-COVID-19 syndrome: Epidemiology, diagnostic criteria and pathogenic mechanisms involved. Crit. Care Explor. 2021, 3, e0427. [Google Scholar]
- Cabrera Martimbianco, A.L.; Pacheco, R.L.; Bagattini, Â.M.; Riera, R. Frequency, signs and symptoms, and criteria adopted for long COVID: A systematic review. Int. J. Clin. Pract. 2021, 75, e14357. [Google Scholar] [CrossRef]
- Torres-Macho, J.; Ryan, P.; Valencia, J.; Pérez-Butragueño, M.; Jiménez, E.; Fontán-Vela, M.; Izquierdo-García, E.; Fernandez-Jimenez, I.; Álvaro-Alonso, E.; Lazaro, A.; et al. The PANDEMYC Score. An Easily Applicable and Interpretable Model for Predicting Mortality Associated With COVID-19. J. Clin. Med. 2020, 9, 3066. [Google Scholar] [CrossRef]
- Arnold, D.T.; Attwood, M.; Barratt, S.; Morley, A.; Elvers, K.T.; McKernon, J.; Donald, C.; Oates, A.; Noel, A.; MacGowan, A.; et al. Predicting outcomes of COVID-19 from admission biomarkers: A prospective UK cohort study. Emerg. Med. J. 2021, 38, 543–548. [Google Scholar] [CrossRef]
- Kaftan, A.N.; Hussain, M.K.; Algenabi, A.A.; Naser, F.H.; Enaya, M.A. Predictive value of c⇓reactive protein, lactate dehydrogenase, ferritin and D-Dimer levels in diagnosing COVID-19 patients: A retrospective study. Acta Inform. Medica. 2021, 29, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, S.; Kuroda, S.; Sano, T.; Kitai, T.; Yonetsu, T.; Kohsaka, S.; Torii, S.; Kishi, T.; Komuro, I.; Hirata, K.; et al. Clinical and Biomarker Profiles and Prognosis of Elderly Patients With Coronavirus Disease 2019 (COVID-19) With Cardiovascular Diseases and/or Risk Factors. Circ. J. 2021, 85, 921–928. [Google Scholar] [CrossRef]
- Myhre, P.L.; Prebensen, C.; Jonassen, C.M.; Berdal, J.E.; Omland, T. SARS-CoV-2 Viremia is Associated with Inflammatory, But Not Cardiovascular Biomarkers, in Patients Hospitalized for COVID-19. J. Am. Heart Assoc. 2021, 10, e019756. [Google Scholar] [CrossRef] [PubMed]
- Cappa, G.; Secco, G.; Nganso, A.; Ruzga, R.; Perlini, S. The Role of Lung Ultrasound in Low-Resource Settings during the Coronavirus (SARS-CoV-2) Pandemic. J. Ultrasound Med. 2021, 24. [Google Scholar] [CrossRef]
- Lerchbaumer, M.H.; Lauryn, J.H.; Bachmann, U.; Enghard, P.; Fischer, T.; Grune, J.; Hegemann, N.; Khadzhynov, D.; Kruse, J.M.; Lehner, L.J.; et al. Point-of-care lung ultrasound in COVID-19 patients: Inter- and intra-observer agreement in a prospective observational study. Sci. Rep. 2021, 11, 10678. [Google Scholar] [CrossRef] [PubMed]
- Mejía, F.; Medina, C.; Cornejo, E.; Morello, E.; Vásquez, S.; Alave, J.; Schwalb, A.; Málaga, G. Oxygen saturation as a predictor of mortality in hospitalized adult patients with COVID-19 in a public hospital in Lima, Peru. PLoS ONE 2020, 15, e0244171. [Google Scholar] [CrossRef]
- Rubio-Gracia, J.; Giménez-López, I.; Garcés-Horna, V.; López-Delgado, D.; Sierra-Monzón, J.L.; Martínez-Lostao, L.; Josa-Laorden, C.; Ruiz-Laiglesia, F.; Pérez-Calvo, J.I.; Crespo-Aznarez, S.; et al. Point-of-care lung ultrasound assessment for risk stratification and therapy guiding in COVID-19 patients. A prospective non-interventional study. Eur. Respir. J. 2021, 58, 2004283. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Marteles, M.; Rubio-Gracia, J.; Peña-Fresneda, N.; Garcés-Horna, V.; Gracia-Tello, B.; Martínez-Lostao, L.; Crespo-Aznárez, S.; Pérez-Calvo, J.I.; Giménez-López, I. Early measurement of blood sST2 is a good predictor of death and poor outcomes in patients admitted for COVID-19 infection. J. Clin. Med. 2021, 10, 3534. [Google Scholar] [CrossRef] [PubMed]
- Harrell, F.E.; Califf, R.M.; Pryor, D.B.; Lee, K.L.; Rosati, R.A. Evaluating the Yield of Medical Tests. JAMA J. Am. Med. Assoc. 1982, 247, 2543–2546. [Google Scholar] [CrossRef]
- Molodianovitch, K.; Faraggi, D.; Reiser, B. Comparing the areas under two correlated ROC curves: Parametric and non-parametric approaches. Biometrical. J. 2006, 48, 745–757. [Google Scholar] [CrossRef] [PubMed]
- Linda, A.; Clark, D.P. Tree-Based Models. In Statistical Models in S, 1st ed.; Routledge: Oxfordshire, UK, 1992; p. 43. [Google Scholar]
- Seni, G.; Elder, J.F. Ensemble Methods in Data Mining: Improving Accuracy through Combining Predictions. Synth. Lect. Data Min. Knowl. Discov. 2010, 2, 1–126. [Google Scholar] [CrossRef]
- Knight, S.R.; Ho, A.; Pius, R.; Buchan, I.; Carson, G.; Drake, T.M.; Dunning, J.; Fairfield, C.J.; Gamble, C.; Green, C.A.; et al. Risk stratification of patients admitted to hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: Development and validation of the 4C Mortality Score. BMJ 2020, 370, m3339. [Google Scholar] [CrossRef]
- Hu, H.; Kong, W.; Yao, N.; Qiu, Y.; Yao, R. Prognostic value of three rapid scoring scales and combined predictors for the assessment of patients with coronavirus disease 2019. Nurs. Open 2021, 3. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, S.; Liu, Y.; Zhang, Y.; Zheng, C.; Zheng, Y.; Zhang, C.; Min, W.; Yu, M.; Hu, M. A preliminary study on the ultrasonic manifestations of peripulmonary lesions of non-critical novel coronavirus pneumonia (COVID-19). Res. Sq. 2020, 10–24. [Google Scholar] [CrossRef]
- Castelao, J.; Graziani, D.; Soriano, J.B.; Izquierdo, J.L. Findings and Prognostic Value of Lung Ultrasound in COVID-19 Pneumonia. J. Ultrasound Med. 2021, 40, 1315–1324. [Google Scholar] [CrossRef]
- Tung-Chen, Y.; Martí de Gracia, M.; Díez-Tascón, A.; Alonso-González, R.; Agudo-Fernández, S.; Parra-Gordo, M.L.; Ossaba-Vélez, S.; Rodríguez-Fuertes, P.; Llamas-Fuentes, R. Correlation between Chest Computed Tomography and Lung Ultrasonography in Patients with Coronavirus Disease 2019 (COVID-19). Ultrasound Med. Biol. 2020, 46, 2918–2926. [Google Scholar] [CrossRef]
- Pivetta, E.; Goffi, A.; Nazerian, P.; Castagno, D.; Tozzetti, C.; Tizzani, P.; Tizzani, M.; Porrino, G.; Ferreri, E.; Busso, V.; et al. Lung ultrasound integrated with clinical assessment for the diagnosis of acute decompensated heart failure in the emergency department: A randomized controlled trial. Eur. J. Heart Fail. 2019, 21, 754–766. [Google Scholar] [CrossRef] [Green Version]
- Pascual-Figal, D.A.; Pérez-Martínez, M.T.; Asensio-Lopez, M.C.; Sanchez-Más, J.; García-García, M.E.; Martinez, C.M.; Lencina, M.; Jara, R.; Januzzi, J.L.; Lax, A. Pulmonary Production of Soluble ST2 in Heart Failure. Circ. Heart Fail. 2018, 11, e005488. [Google Scholar] [CrossRef]
- Grasselli, G.; Zangrillo, A.; Zanella, A.; Antonelli, M.; Cabrini, L.; Castelli, A.; Cereda, D.; Coluccello, A.; Foti, G.; Fumagalli, R.; et al. Baseline Characteristics and Outcomes of 1591 Patients Infected with SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA-J. Am. Med. Assoc. 2020, 323, 1574–1581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zizzo, G.; Cohen, P.L. Imperfect storm: Is interleukin-33 the Achilles heel of COVID-19? Lancet Rheumatol. 2020, 2, e779–e790. [Google Scholar] [CrossRef]
- Villacorta, H.; Maisel, A.S. Teste com ST2 solúvel: Um biomarcador promissor no tratamento da insuficiência cardíaca. Arq. Bras. Cardiol. 2016, 106, 145–152. [Google Scholar] [PubMed]
- Colbert, R.; Mital, R.; Marston, N. Biomarkers in heart failure with preserved ejection fraction. Card. Biomark. Case Stud. Clin. Correl. 2016, 24, 357–365. [Google Scholar]
- Pascual-Figal, D.A.; Manzano-Fernández, S.; Boronat, M.; Casas, T.; Garrido, I.P.; Bonaque, J.C.; Pastor-Perez, F.; Valdés, M.; Januzzi, J.L. Soluble ST2, high-sensitivity troponin T- and N-terminal pro-B-type natriuretic peptide: Complementary role for risk stratification in acutely decompensated heart failure. Eur. J. Heart Fail. 2011, 13, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Giustino, G.; Croft, L.B.; Stefanini, G.G.; Bragato, R.; Silbiger, J.J.; Vicenzi, M.; Danilov, T.; Kukar, N.; Shaban, N.; Kini, A.; et al. Characterization of Myocardial Injury in Patients With COVID-19. J. Am. Coll. Cardiol. 2020, 76, 2043–2055. [Google Scholar] [CrossRef] [PubMed]
- Crnko, S.; Printezi, M.I.; Jansen, T.P.J.; Leiteris, L.; van der Meer, M.G.; Schutte, H.; van Faassen, M.; du Pré, B.C.; de Jonge, N.; Asselbergs, F.W.; et al. Prognostic biomarker soluble ST2 exhibits diurnal variation in chronic heart failure patients. ESC Heart Fail. 2020, 7, 1224–1233. [Google Scholar] [CrossRef]
- Hernández-Píriz, A.; Tung-Chen, Y.; Jiménez-Virumbrales, D.; Ayala-Larrañaga, I.; Barba-Martín, R.; Canora-Lebrato, J.; Zapatero-Gaviria, A.; García De Casasola-Sánchez, G. Usefulness of lung ultrasound in the early identification of severe COVID-19: Results from a prospective study. Med. Ultrason. 2021, 10. [Google Scholar] [CrossRef]
- Torres-Macho, J.; Sánchez-Fernández, M.; Arnanz-González, I.; Tung-Chen, Y.; Franco-Moreno, A.I.; Duffort-Falcó, M.; Beltrán-Romero, L.; Rodríguez-Suaréz, S.; Bernabeu-Wittel, M.; Urbano, E.; et al. Prediction accuracy of serial lung ultrasound in COVID-19 hospitalized patients (Pred-echovid study). J. Clin. Med. 2021, 10, 4818. [Google Scholar] [CrossRef] [PubMed]

| Variable | TOTAL | p < 25 (<214 Points) | p 25 to 75 (214–266 Points) | p > 75 (>266 Points) | p-Value |
|---|---|---|---|---|---|
| Total size (N) | 144 | ||||
| Age (years) * | 57.5 ± 12.8 | 42.7 ± 9.2 | 59.6 ± 9.3 | 68.1 ± 7.9 | <0.001 |
| Gender-Male (n (%)) | 87 (60.4) | 21 (58.3) | 42 (58.3) | 24 (66.7) | 0.471 |
| Duration of symptom (days) | 6.5 ± 3.3 | 6.6 ± 3.3 | 6.6 ± 3.3 | 6.0 ± 3.3 | 0.677 |
| Time until COVID-19 confirmation (Days) | 3 (7) | 2 (6) | 3 (7) | 3 (8) | 0.832 |
| Comorbidities (n (%)): | |||||
| • Hypertension | 54 (37.5) | 4 (11.1) | 28 (38.9) | 22 (61.1) | <0.001 |
| • Heart failure | 4 (2.8) | 0 (0.0) | 1 (1.4) | 3 (8.3) | 0.033 |
| • Dyslipidemia | 42 (29.2) | 7 (19.4) | 15 (20.8) | 20 (55.6) | 0.001 |
| • Coronary artery disease | 5 (3.5) | 1 (2.8) | 3 (4.2) | 1 (2.8) | 1.000 |
| • Diabetes | 25 (17.4) | 2 (5.6) | 17 (23.6) | 6 (16.7) | 0.215 |
| • History of smoking * | 48 (33.6) | 6 (16.7) | 26 (36.1) | 16 (45.7) | 0.010 |
| • COPD/Asthma | 16 (11.1) | ||||
| • Atrial/flutter fibrillation | 5 (3.6) | 0 (0.0) | 2 (2.9) | 3 (8.3) | 0.059 |
| • CKD | 7 (4.9) | 1 (2.8) | 1 (1.4) | 5 (13.9) | 0.029 |
| Clinical variables | |||||
| • BMI (Kgs/m2) | 28.9 (6.4) | 30.2 (7.8) | 29.1 (6.6) | 28.2 (4.9) | 0.568 |
| • SBP (mmHg) | 126.9 ± 16.7 | 124.6 ± 15.3 | 126.2 ± 17.9 | 130.5 ± 15.1 | 0.301 |
| • DBP (mmHg) | 77.2 ± 10.9 | 76.9 ± 11.4 | 76.5 ± 10.8 | 79.2 ± 10.5 | 0.480 |
| • HR (bpm) | 80.9 ± 12.8 | 83.1 ± 13.7 | 80.0 ± 13.4 | 80.5 ± 10.4 | 0.490 |
| • Estimated PAFI (mmHg) | 367 (92) | 429 (74) | 403 (94) | 340 (76) | 0.001 |
| • Borg scale for dyspnea (points) | 4 (6) | 5 (6) | 5 (4) | 4 (5) | 0.844 |
| Laboratory: | |||||
| • Urea (mg/dL) | 33 (19) | 28 (16) | 31 (14) | 40 (23) | 0.002 |
| • Creatinine (mg/dL) * | 0.94 (0.29) | 0.82 (0.26) | 0.89 (0.28) | 1.05 (0.51) | <0.001 |
| Variable (Continue) | TOTAL | p < 25 | p 25 to 75 | p > 75 | p-Value |
| Laboratory: | |||||
| • Aspartate transaminase (U/L) | 37 (27) | 38 (48) | 34 (20) | 41 (21) | 0.338 |
| • Alanine transaminase (U/L) | 31 (28) | 40 (56) | 31 (20) | 28 (25) | 0.175 |
| • Creatin phophokinase (U/L) | 94 (92) | 103 (116) | 83 (63) | 129 (92) | 0.048 |
| • Lactate deshidrogenase (U/L) | 306 (145) | 282 (94) | 306 (114) | 369 (202) | 0.007 |
| • C-Reactive Protein (mg/L) * | 63 (81) | 38 (77) | 53 (70) | 91 (98) | 0.002 |
| • Ferritin (ng/mL) | 707 (908) | 682 (917) | 710 (914) | 699 (1022) | 0.666 |
| • Hemoglobin (g/dL) * | 14.2 ± 1.5 | 14.3 ± 1.1 | 14.2 ± 1.6 | 14.1 ± 1.7 | 0.707 |
| • Total leucocytes (×1000) | 5.6 (3.1) | 5.0 (1.9) | 5.8 (3.6) | 6.1 (3.1) | 0.407 |
| • Total lymphocytes (×1000) * | 0.9 (0.7) | 1.1 (0.6) | 1.0 (0.6) | 0.7 (0.5) | 0.019 |
| • Total platelets (×1000) * | 173 (100) | 189 (75) | 176 (118) | 147 (87) | 0.016 |
| • D-Dimer (ng/mL) | 688 (633) | 664 (560) | 654 (519) | 802 (820) | 0.195 |
| • Fibrinogen (mg/dL) | 775 (208) | 783 (193) | 763 (212) | 779 (243) | 0.976 |
| • Interleukine-6 (pg/mL) | 40 (30) | 39 (27) | 29 (31) | 50 (57) | 0.041 |
| • sST2 (ng/L) | 53.1 (30.9) | 49.3 (24.9) | 50.8 (32.0) | 62.1 (36.6) | 0.060 |
| X-rays (n (%)) | 0.192 | ||||
| • Normal | 25 (17.9) | 8 (22.9) | 12 (16.9) | 5 (14.7) | |
| • Unilateral consolidation | 35 (25.0) | 9 (25.7) | 20 (28.2) | 6 (17.6) | |
| • Bilateral consolidations | 80 (57.1) | 18 (51.4) | 39 (54.9) | 23 (67.6) | |
| Lung ultrasound (LUZ-score) | 21 (10) | 18 (12) | 21 (10) | 22 (10) | 0.024 |
| Therapies (n (%)) | |||||
| • Colchicine | 10 (6.9) | 4 (11.1) | 4 (5.6) | 2 (5.6) | 0.525 |
| • Remdesivir | 46 (31.9) | 10 (27.8) | 18 (25.0) | 18 (50.0) | 0.026 |
| • Systemic corticosteroids | 113 (78.5) | 28 (77.8) | 52 (72.2) | 33 (91.7) | 0.153 |
| • Medium dose of corticosteroids (Dexametasone (mg)) | 6 (3) | 6 (0) | 6 (3) | 6 (3) | 0.156 |
| Univariable | Multivariable | ||||
|---|---|---|---|---|---|
| Variable | OR (CI 95%) | p-Value | Variable | OR (CI 95%) | p-Value |
| PANDEMYC score (points) | 1.03 (1.01–1.05) | 0.002 | PANDEMYC score (points) | 1.02 (1.01–1.04) | 0.034 |
| sST2 (ng/mL) | 1.02 (1.01–1.03) | 0.016 | sST2 (ng/mL) | 1.02 (1.01–1.03) | 0.038 |
| LUZ-score (points) | 1.13 (1.04–1.22) | 0.004 | LUZ-score (points) | 1.12 (1.02–1.22) | 0.014 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubio-Gracia, J.; Sánchez-Marteles, M.; Garcés-Horna, V.; Martínez-Lostao, L.; Ruiz-Laiglesia, F.; Crespo-Aznarez, S.; Peña-Fresneda, N.; Gracia-Tello, B.; Cebollada, A.; Carrera-Lasfuentes, P.; et al. Multiple Approaches at Admission Based on Lung Ultrasound and Biomarkers Improves Risk Identification in COVID-19 Patients. J. Clin. Med. 2021, 10, 5478. https://doi.org/10.3390/jcm10235478
Rubio-Gracia J, Sánchez-Marteles M, Garcés-Horna V, Martínez-Lostao L, Ruiz-Laiglesia F, Crespo-Aznarez S, Peña-Fresneda N, Gracia-Tello B, Cebollada A, Carrera-Lasfuentes P, et al. Multiple Approaches at Admission Based on Lung Ultrasound and Biomarkers Improves Risk Identification in COVID-19 Patients. Journal of Clinical Medicine. 2021; 10(23):5478. https://doi.org/10.3390/jcm10235478
Chicago/Turabian StyleRubio-Gracia, Jorge, Marta Sánchez-Marteles, Vanesa Garcés-Horna, Luis Martínez-Lostao, Fernando Ruiz-Laiglesia, Silvia Crespo-Aznarez, Natacha Peña-Fresneda, Borja Gracia-Tello, Alberto Cebollada, Patricia Carrera-Lasfuentes, and et al. 2021. "Multiple Approaches at Admission Based on Lung Ultrasound and Biomarkers Improves Risk Identification in COVID-19 Patients" Journal of Clinical Medicine 10, no. 23: 5478. https://doi.org/10.3390/jcm10235478
APA StyleRubio-Gracia, J., Sánchez-Marteles, M., Garcés-Horna, V., Martínez-Lostao, L., Ruiz-Laiglesia, F., Crespo-Aznarez, S., Peña-Fresneda, N., Gracia-Tello, B., Cebollada, A., Carrera-Lasfuentes, P., Pérez-Calvo, J. I., & Giménez-López, I. (2021). Multiple Approaches at Admission Based on Lung Ultrasound and Biomarkers Improves Risk Identification in COVID-19 Patients. Journal of Clinical Medicine, 10(23), 5478. https://doi.org/10.3390/jcm10235478






